Berry Pomace Extracts as a Natural Washing Aid to Mitigate Enterohaemorrhagic E. coli in Fresh Produce
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Growth Conditions
2.2. Preparation of the Bacterial Inoculum
2.3. Preparation of Berry Pomace Extract and Determination of Phenolic Content
2.4. Determination of Minimum Inhibitory/Bactericidal Concentration of BPE against EHEC EDL-933
2.5. Inhibition of EHEC EDL-933 in Fresh Produce Using BPE Supplemented Water
2.6. RNA Extraction and cDNA Synthesis
2.7. Determination of the Expression of Genes Due to BPE Treatment Using qRT-PCR
2.8. Statistical Analyses
3. Results
3.1. MIC and MBC of BPE against EHEC EDL-933
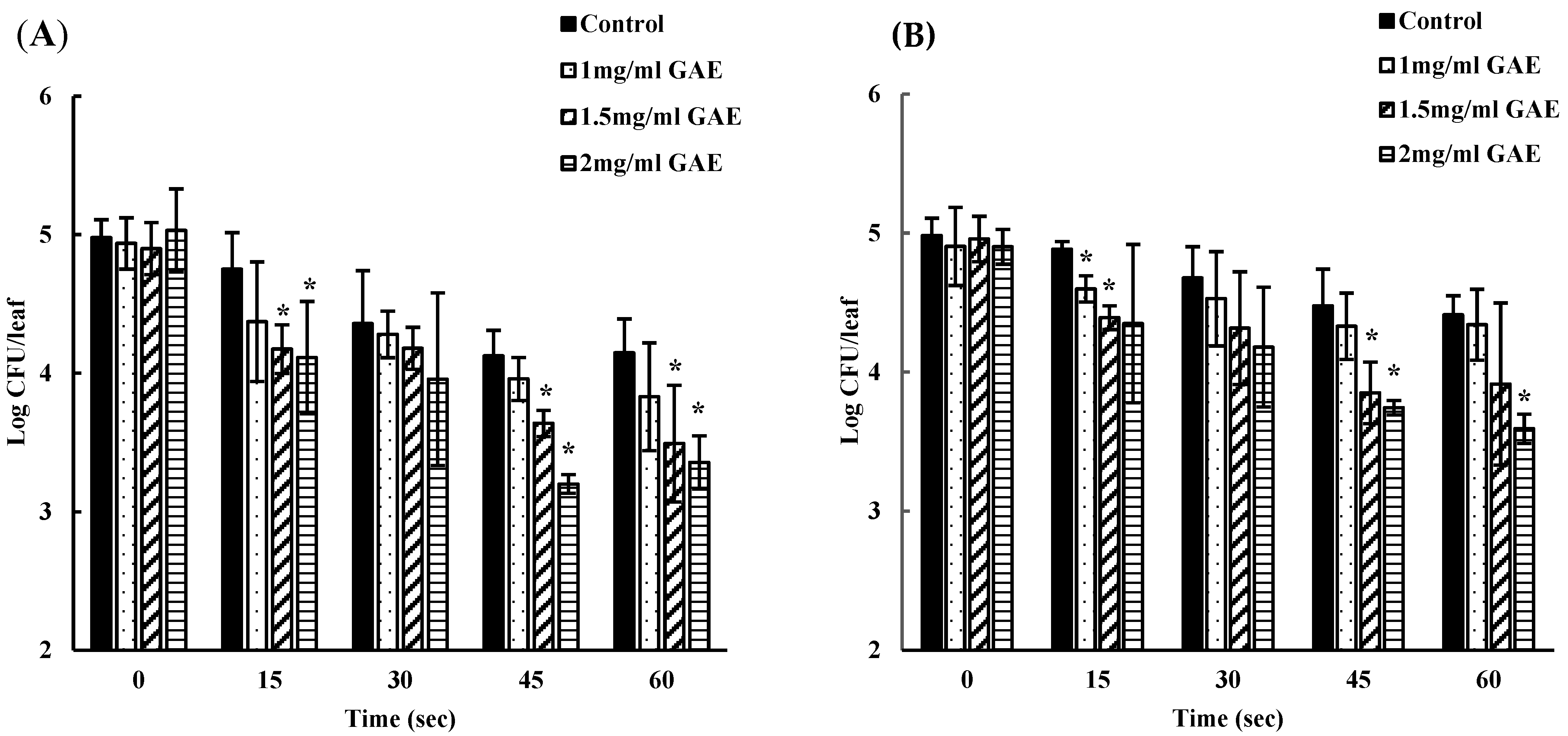
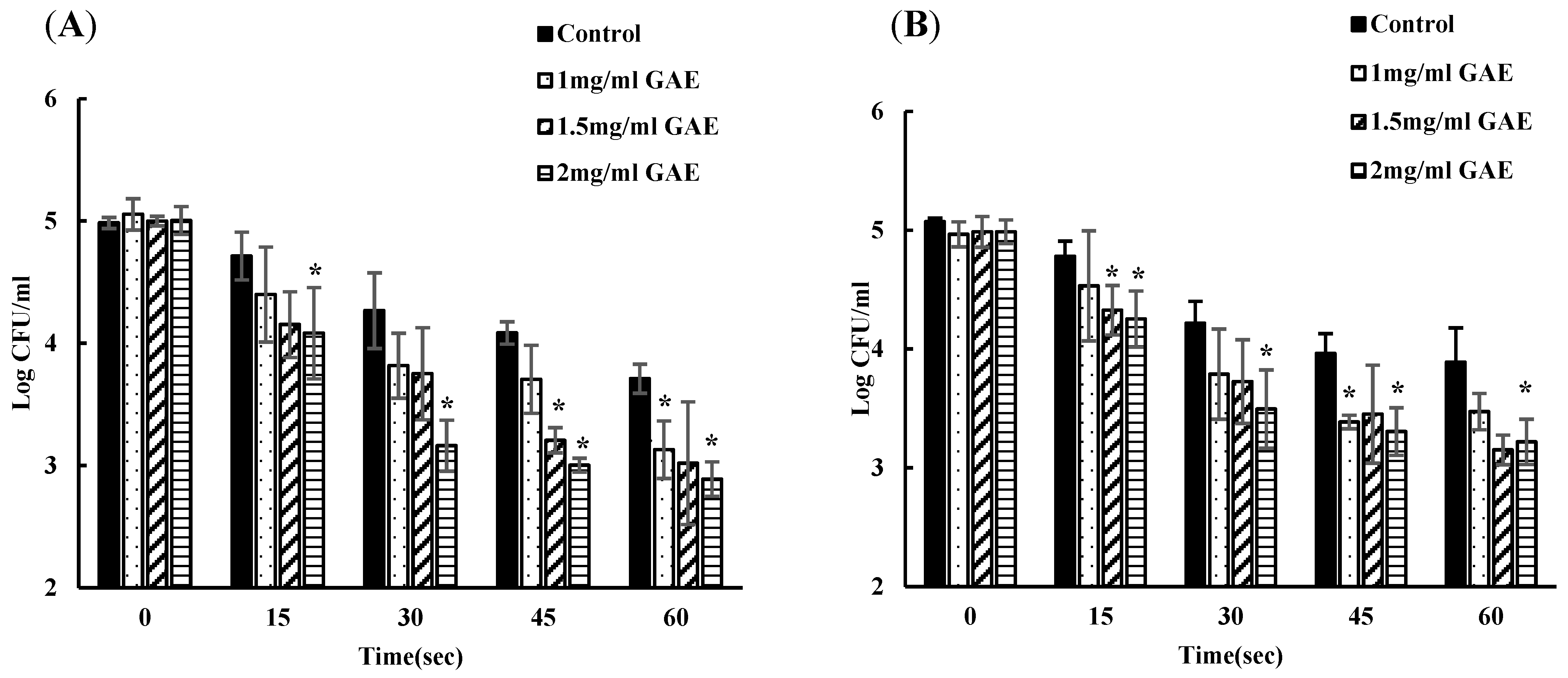
3.2. Inhibition of EHEC by Using a Single Batch of BPE-Containing Water at Various Time Points
3.3. Inhibition of EHEC by Using a New Batch of BPE at Each Time Point
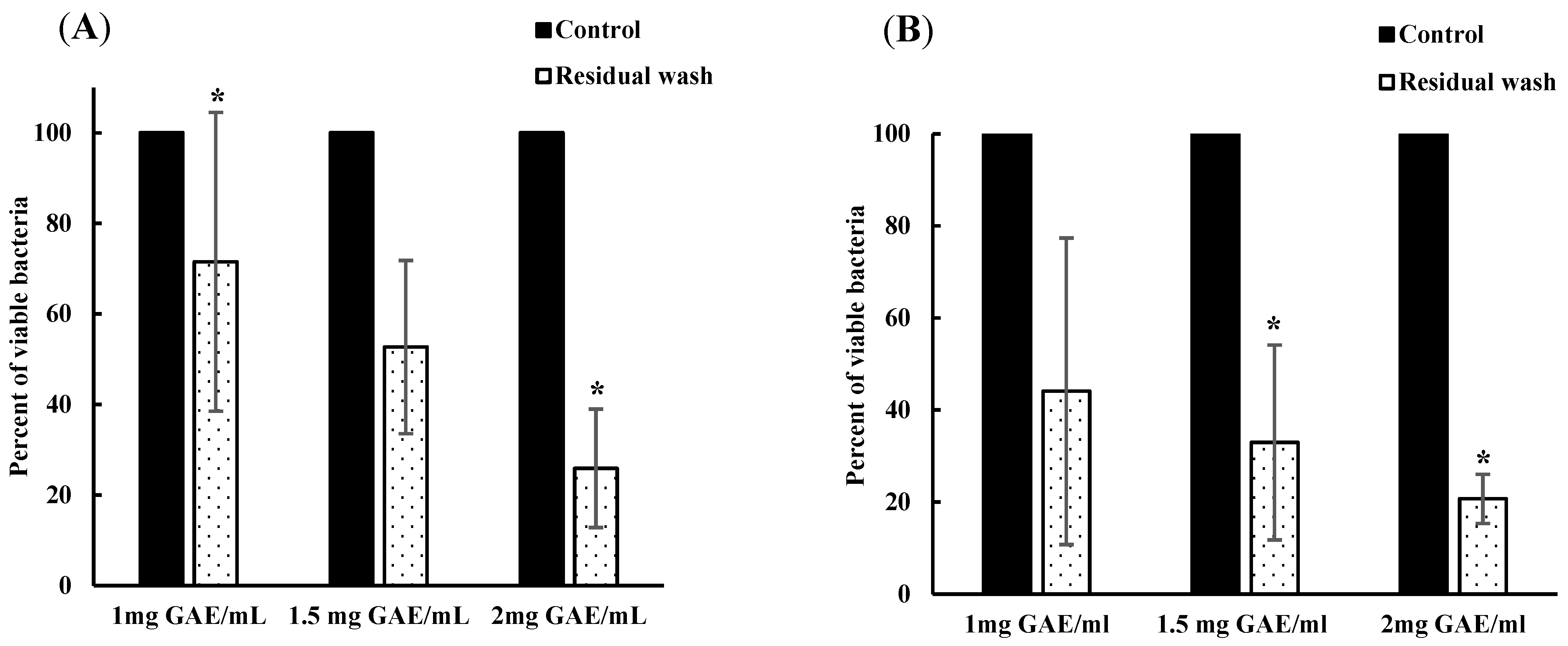
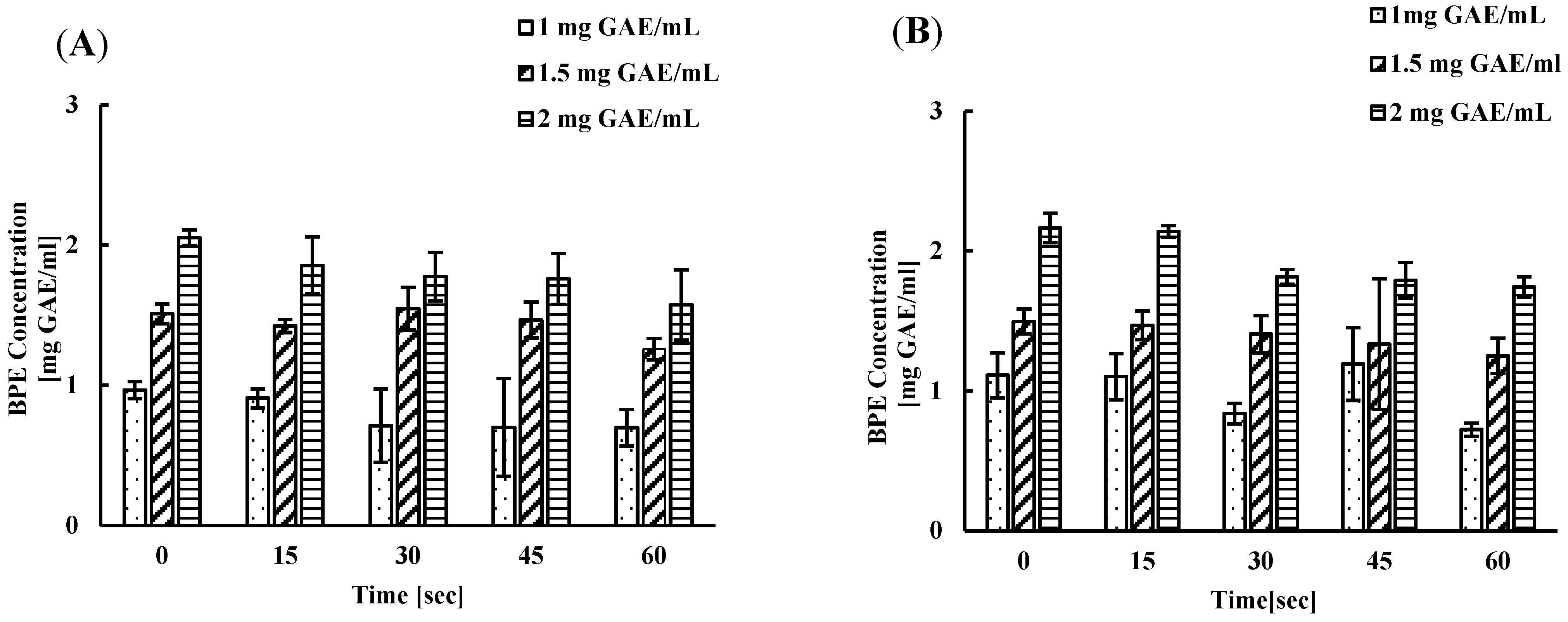
3.4. Remaining Phenolic Content of BPE after Subsequent Wash
3.5. Alteration of EHEC EDL-933 Gene Expressions in Response to BPE Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- CDC. Summary of Possible Multistate Enteric (Intestinal) Disease Outbreaks. 2021. Available online: https://www.cdc.gov/foodborne-outbreaks/php/data-research/annual-summaries/?CDC_AAref_Val=https://www.cdc.gov/foodsafety/outbreaks/lists/annual-summaries.html (accessed on 13 February 2024).
- United States Department of Health & Human Services, Centers for Disease Control and Prevention. List of Selected Multistate Foodborne Outbreak Investigations. 2021. Available online: https://www.cdc.gov/foodborne-outbreaks/active-investigations/all-foodborne-outbreak-notices.html (accessed on 6 February 2024).
- Ameer, M.A.; Wasey, A.; Salen, P. Escherichia coli (e Coli 0157 H7) [Updated 2023 August 8]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK430685/ (accessed on 10 February 2024).
- Cordonnier, C.; Etienne-Mesmin, L.; Thévenot, J.; Rougeron, A.; Rénier, S.; Chassaing, B.; Darfeuille-Michaud, A.; Barnich, N.; Blanquet-Diot, S.; Livrelli, V. Enterohemorrhagic Escherichia coli pathogenesis: Role of Long polar fimbriae in Peyer’s patches interactions. Sci. Rep. 2017, 7, 44655. [Google Scholar] [CrossRef]
- Gambushe, S.M.; Zishiri, O.T.; El Zowalaty, M.E. Review of Escherichia coli O157:H7 Prevalence, Pathogenicity, Heavy Metal and Antimicrobial Resistance, African Perspective. Infect. Drug Resist. 2022, 15, 4645–4673. [Google Scholar] [CrossRef]
- Sun, H.; Wang, M.; Liu, Y.; Wu, P.; Yao, T.; Yang, W.; Yang, Q.; Yan, J.; Yang, B. Regulation of flagellar motility and biosynthesis in enterohemorrhagic Escherichia coli O157:H7. Gut Microbes 2022, 14, 2110822. [Google Scholar] [CrossRef] [PubMed]
- Bielaszewska, M.; Mellmann, A.; Zhang, W.; Köck, R.; Fruth, A.; Bauwens, A.; Peters, G.; Karch, H. Characterisation of the Escherichia coli strain associated with an outbreak of haemolytic uraemic syndrome in Germany, 2011: A microbiological study. Lancet Infect. Dis. 2011, 11, 671–676. [Google Scholar] [CrossRef]
- Kozak, G.K.; Macdonald, D.; Landry, L.; Farber, J.M. Foodborne outbreaks in Canada linked to produce: 2001 through 2009. J. Food Prot. 2013, 76, 173–183. [Google Scholar] [CrossRef]
- Mukherjee, A.; Speh, D.; Jones, A.T.; Buesing, K.M.; Diez-Gonzalez, F. Longitudinal Microbiological Survey of Fresh Produce Grown by Farmers in the Upper Midwest. J. Food Prot. 2006, 69, 1928–1936. [Google Scholar] [CrossRef]
- Alvarado-Martinez, Z.; Tabashsum, Z.; Salaheen, S.; Mui, C.; Lebovic, A.; Gaspard, S.; Dattilio, A.; Young, A.; Kennedy, N.-F.; Biswas, D. Growth Inhibition and Alternation of Virulence Genes of Salmonella on Produce Products Treated with Polyphenolic Extracts from Berry Pomace. J. Food Prot. 2020, 83, 1463–1471. [Google Scholar] [CrossRef]
- Brodt, S.; Six, J.; Feenstra, G.; Ingels, C.; Campbell, D. Sustainable Agriculture. Nature Education Knowledge. 2011. Available online: https://www.nature.com/scitable/knowledge/library/sustainable-agriculture-23562787/ (accessed on 6 February 2024).
- Sarma, U.; Bhavya, T.R. Dietary phytonutrients in common green leafy vegetables and the significant role of processing techniques on spinach: A review. Food Prod. Process. Nutr. 2024, 6, 10. [Google Scholar] [CrossRef]
- Mashitoa, F.M.; Shoko, T.; Shai, J.L.; Slabbert, R.M.; Sultanbawa, Y.; Sivakumar, D. Influence of Different Types of Drying Methods on Color Properties, Phenolic Metabolites and Bioactivities of Pumpkin Leaves of var. Butternut squash (Cucurbita moschata Duchesne ex Poir). Front. Nutr. 2021, 8, 694649. [Google Scholar] [CrossRef]
- Delaquis, P.; Bach, S.; Dinu, L.D. Behavior of Escherichia coli O157:H7 in leafy vegetables. J. Food Prot. 2007, 70, 1966–1974. [Google Scholar] [CrossRef]
- Chauret, C. Survival and control of Escherichia coli O157:H7 in foods, beverages, soil and water. Virulence 2011, 2, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Santamaría, J.; Toranzos, G.A. Enteric pathogens and soil: A short review. Int. Microbiol. 2003, 6, 5–9. [Google Scholar] [CrossRef]
- Summerlin, H.N.; Pola, C.C.; McLamore, E.S.; Gentry, T.; Karthikeyan, R.; Gomes, C.L. Prevalence of Escherichia coli and Antibiotic-Resistant Bacteria During Fresh Produce Production (Romaine Lettuce) Using Municipal Wastewater Effluents. Front. Microbiol. 2021, 12, 660047. [Google Scholar] [CrossRef] [PubMed]
- Brackett, R.E. Incidence, Contributing Factors, and Control of Bacterial Pathogens in Produce. Postharvest Biology and Technology 1999, 15, 305–311. [Google Scholar] [CrossRef]
- Possas, A.; Pérez-Rodríguez, F. New insights into cross-contamination of fresh-produce. Curr. Opin. Food Sci. 2023, 49, 100954. [Google Scholar] [CrossRef]
- Rosberg, A.K.; Darlison, J.; Mogren, L.; Alsanius, B.W. Commercial wash of leafy vegetables do not significantly decrease bacterial load but leads to shifts in bacterial species composition. Food Microbiol. 2021, 94, 103667. [Google Scholar] [CrossRef]
- Murray, K.; Wu, F.; Shi, J.; Jun Xue, S.; Warriner, K. Challenges in the microbiological food safety of fresh produce: Limitations of post-harvest washing and the need for alternative interventions. Food Qual. Saf. 2017, 1, 289–301. [Google Scholar] [CrossRef]
- Gombas, D.; Luo, Y.; Brennan, J.; Shergill, G.; Petran, R.; Walsh, R.; Hau, H.; Khurana, K.; Zomorodi, B.; Rosen, J.; et al. Guidelines to validate control of cross-contamination during washing of fresh-cut leafy vegetables. J. Food Prot. 2017, 80, 312–330. [Google Scholar] [CrossRef]
- Gil, M.I.; Selma, M.V.; López-Gálvez, F.; Allende, A. Fresh-cut product sanitation and wash water disinfection: Problems and solutions. Int. J. Food Microbiol. 2009, 134, 37–45. [Google Scholar] [CrossRef]
- Kang, J.H.; Song, K.B. Inhibitory effect of plant essential oil nanoemulsions against Listeria monocytogenes, Escherichia coli O157:H7, and Salmonella Typhimurium on red mustard leaves. Innov. Food Sci. Emerg. Technol. 2018, 45, 447–454. [Google Scholar] [CrossRef]
- Poimenidou, S.V.; Bikouli, V.C.; Gardeli, C.; Mitsi, C.; Tarantilis, P.A.; Nychas, G.-J.; Skandamis, P.N. Effect of single or combined chemical and natural antimicrobial interventions on Escherichia coli O157:H7, total microbiota and color of packaged spinach and lettuce. Int. J. Food Microbiol. 2016, 220, 6–18. [Google Scholar] [CrossRef] [PubMed]
- Salaheen, S.; Nguyen, C.; Hewes, D.; Biswas, D. Cheap extraction of antibacterial compounds of berry pomace and their mode of action against the pathogen Campylobacter jejuni. Food Control 2014, 46, 174–181. [Google Scholar] [CrossRef]
- Salaheen, S.; Nguyen, C.; Mui, C.; Biswas, D. Bioactive berry juice byproducts as alternative and natural inhibitors for Salmonella Gallinarum and Salmonella Pullorum. J. Appl. Poult. Res. 2015, 24, 186–197. [Google Scholar] [CrossRef]
- Puupponen-Pimiä, R.; Nohynek, L.; Alakomi, H.L.; Oksman-Caldentey, K.M. The action of berry phenolics against human intestinal pathogens. BioFactors 2005, 23, 243–251. [Google Scholar] [CrossRef]
- Singh, H.; Bhardwaj, S.K.; Khatri, M.; Kim, K.H.; Bhardwaj, N. UVC radiation for food safety: An emerging technology for the microbial disinfection of food products. Chem. Eng. J. 2021, 417, 128084. [Google Scholar] [CrossRef]
- Kim, D.K.; Kang, D.H. Inactivation efficacy of a sixteen UVC LED module to control foodborne pathogens on selective media and sliced deli meat and spinach surfaces. LWT 2020, 130, 109422. [Google Scholar] [CrossRef]
- Yaun, B.R.; Sumner, S.S.; Eifert, J.D.; Marcy, J.E. Inhibition of pathogens on fresh produce by ultraviolet energy. Int. J. Food Microbiol. 2004, 90, 1–8. [Google Scholar] [CrossRef]
- Birmpa, A.; Sfika, V.; Vantarakis, A. Ultraviolet light and Ultrasound as non-thermal treatments for the inactivation of microorganisms in fresh ready-to-eat foods. Int. J. Food Microbiol. 2013, 167, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Zheng, H.; Ho, P.-Y.; Jiang, M.; Tang, B.; Liu, W.; Li, D.; Yu, X.; Kleckner, N.E.; Amir, A.; Liu, C. Interrogating the Escherichia coli cell cycle by cell dimension perturbations. Proc. Natl. Acad. Sci. USA 2016, 113, 15000–15005. [Google Scholar] [CrossRef]
- Aditya, A.; Alvarado-Martinez, Z.; Nagarajan, V.; Peng, M.; Biswas, D. Antagonistic effects of phenolic extracts of Chokeberry pomace on E. coli O157: H7 but not on probiotic and normal bacterial flora. J. Berry Res. 2019, 9, 459–472. [Google Scholar] [CrossRef]
- Shaw, R.K.; Berger, C.N.; Feys, B.; Knutton, S.; Pallen, M.J.; Frankel, G. Enterohemorrhagic Escherichia coli exploits EspA filaments for attachment to salad leaves. Appl. Environ. Microbiol. 2008, 74, 2908–2914. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.G.; Lee, J.H.; Gwon, G.; Kim SIl Park, J.G.; Lee, J. Essential Oils and Eugenols Inhibit Biofilm Formation and the Virulence of Escherichia coli O157:H7. Sci. Rep. 2016, 6, 36377. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-S.; Yang, H.J.; Choi, E.-K.; Park, Y.J.; Cho, D.H.; Ahn, K.S.; Lee, J.H.; Lee, S.-G.; Um, J.Y.; Jung, H.-J.; et al. The multi-target antibiotic efficacy of Angelica dahurica Bentham et Hooker extract exposed to the Escherichia coli O157:H7. BioChip J. 2011, 5, 333–342. [Google Scholar] [CrossRef]
- Rizzello, L.; Galeone, A.; Vecchio, G.; Brunetti, V.; Sabella, S.; Pompa, P.P. Molecular response of Escherichia coli adhering onto nanoscale topography. Nanoscale Res. Lett. 2012, 7, 575. [Google Scholar] [CrossRef]
- Dávila-Aviña, J.; Gil-Solís, C.; Merino-Mascorro, J.; García, S.; Heredia, N. Phenolics with Bactericidal Activity Alter Motility and Biofilm Formation in Enterotoxigenic, Enteropathogenic, and Enterohemorrhagic Escherichia coli. Foodborne Pathog. Dis. 2020, 17, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.K.; Bearson, B.L. Hha controls Escherichia coli O157:H7 biofilm formation by differential regulation of global transcriptional regulators FlhDC and CsgD. Appl. Environ. Microbiol. 2013, 79, 2384–2396. [Google Scholar] [CrossRef]
- Pankey, G.A.; Sabath, L.D. Clinical Relevance of Bacteriostatic versus Bactericidal Mechanisms of Action in the Treatment of Gram-Positive Bacterial Infections. Clin. Infect. Dis. 2004, 38, 864–870. [Google Scholar] [CrossRef]
- Harris, L.; Farber, J.; Beuchat, L.; Parish, M.; Suslow, T.; Garrett, E.; Busta, F. Outbreaks associated with fresh produce: Incidence, growth, and survival of pathogens in fresh and fresh-cut produce. Compr. Rev. Food Sci. Food Saf. 2003, 2 (Suppl. S1), 78–141. [Google Scholar] [CrossRef]
- Alharbi, M.G.; Al-Hindi, R.R.; Esmael, A.; Alotibi, I.A.; Azhari, S.A.; Alseghayer, M.S.; Teklemariam, A.D. The “Big Six”: Hidden Emerging Foodborne Bacterial Pathogens. Trop. Med. Infect. Dis. 2022, 7, 356. [Google Scholar] [CrossRef]
- Keskinen, L.A.; Burke, A.; Annous, B.A. Efficacy of chlorine, acidic electrolyzed water and aqueous chlorine dioxide solutions to decontaminate Escherichia coli O157:H7 from lettuce leaves. Int. J. Food Microbiol. 2009, 132, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Pinto, L.; Baruzzi, F.; Ippolito, A. Recent advances to control spoilage microorganisms in washing water of fruits and vegetables: The use of electrolyzed water. Acta Hortic. 2016, 1144, 379–384. [Google Scholar] [CrossRef]
- Gibson, K.E.; Almeida, G.; Jones, S.L.; Wright, K.; Lee, J.A. Inactivation of bacteria on fresh produce by batch wash ozone sanitation. Food Control 2019, 106, 106747. [Google Scholar] [CrossRef]
- de Siqueira Oliveira, L.; Eça, K.S.; de Aquino, A.C.; Vasconcelos, L.B. Hydrogen Peroxide (H2O2) for Postharvest Fruit and Vegetable Disinfection. In Postharvest Disinfection of Fruits and Vegetables; Elsevier: Amsterdam, The Netherlands, 2018; pp. 91–99. [Google Scholar] [CrossRef]
- Cueva, C.; Moreno-Arribas, M.V.; Martín-Álvarez, P.J.; Bills, G.; Vicente, M.F.; Basilio, A.; Rivas, C.L.; Requena, T.; Rodríguez, J.M.; Bartolomé, B. Antimicrobial activity of phenolic acids against commensal, probiotic and pathogenic bacteria. Res. Microbiol. 2010, 161, 372–382. [Google Scholar] [CrossRef]
- Lee, O.H.; Lee, B.Y. Antioxidant and antimicrobial activities of individual and combined phenolics in Olea europaea leaf extract. Bioresour. Technol. 2010, 101, 3751–3754. [Google Scholar] [CrossRef]
- Salaheen, S.; Jaiswal, E.; Joo, J.; Peng, M.; Ho, R.; Oconnor, D.; Adlerz, K.; Aranda-Espinoza, J.H.; Biswas, D. Bioactive extracts from berry byproducts on the pathogenicity of Salmonella Typhimurium. Int. J. Food Microbiol. 2016, 237, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Salaheen, S.; Peng, M.; Joo, J.; Teramoto, H.; Biswas, D. Eradication and sensitization of methicillin resistant Staphylococcus aureus to methicillin with bioactive extracts of berry pomace. Front. Microbiol. 2017, 8, 253. [Google Scholar] [CrossRef]
- Salaheen, S.; Tabashsum, Z.; Gaspard, S.; Dattilio, A.; Tran, T.H.; Biswas, D. Reduced Campylobacter jejuni colonization in poultry gut with bioactive phenolics. Food Control 2018, 84, 1–7. [Google Scholar] [CrossRef]
- Takeuchi, K.; Frank, J.F. Penetration of Escherichia coli O157:H7 into Lettuce Tissues as Affected by Inoculum Size and Temperature and the Effect of Chlorine Treatment on Cell Viability. J. Food Prot. 2000, 63, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Sapers, G.M. Efficacy of Washing and Sanitizing Methods for Disinfection of Fresh Fruit and Vegetable Products. Food Technol. Biotechnol. 2001, 39, 305–311. [Google Scholar]
- Burnett, S.L.; Chen, J.; Beuchat, L.R. Attachment of Escherichia coli O157:H7 to the Surfaces and Internal Structures of Apples as Detected by Confocal Scanning Laser Microscopy. Appl. Environ. Microbiol. 2000, 66, 4679–4687. [Google Scholar] [CrossRef] [PubMed]
- Brandl, M.T. Plant lesions promote the rapid multiplication of Escherichia coli O157:H7 on postharvest lettuce. Appl. Environ. Microbiol. 2008, 74, 5285–5289. [Google Scholar] [CrossRef]
- Patel, J.; Sharma, M.; Ravishakar, S. Effect of curli expression and hydrophobicity of Escherichia coli O157:H7 on attachment to fresh produce surfaces. J. Appl. Microbiol. 2011, 110, 737–745. [Google Scholar] [CrossRef]
- Pawar, D.M.; Rossman, M.L.; Chen, J.; Chen, J. Role of curli fimbriae in mediating the cells of enterohaemorrhagic Escherichia coli to attach to abiotic surfaces. J. Appl. Microbiol. 2005, 99, 418–425. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, Y.G.; Lee, J. Antibiofilm activity of lawsone against polymicrobial enterohemorrhagic Escherichia coli O157:H7 and Candida albicans by suppression of curli production and hyphal growth. Phytomedicine 2024, 124, 155306. [Google Scholar] [CrossRef]
- Barnhart, M.M.; Lynem, J.; Chapman, M.R. GlcNAc-6P levels modulate the expression of curli fibers by Escherichia coli. J. Bacteriol. 2006, 188, 5212–5219. [Google Scholar] [CrossRef]
- Scalbert, A. Antimicrobial Properties of Tannins. Phytochemistry 1991, 30, 3875–3883. [Google Scholar] [CrossRef]
- Lacombe, A.; Tadepalli, S.; Hwang, C.A.; Wu, V.C.H. Phytochemicals in lowbush wild blueberry inactivate Escherichia coli O157:H7 by damaging its cell membrane. Foodborne Pathog. Dis. 2013, 10, 944–950. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.P.; Mcmurry, L.M.; Levy, S.B. MarA Locus Causes Decreased Expression of OmpF Porin in Multiple-Antibiotic-Resistant (Mar) Mutants of Escherichia coli. J. Bacteriol. 1988, 170, 5416–5422. [Google Scholar] [CrossRef]
- Alekshun, M.N.; Levy, S.B. The mar regulon: Multiple resistance to antibiotics and other toxic chemicals. Trends Microbiol. 1999, 7, 410–413. [Google Scholar] [CrossRef] [PubMed]
- Carey, C.M.; Kostrzynska, M.; Thompson, S. Escherichia coli O157:H7 stress and virulence gene expression on Romaine lettuce using comparative real-time PCR. J. Microbiol. Methods 2009, 77, 235–242. [Google Scholar] [CrossRef] [PubMed]
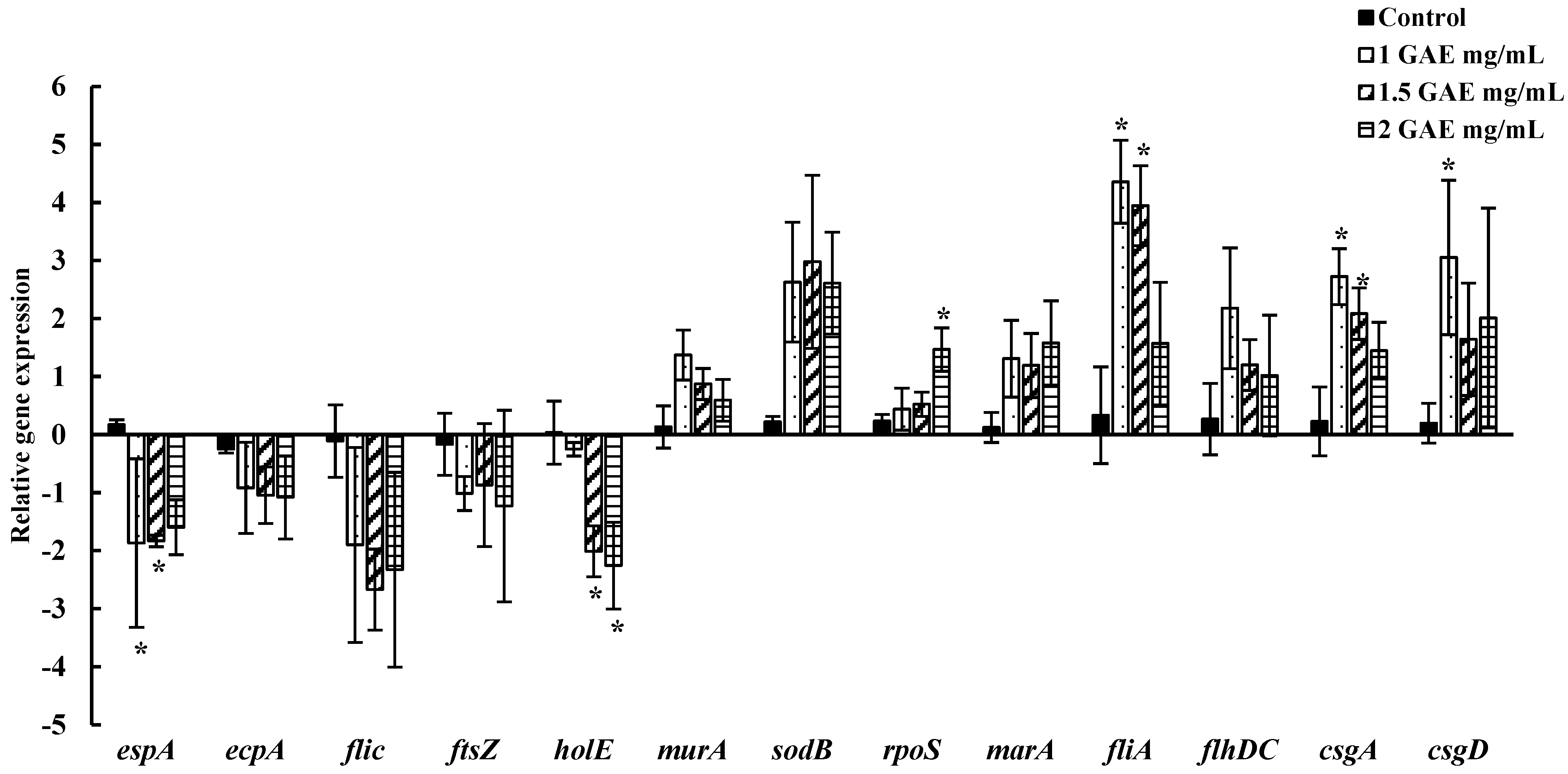
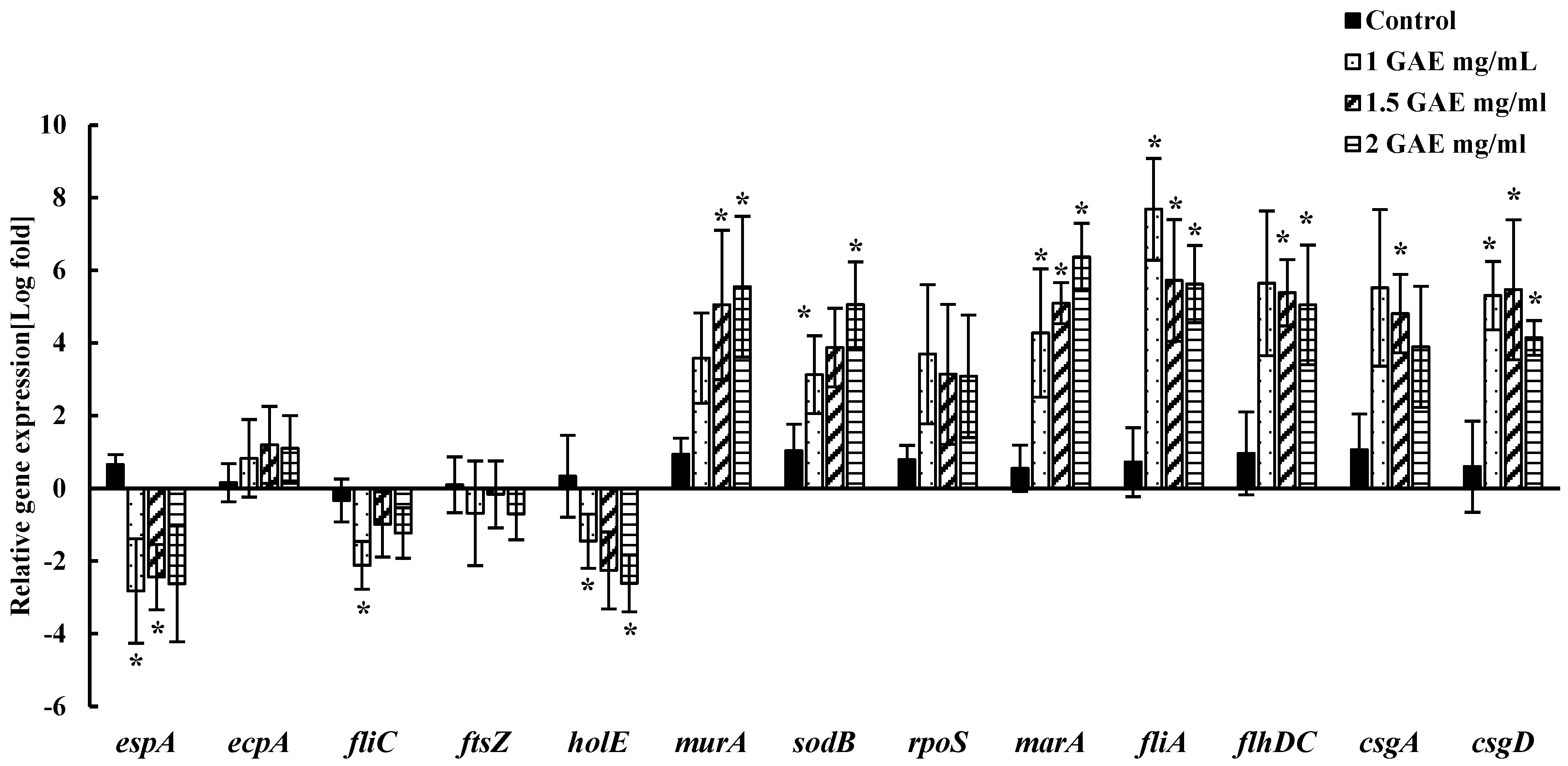
| Gene | Primer Sequence [5′-3] | Function | Reference |
|---|---|---|---|
| 16s RNA | F: CGTTACCCGCAGAAGAAGC R: GTGGACTACCAGGGTATCTAATCC | Housekeeping gene | [35] |
| espA | F: GTGAGCAGAGAGAGAATGC R: GTAAATCCAGCGATAAACCCG | Adherence and colonization | [36] |
| ecpA | F: CGCGGATCCATGAAAAAAAAGGTTCTGGC R: CGCGAATTCTAACTGGTCCAGGTCGCGTCG | E. coli common pilus | [37] |
| fliC | F: TACCATCGCAAAAGCAACTCCR: GTCGGCAACGTTAGTGATACC | Flagellar filament protein | [35] |
| ftsZ | F: CTTCTCTTGACCCGGATATG R: CATTCACGACTTTAGCAACC | Cell division | [34] |
| holE | F: ACGTGAACAGCCTGAACATTTGCG R: TGGGCTCGTAAGGTAAACGTGACA | DNA polymerase III subunit θ | [38] |
| murA | F: AACGAAGCTCCAGGGCGAAG R: TTCGCACCCAGCTGGCTTAG | Peptidoglycan synthesis | [39] |
| sodB | F: GCGATCAAAAACTTTGGTT R: CCAGAAGTGCTCAAGAT | Multi-defense system against oxidative stress | [38] |
| rpoS | F: CGCCGGATGATCGAGAGTAA R: GAGGCCAATTTCACGACCTA | Survival against stress | [38] |
| marA | F: TTAGGCCAATACATCCGCAG R: AAGGTTCGGGTCAGAGTTTG | Antibiotic efflux system | [38] |
| fliA | F: TTAGGGATCGATATTGCCGATT R: CGTAGGAGAAGAGCTGGCTGTT | Flagellar biosynthesis sigma factor | [40] |
| flhDC | F: ACAACATTAGCGGCACTGAC R: AGAGTAATCGTCTGGTGGCTG | Master regulator for flagellar synthesis | [41] |
| csgA | F: AGATGTTGGTCAGGGCTCAG R: CGTTGTTACCAAAGCCAACC | Curli subunit | [38] |
| csgD | F: CCGCTTGTGTCCGGTTTT R: GAGATCGCTCGTTCGTTGTTC | Curli subunit | [37] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thapa, K.; Julianingsih, D.; Tung, C.-W.; Phan, A.; Hashmi, M.A.; Bleich, K.; Biswas, D. Berry Pomace Extracts as a Natural Washing Aid to Mitigate Enterohaemorrhagic E. coli in Fresh Produce. Foods 2024, 13, 2746. https://doi.org/10.3390/foods13172746
Thapa K, Julianingsih D, Tung C-W, Phan A, Hashmi MA, Bleich K, Biswas D. Berry Pomace Extracts as a Natural Washing Aid to Mitigate Enterohaemorrhagic E. coli in Fresh Produce. Foods. 2024; 13(17):2746. https://doi.org/10.3390/foods13172746
Chicago/Turabian StyleThapa, Kanchan, Dita Julianingsih, Chuan-Wei Tung, Anna Phan, Muhammad Abrar Hashmi, Kayla Bleich, and Debabrata Biswas. 2024. "Berry Pomace Extracts as a Natural Washing Aid to Mitigate Enterohaemorrhagic E. coli in Fresh Produce" Foods 13, no. 17: 2746. https://doi.org/10.3390/foods13172746





