Influence of Thermal Treatment and Granulometry on Physicochemical, Techno-Functional and Nutritional Properties of Lentil Flours
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Obtention of Lentil Flour
2.2.2. Particle Size Distribution of Flour
2.2.3. Color of Lentil Flours
2.2.4. Hydration and Absorptive Properties of Flours
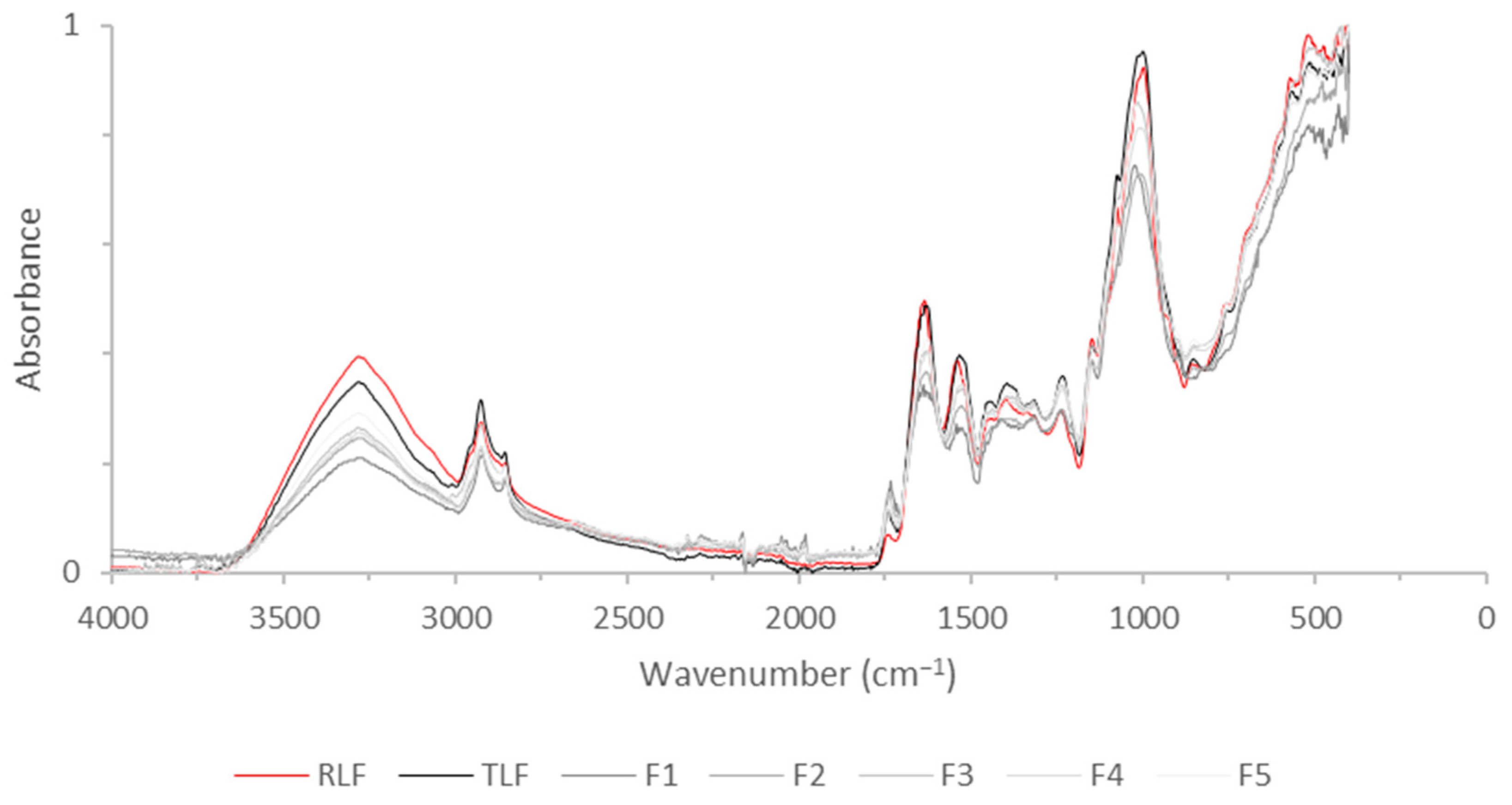
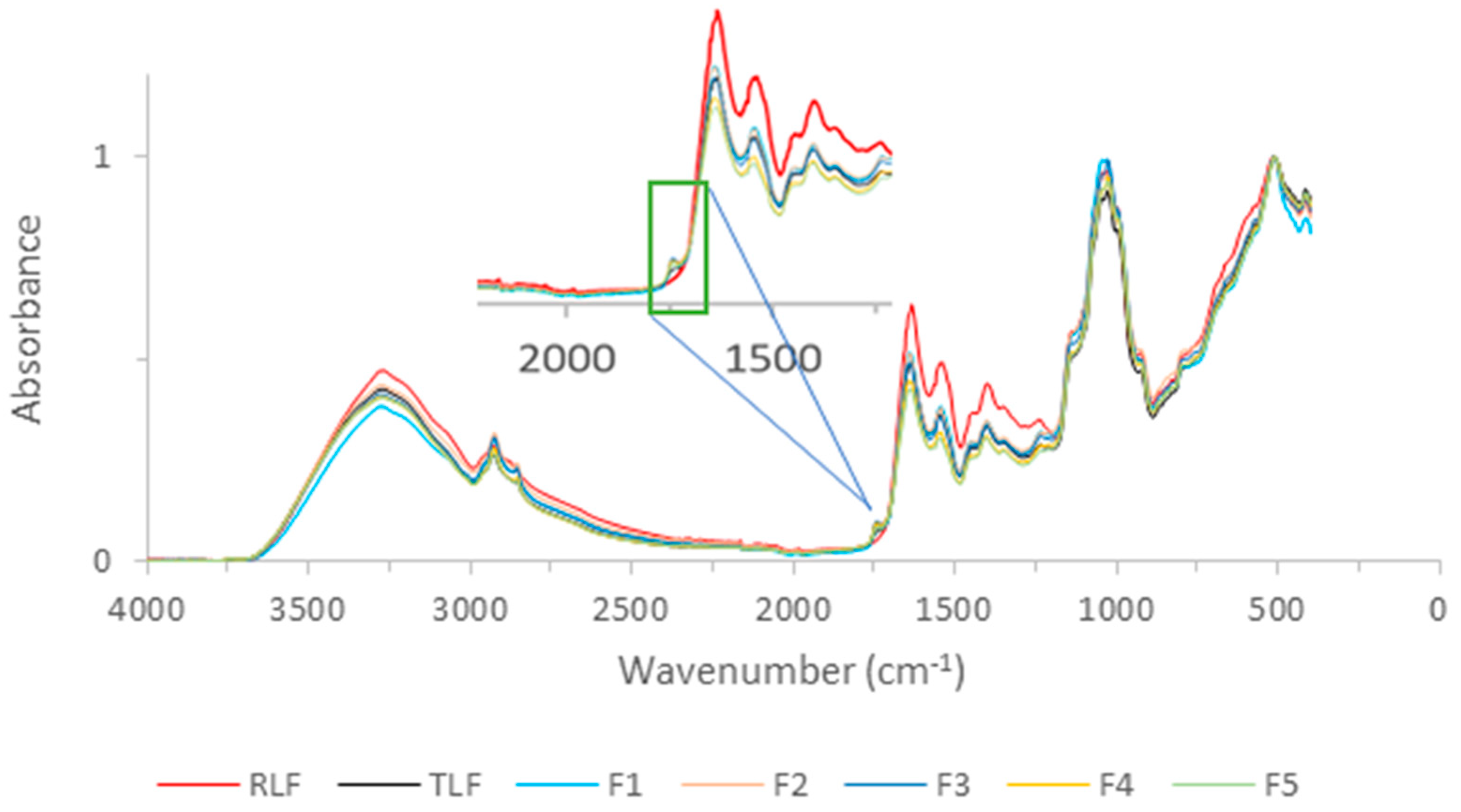
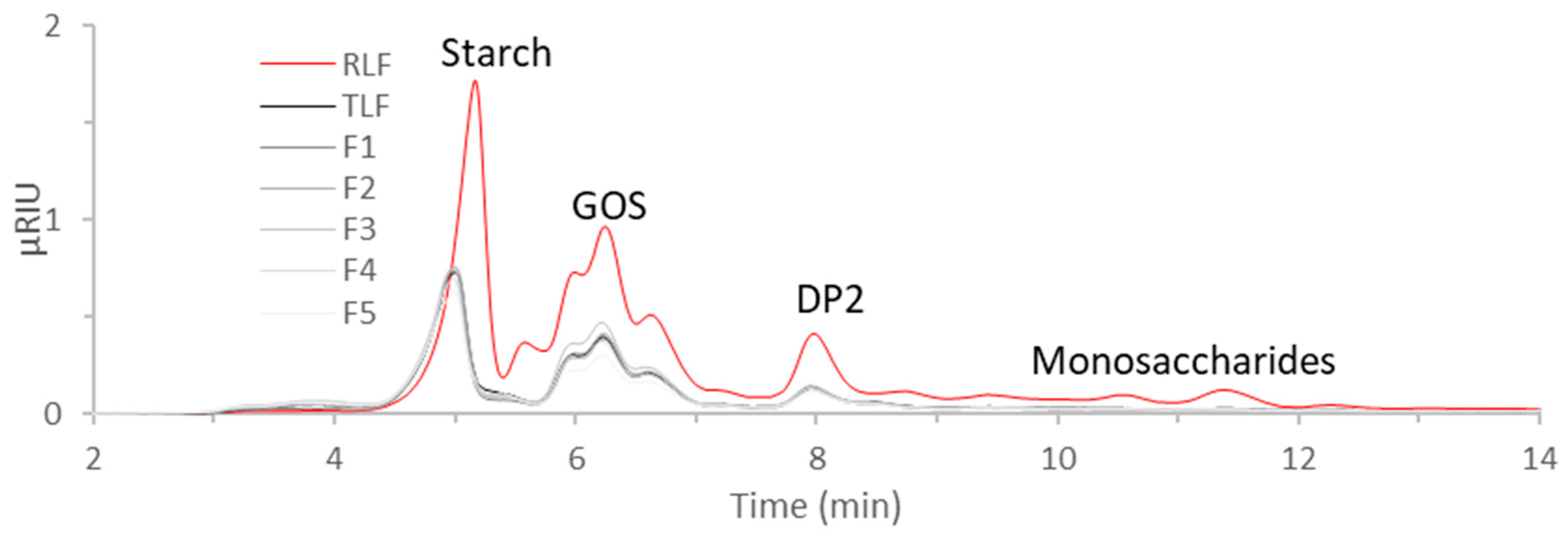
2.2.5. FTIR of Flours and Carbohydrate Analysis
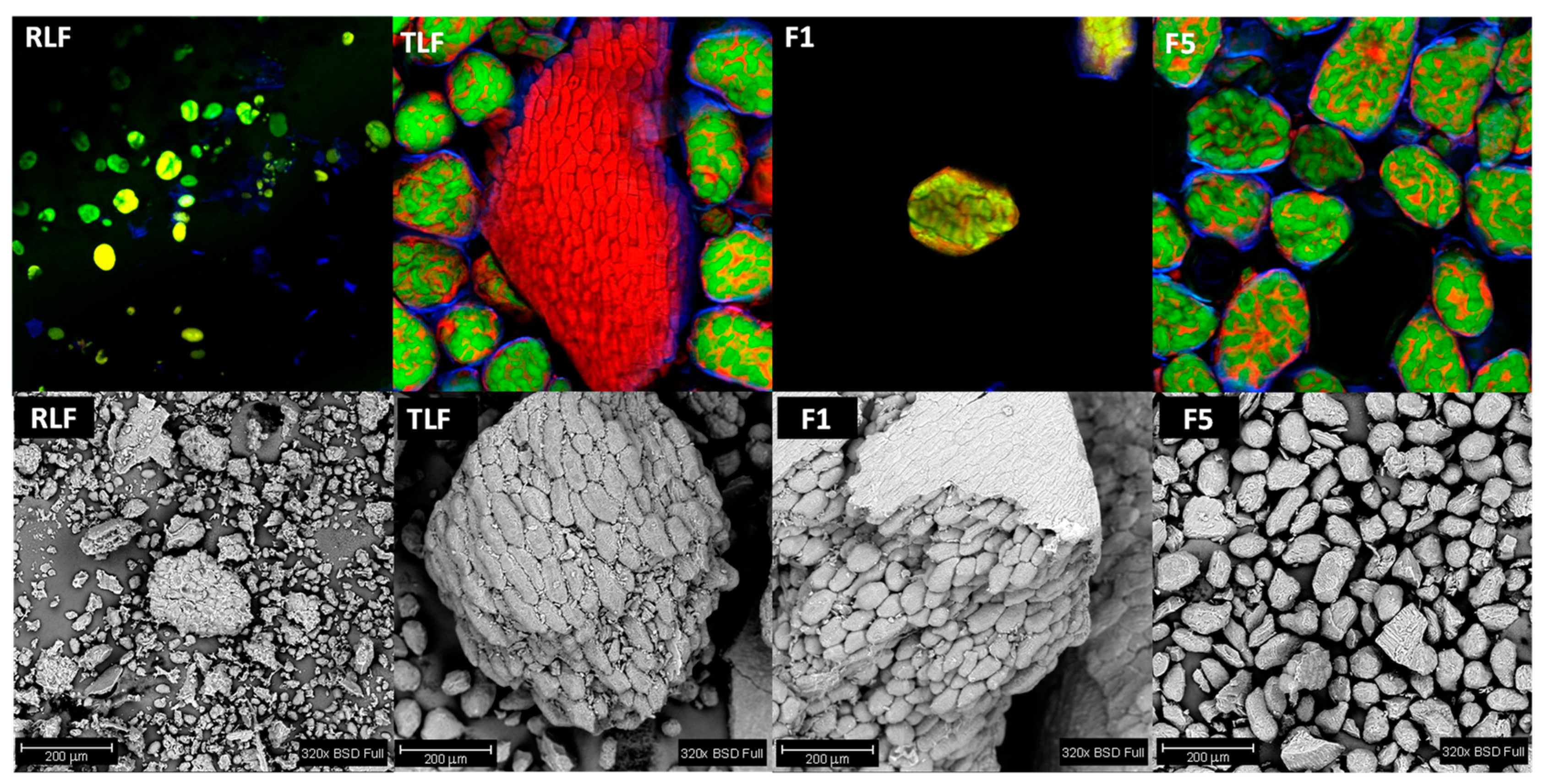
2.2.6. Microscopy Analysis of Flours
2.2.7. Thermal Analysis
2.2.8. Nutritional Profile of Flours
2.3. Statistical Analysis
3. Results and Discussion
3.1. Particle Size Distribution of Lentil Flour
3.2. Color of Lentil Flours
3.3. Hydration and Absorptive Properties of Lentil Flours
3.4. FTIR of Flours and Carbohydrate Analysis
3.5. Microstructure of Flours
3.6. Nutritional Profile of Lentil Flours
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gallego, C.; Belorio, M.; Guerra-Oliveira, P.; Gómez, M. Effects of adding chickpea and chestnut flours to layer cakes. Int. J. Food Sci. Technol. 2022, 57, 4840–4846. [Google Scholar] [CrossRef]
- Cichońska, P.; Ziarno, M. Legumes and legume-based beverages fermented with lactic acid bacteria as a potential carrier of probiotics and prebiotics. Microorganisms 2021, 10, 91. [Google Scholar] [CrossRef] [PubMed]
- Proserpio, C.; Bresciani, A.; Marti, A.; Pagliarini, E. Legume flour or bran: Sustainable, fiber-rich ingredients for extruded snacks? Foods 2020, 9, 1680. [Google Scholar] [CrossRef]
- Carboni, A.D.; Salinas, M.V.; Puppo, M.C. Production of legume-wheat dough of optimum quality for breadmaking: Essential analyses required. Curr. Opin. Food Sci. 2023, 49, 100970. [Google Scholar] [CrossRef]
- Odabas, E.; Aktas-Akyildiz, E.; Cakmak, H. Effect of raw and heat-treated yellow lentil flour on starch-based gluten-free noodle quality. J. Food Process. Preserv. 2022, 46, e16825. [Google Scholar] [CrossRef]
- Wang, N.; Hatcher, D.W.; Toews, R.; Gawalko, E.J. Influence of cooking and dehulling on nutritional composition of several varieties of lentils (Lens culinaris). LWT 2009, 42, 842–848. [Google Scholar] [CrossRef]
- Abbas, Y.; Ahmad, A. Impact of processing on nutritional and antinutritional factors of legumes: A review. Ann. Food Sci. Technol. 2018, 19, 2. [Google Scholar]
- Nosworthy, M.G.; Medina, G.; Franczyk, A.J.; Neufeld, J.; Appah, P.; Utioh, A.; Frohlich, P.; House, J.D. Effect of processing on the in vitro and in vivo protein quality of red and green lentils (Lens culinaris). Food Chem. 2018, 240, 588–593. [Google Scholar] [CrossRef]
- Roland, W.S.; Pouvreau, L.; Curran, J.; van de Velde, F.; de Kok, P.M. Flavor aspects of pulse ingredients. Cereal Chem. 2017, 94, 58–65. [Google Scholar] [CrossRef]
- Jiang, Z.Q.; Pulkkinen, M.; Wang, Y.J.; Lampi, A.M.; Stoddard, F.L.; Salovaara, H.; Sontag-Strohm, T. Faba bean flavour and technological property improvement by thermal pre-treatments. LWT 2016, 68, 295–305. [Google Scholar] [CrossRef]
- Samaranayaka, A. Lentil: Revival of poor man’s meat. In Sustainable Protein Sources; Nadathur, S.R., Wanasundara, J.P.D., Scanlin, L., Eds.; Academic Press: New York, NY, USA, 2017; pp. 185–196. [Google Scholar] [CrossRef]
- Siva, N.; Johnson, C.R.; Duckett, S.; Jesch, E.D.; Thavarajah, P. Can lentil (Lens culinaris Medikus) reduce the risk of obesity? J. Funct. Foods 2017, 38, 706–715. [Google Scholar] [CrossRef]
- Didinger, C.; Thompson, H.J. The role of pulses in improving human health: A review. Legume Sci. 2022, 4, e147. [Google Scholar] [CrossRef]
- Dahl, W.J.; Alvarez, M.M. Whole pulses and pulse fiber: Modulating gastrointestinal function and the microbiome. In Health Benefits of Pulses, 1st ed.; Dahl, W.J., Ed.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 91–108. [Google Scholar] [CrossRef]
- Jarpa-Parra, M. Lentil protein: A review of functional properties and food application. An overview of lentil protein functionality. Int. J. Food Sci. Technol. 2018, 53, 892–903. [Google Scholar] [CrossRef]
- Sharma, N.; Sahu, J.K.; Joshi, S.; Khubber, S.; Bansal, V.; Bhardwaj, A.; Bangar, S.P.; Bal, L.M. Modulation of lentil antinutritional properties using non-thermal mediated processing techniques–A review. J. Food Compos. Anal. 2022, 109, 104498. [Google Scholar] [CrossRef]
- Friedman, M.; Brandon, D.L. Nutritional and health benefits of soy proteins. J. Agric. Food Chem. 2001, 49, 1069–1086. [Google Scholar] [CrossRef]
- Gallo, V.; Romano, A.; Ferranti, P.; D’Auria, G.; Masi, P. Properties and in vitro digestibility of a bread enriched with lentil flour at different leavening times. Food Struct. 2022, 33, 100284. [Google Scholar] [CrossRef]
- Carboni, A.D.; Weisstaub, A.; Ferrero, C.; Zuleta, A.; Puppo, M.C. Impact of lentil-wheat bread on calcium metabolism, cecal and serum parameters in growing Wistar rats. Food Biosci. 2022, 48, 101805. [Google Scholar] [CrossRef]
- de la Hera, E.; Ruiz-París, E.; Oliete, B.; Gómez, M. Studies of the quality of cakes made with wheat-lentil composite flours. LWT 2012, 49, 48–54. [Google Scholar] [CrossRef]
- Kerr, W.L.; Ward, C.D.W.; McWatters, K.H.; Resurreccion, A.V.A. Effect of milling and particle size on functionality and physicochemical properties of cowpea flour. Cereal Chem. 2000, 77, 213–219. [Google Scholar] [CrossRef]
- Marchini, M.; Carini, E.; Cataldi, N.; Boukid, F.; Blandino, M.; Ganino, T.; Pellegrini, N. The use of red lentil flour in bakery products: How do particle size and substitution level affect rheological properties of wheat bread dough? LWT 2021, 136, 110299. [Google Scholar] [CrossRef]
- Kathirvel, P.; Yamazaki, Y.; Zhu, W.; Luhovyy, B.L. Glucose release from lentil flours digested in vitro: The role of particle size. Cereal Chem. 2019, 96, 1126–1136. [Google Scholar] [CrossRef]
- Suo, X.; Dall’Asta, M.; Giuberti, G.; Minucciani, M.; Wang, Z.; Vittadini, E. Effect of “shape” on technological properties and nutritional quality of chickpea-corn-rice gluten free pasta. LWT 2024, 192, 115661. [Google Scholar] [CrossRef]
- Anuntagool, J.; Soonthonsun, S. Effect of particle size classification on properties of flour from jack bean: An under-utilized high protein legumes. LWT 2023, 189, 115418. [Google Scholar] [CrossRef]
- Contini, G.K.; Simões, D.R.S.; Los, P.R.; Kotovicz, V.; Bedin, A.C.; Martins, A.; Demiate, I.M. High nutritional value muffins produced with wholemeal rye (Secale cereale L.) and wholemeal bean (Phaseolus vulgaris L.) flour mix. Food Sci. Technol. 2022, 42, e30022. [Google Scholar] [CrossRef]
- Thakur, S.; Scanlon, M.G.; Tyler, R.T.; Milani, A.; Paliwal, J. Pulse flour characteristics from a wheat flour miller’s perspective: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 775–797. [Google Scholar] [CrossRef] [PubMed]
- Millar, K.A.; Gallagher, E.; Burke, R.; McCarthy, S.; Barry-Ryan, C. Proximate composition and anti-nutritional factors of fava-bean (Vicia faba), green-pea and yellow-pea (Pisum sativum) flour. J. Food Compos. Anal. 2019, 82, 103233. [Google Scholar] [CrossRef]
- Pulivarthi, M.K.; Nkurikiye, E.; Watt, J.; Li, Y.; Siliveru, K. Comprehensive understanding of roller milling on the physicochemical properties of red lentil and yellow pea flours. Processes 2021, 9, 1836. [Google Scholar] [CrossRef]
- Bourré, L.; Frohlich, P.; Young, G.; Borsuk, Y.; Sopiwnyk, E.; Sarkar, A.; Malcolmson, L. Influence of particle size on flour and baking properties of yellow pea, navy bean, and red lentil flours. Cereal Chem. 2019, 96, 655–667. [Google Scholar] [CrossRef]
- Ahmed, J.; Taher, A.; Mulla, M.Z.; Al-Hazza, A.; Luciano, G. Effect of sieve particle size on functional, thermal, rheological and pasting properties of Indian and Turkish lentil flour. J. Food Eng. 2016, 186, 34–41. [Google Scholar] [CrossRef]
- Buera, M.P.; Retriella, C.; Lozano, R.D. Definition of colour in the nonenzymatic browning. Die Farbe 1985, 33, 316–326. [Google Scholar]
- Mesías, M.; Morales, F.J. Effect of different flours on the formation of hydroxymethylfurfural, furfural, and dicarbonyl compounds in heated glucose/flour systems. Foods 2017, 6, 14. [Google Scholar] [CrossRef] [PubMed]
- Frühwirth, S.; Call, L.; Maier, F.A.; Hebenstreit, V.; D’Amico, S.; Pignitter, M. LC–MS/MS method validation for the quantitation of 1-kestose in wheat flour. J. Food Compos. Anal. 2021, 100, 103930. [Google Scholar] [CrossRef]
- Prieto-Santiago, V.; del Mar Cavia, M.; Barba, F.J.; Alonso-Torre, S.R.; Carrillo, C. Multiple reaction monitoring for identification and quantification of oligosaccharides in legumes using a triple quadrupole mass spectrometer. Food Chem. 2022, 368, 130761. [Google Scholar] [CrossRef]
- Martins, G.N.; Carboni, A.D.; Hugo, A.A.; Castilho, P.C.; Gómez-Zavaglia, A. Chickpeas’ and Lentils’ Soaking and Cooking Wastewaters Repurposed for Growing Lactic Acid Bacteria. Foods 2023, 12, 2324. [Google Scholar] [CrossRef] [PubMed]
- AACC. Approved Methods of the AACC: Methods 46-12.01, 30-10.01, 32–05.01, 08-01.01, 44-15.02, 10th ed.; AACC: St. Paul, MN, USA, 2000. [Google Scholar]
- Bragança, G.; Ávila, B.; Rockenbach, R.; Santos, M.; Alves, G.; de los Santos, M. Effects of different hydration treatments on technological, physical, nutritional, and bioactive parameters of lentils. Rev. Chil. Nutr. 2020, 47, 658–668. [Google Scholar] [CrossRef]
- Gyawali, R.; Ibrahim, S.A. Effects of hydrocolloids and processing conditions on acid whey production with reference to Greek yogurt. Trends Food Sci. Technol. 2016, 56, 61–76. [Google Scholar] [CrossRef]
- Sahni, C.; Gupta, R.K.; Nand, P. Insignificant viability of the granules of probiotic and prebiotic with skimmed milk powder. Biomed. Prev. Nutr. 2014, 4, 603–605. [Google Scholar] [CrossRef]
- Troller, J.A.; Christian, J.H.B. Water Activity and Food, 1st ed.; Academic Press: New York, NY, USA, 1978. [Google Scholar]
- Pittia, P.; Paparella, A. Safety by control of water activity: Drying, smoking, and salt or sugar addition. In Regulating Safety of Traditional and Ethnic Foods; Vishweshwaraiah, P., Martín-Belloso, O., Keener, Y.L., Eds.; Academic Press: Nueva York, NY, USA, 2016. [Google Scholar] [CrossRef]
- Nagai, N.F.; Argel, N.S.; Andrés, S.C. Physicochemical characterization and functional properties of flours from North-Western Argentina bean (Phaseolus vulgaris L.) cultivars. Cereal Chem. 2022, 99, 850–859. [Google Scholar] [CrossRef]
- Ma, Z.; Boye, J.I.; Simpson, B.K.; Prasher, S.O.; Monpetit, D.; Malcolmson, L. Thermal processing effects on the functional properties and microstructure of lentil, chickpea, and pea flours. Food Res. Int. 2011, 44, 2534–2544. [Google Scholar] [CrossRef]
- Aguilera, Y.; Esteban, R.M.; Benitez, V.; Molla, E.; Martin-Cabrejas, M.A. Starch, functional properties, and microstructural characteristics in chickpea and lentil as affected by thermal processing. J. Agric. Food Chem. 2009, 57, 10682–10688. [Google Scholar] [CrossRef]
- Chávez-Murillo, C.E.; Veyna-Torres, J.I.; Cavazos-Tamez, L.M.; de la Rosa-Millán, J.; Serna-Saldívar, S.O. Physicochemical characteristics, ATR-FTIR molecular interactions and in vitro starch and protein digestion of thermally-treated whole pulse flours. Food Res. Int. 2018, 105, 371–383. [Google Scholar] [CrossRef] [PubMed]
- Kotsiou, K.; Sacharidis, D.D.; Matsakidou, A.; Biliaderis, C.G.; Lazaridou, A. Physicochemical and functional aspects of composite wheat-roasted chickpea flours in relation to dough rheology, bread quality and staling phenomena. Food Hydrocoll. 2022, 124, 107322. [Google Scholar] [CrossRef]
- Rodriguez-Saona, L.; Ayvaz, H.; Wehling, R.L. Infrared and Raman Spectroscopy. In Food Analysis. Food Science Text Series; Nielsen, S.S., Ed.; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Bhatty, R.S. Cooking quality of lentils: The role of structure and composition of cell walls. J. Agric. Food Chem. 1990, 38, 376–383. [Google Scholar] [CrossRef]
- Gilani, G.S.; Xiao, C.W.; Cockell, K.A. Impact of antinutritional factors in food proteins on the digestibility of protein and the bioavailability of amino acids and on protein quality. Br. J. Nutr. 2012, 108, S315–S332. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wang, Y.; Liu, X.; Liu, Y.; Ji, L.; Zhou, Y.; Sun, L. Comparison of analytical methods for determining methylesterification and acetylation of pectin. Appl. Sci. 2021, 11, 4461. [Google Scholar] [CrossRef]
- Hans Tromp, R.; Van de Velde, F.; Van Riel, J.; Paques, M. Confocal scanning light microscopy (CSLM) on mixtures of gelatine and polysaccharides. Food Res. Int. 2001, 34, 931–938. [Google Scholar] [CrossRef]
- Correa, M.J.; Ferrer, E.; Añón, M.C.; Ferrero, C. Interaction of modified celluloses and pectins with gluten proteins. Food Hydrocoll. 2014, 35, 91–99. [Google Scholar] [CrossRef]
- Maçãs, M.; Ferragina, A.; Biduski, B.; Hussey, K.; Vahedikia, N.; Arendt, E.; Gallagher, E. A study of the milling process of Irish-grown peas: NIR spectroscopy, flour pasting properties and dough rheology. Food Struct. 2023, 38, 100351. [Google Scholar] [CrossRef]
- USDA. U.S. Department of Agriculture. FoodData Central. 2018. Available online: https://fdc.nal.usda.gov/ (accessed on 25 August 2023).
- Hefnawy, T.H. Effect of processing methods on nutritional composition and anti-nutritional factors in lentils (Lens culinaris). Ann. Agric. Sci. 2011, 56, 57–61. [Google Scholar] [CrossRef]
- Wang, C.; Cao, H.; Wang, P.; Dai, Z.; Guan, X.; Huang, K.; Song, H. Changes of components and organizational structure induced by different milling degrees on the physicochemical properties and cooking characteristics of quinoa. Food Struct. 2023, 36, 100316. [Google Scholar] [CrossRef]
- Shafi, M.; Baba, W.N.; Masoodi, F.A. Composite flour blends: Influence of particle size of water chestnut flour on nutraceutical potential and quality of Indian flat breads. J. Food Meas. Charact. 2017, 11, 1094–1105. [Google Scholar] [CrossRef]
- Carboni, A.D. Desarrollo De Formulaciones De Panificados Funcionales (Sin Y Con Gluten) a Base De Harina De Lenteja (Lens culinaris): Caracterización Tecnológica Y Nutricional. Ph.D. Thesis, Universidad Nacional de La Plata, La Plata, Argentina, April 2024. [Google Scholar] [CrossRef]

| Fraction and Particle Size (mm) | % |
|---|---|
| F1 ≥ 1 | 17.76 |
| 1 > F2 ≥ 0.5 | 28.73 |
| 0.5 > F3 ≥ 0.250 | 17.81 |
| 0.250 > F4 ≥ 0.125 | 9.64 |
| F5 < 0.125 | 26.07 |
| Sample | Parameter | ||||
|---|---|---|---|---|---|
| L* | a* | b* | BI | Color | |
| RLF | 66.78 ± 0.42 c | 2.33 ± 0.18 a | 25.08 ± 0.14 d | 57.63 ± 1.26 d | |
| TLF | 58.93 ± 1.61 b | 2.36 ± 0.14 a | 15.53 ± 0.10 b | 40.68 ± 1.28 b | |
| F1 | 48.24 ± 0.93 a | 3.18 ± 0.38 c | 16.68 ± 0.27 c | 54.32 ± 1.16 c | |
| F2 | 47.67 ± 1.33 a | 4.03 ± 0.16 d | 16.57 ± 0.43 c | 57.61 ± 1.48 d | |
| F3 | 47.35 ± 0.95 a | 5.09 ± 0.12 e | 15.86 ± 0.16 b | 56.44 ± 1.04 cd | |
| F4 | 58.91 ± 0.46 b | 3.74 ± 0.22 d | 14.10 ± 0.25 a | 39.09 ± 1.10 b | |
| F5 | 68.07 ± 0.28 c | 2.78 ± 0.05 b | 15.48 ± 0.13 b | 35.75 ± 0.13 a | |
| p-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| Sample | Moisture (%) * | aw * | WHC (g Water/g Flour) † | OHC (g Oil/g Flour) † |
|---|---|---|---|---|
| RLF | 11.78 ± 0.07 e | 0.596 ± 0.005 e | 2.28 ± 0.02 a | 1.78 ± 0.15 a |
| TLF | 6.78 ± 0.11 c | 0.315 ± 0.007 a | 3.17 ± 0.08 bc | 1.81 ± 0.00 a |
| F1 | 4.60 ± 0.21 a | 0.388 ± 0.007 b | 3.25 ± 0.18 c | 1.91 ± 0.11 a |
| F2 | 5.81 ± 0.11 b | 0.301 ± 0.001 a | 3.18 ± 0.11 bc | 1.85 ± 0.18 a |
| F3 | 6.89 ± 0.13 c | 0.407 ± 0.013 c | 3.21 ± 0.12 bc | 1.71 ± 0.11 a |
| F4 | 7.20 ± 0.18 cd | 0.461 ± 0.001 d | 3.09 ± 0.06 bc | 1.82 ± 0.02 a |
| F5 | 7.61 ± 0.17 d | 0.394 ± 0.002 bc | 2.86 ± 0.02 b | 1.41 ± 0.16 a |
| p-value | <0.0001 | <0.0001 | 0.0002 | 0.1110 |
| Position (cm−1) | Identification |
|---|---|
| 3300 | Amide A |
| 3000–2800 | C-H bending of long-chain fatty acids |
| 1750 | Pectic substances |
| 1640 | Amide I |
| 1540 | Amide II |
| 1000 | Carbohydrates |
| Sample | Total Area | Brix |
|---|---|---|
| RLF | 21.1 ± 2.2 b | 4.6 ± 0.6 b |
| TLF | 8.8 ± 0.7 a | 0.7 ± 0.6 a |
| F1 | 7.7 ± 0.4 a | 0.7 ±0.6 a |
| F2 | 8.0 ± 0.6 a | 0.7 ± 0.6 a |
| F3 | 9.5 ± 0.4 a | 0.7 ± 0.6 a |
| F4 | 9.6 ± 1.3 a | 1.0 ± 0.0 a |
| F5 | 7.1 ± 0.9 a | 0.7 ± 0.6 a |
| p-value | <0.0001 | <0.0001 |
| Sample | Protein * | Lipids * | TDF * | Ash † | Carb • | Energy Content (Kcal/100 g) |
|---|---|---|---|---|---|---|
| RLF | 23.50 ± 1.21 a | 0.87 ± 0.05 a | 25.42 ± 0.21 b | 3.34 ± 0.01 c | 46.87 | 340 |
| TLF | 23.84 ± 0.21 a | 1.64 ± 0.07 c | 27.56 ± 0.08 c | 1.77 ± 0.06 b | 45.19 | 346 |
| F1 | 21.85 ± 0.07 a | 1.28 ± 0.01 b | 29.53 ± 0.06 d | 1.75 ± 0.01 b | 45.59 | 340 |
| F5 | 21.20 ± 0.01 a | 1.55 ± 0.03 c | 18.99 ± 0.23 a | 1.52 ± 0.04 a | 60.59 | 371 |
| p-value | 0.1042 | 0.0002 | <0.0001 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carboni, A.D.; Martins, G.N.; Castilho, P.C.; Puppo, M.C.; Ferrero, C. Influence of Thermal Treatment and Granulometry on Physicochemical, Techno-Functional and Nutritional Properties of Lentil Flours. Foods 2024, 13, 2744. https://doi.org/10.3390/foods13172744
Carboni AD, Martins GN, Castilho PC, Puppo MC, Ferrero C. Influence of Thermal Treatment and Granulometry on Physicochemical, Techno-Functional and Nutritional Properties of Lentil Flours. Foods. 2024; 13(17):2744. https://doi.org/10.3390/foods13172744
Chicago/Turabian StyleCarboni, Angela Daniela, Gonçalo Nuno Martins, Paula Cristina Castilho, María Cecilia Puppo, and Cristina Ferrero. 2024. "Influence of Thermal Treatment and Granulometry on Physicochemical, Techno-Functional and Nutritional Properties of Lentil Flours" Foods 13, no. 17: 2744. https://doi.org/10.3390/foods13172744








