Study on the Extraction of Nervonic Acid from the Oil of Xanthoceras sorbifolium Bunge Seeds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
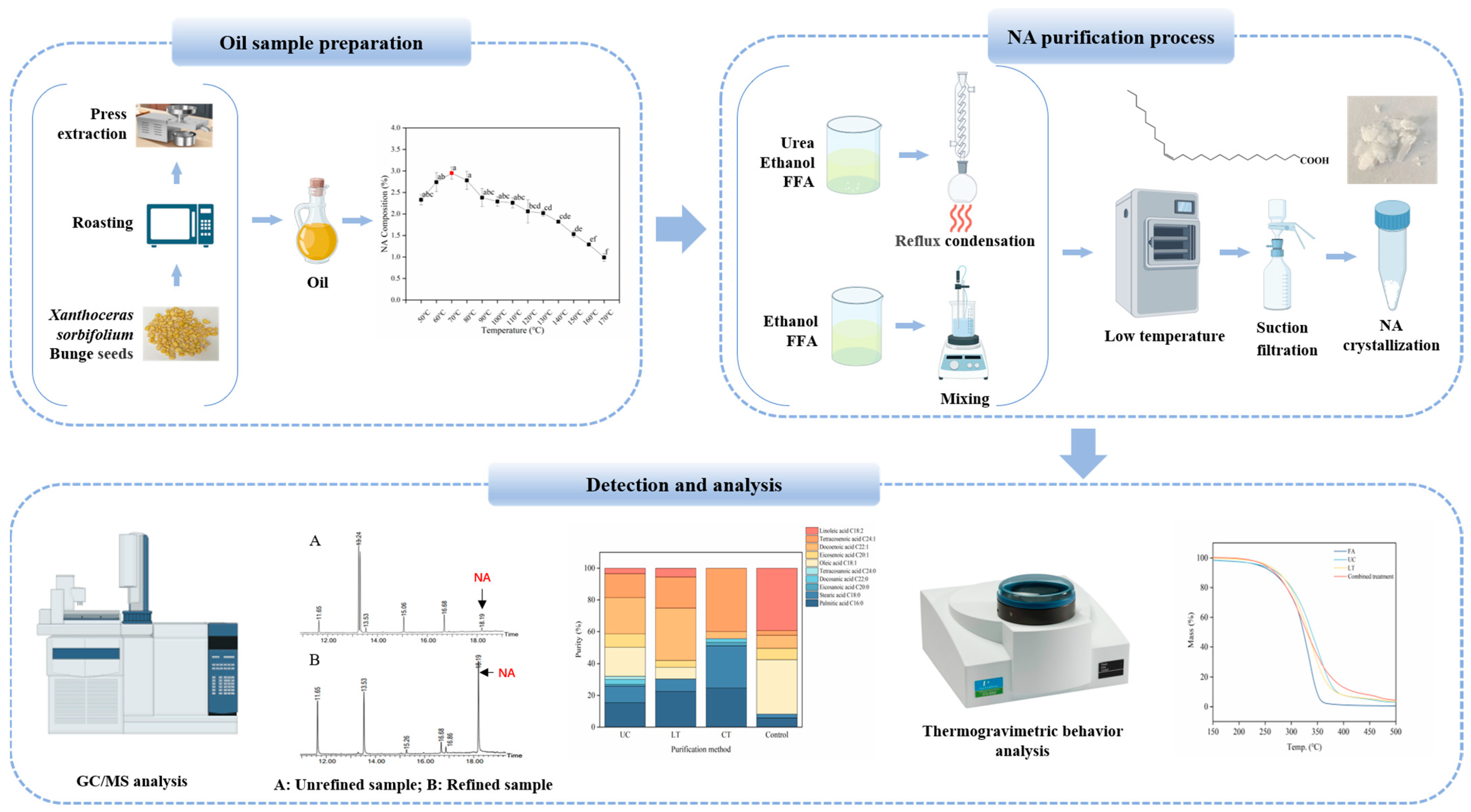
2.2. Preparation of Seed Oil
2.3. Preparation of Free Fatty Acids
2.4. Urea Complexation Method
2.5. Low-Temperature Solvent Crystallization Method
2.6. Combined Treatment Method
2.7. Fatty Acid Compositions Analysis
2.7.1. Methylation of FFA
2.7.2. Identification of Fatty Acid Composition
2.8. Thermogravimetric
2.9. FTIR Spectroscopy
2.10. NMR Spectroscopy
2.11. Statistical Analysis
3. Results and Discussion
3.1. Fatty Acid Composition and Content in Xanthoceras sorbifolium Bunge Seed Oil
3.2. Urea Complexation
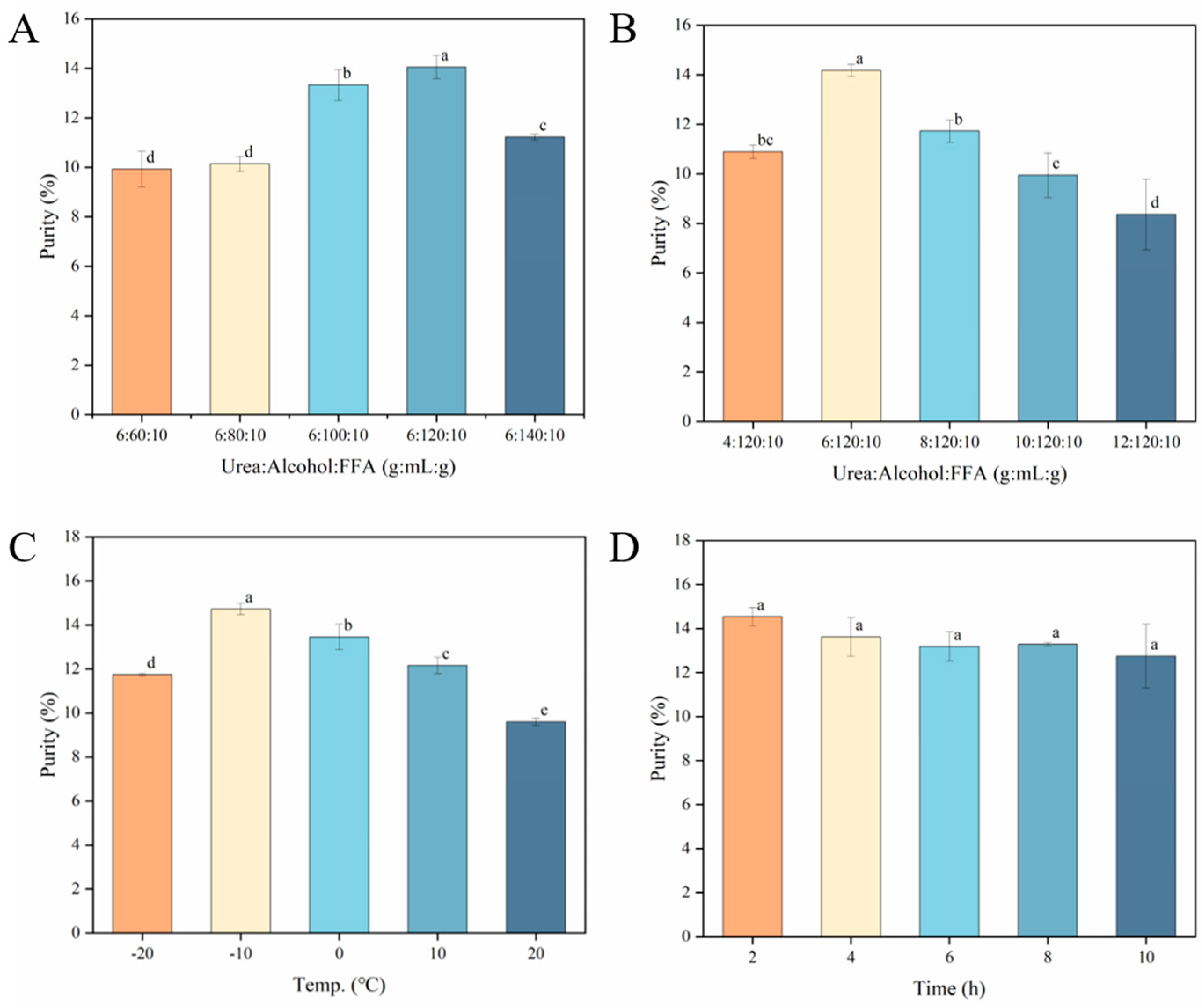
3.2.1. Single Factor of Urea Complexation
Effect of Ethanol Volume and Urea Mass on NA Content
Effect of Temperature and Time on NA Content
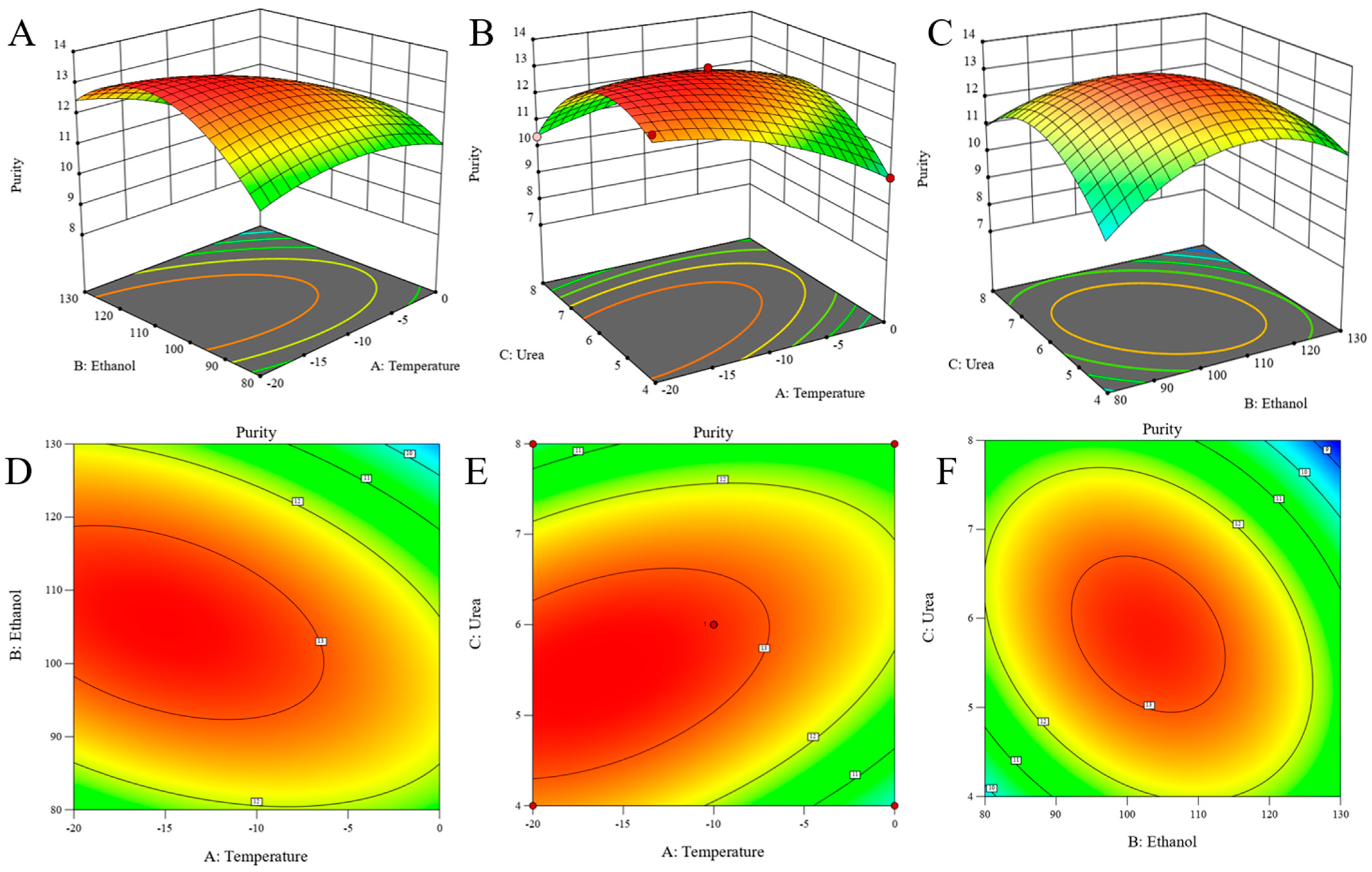
3.2.2. Statistical Analysis Using Response Surface Methodology
3.3. Low-Temperature Solvent Crystallization
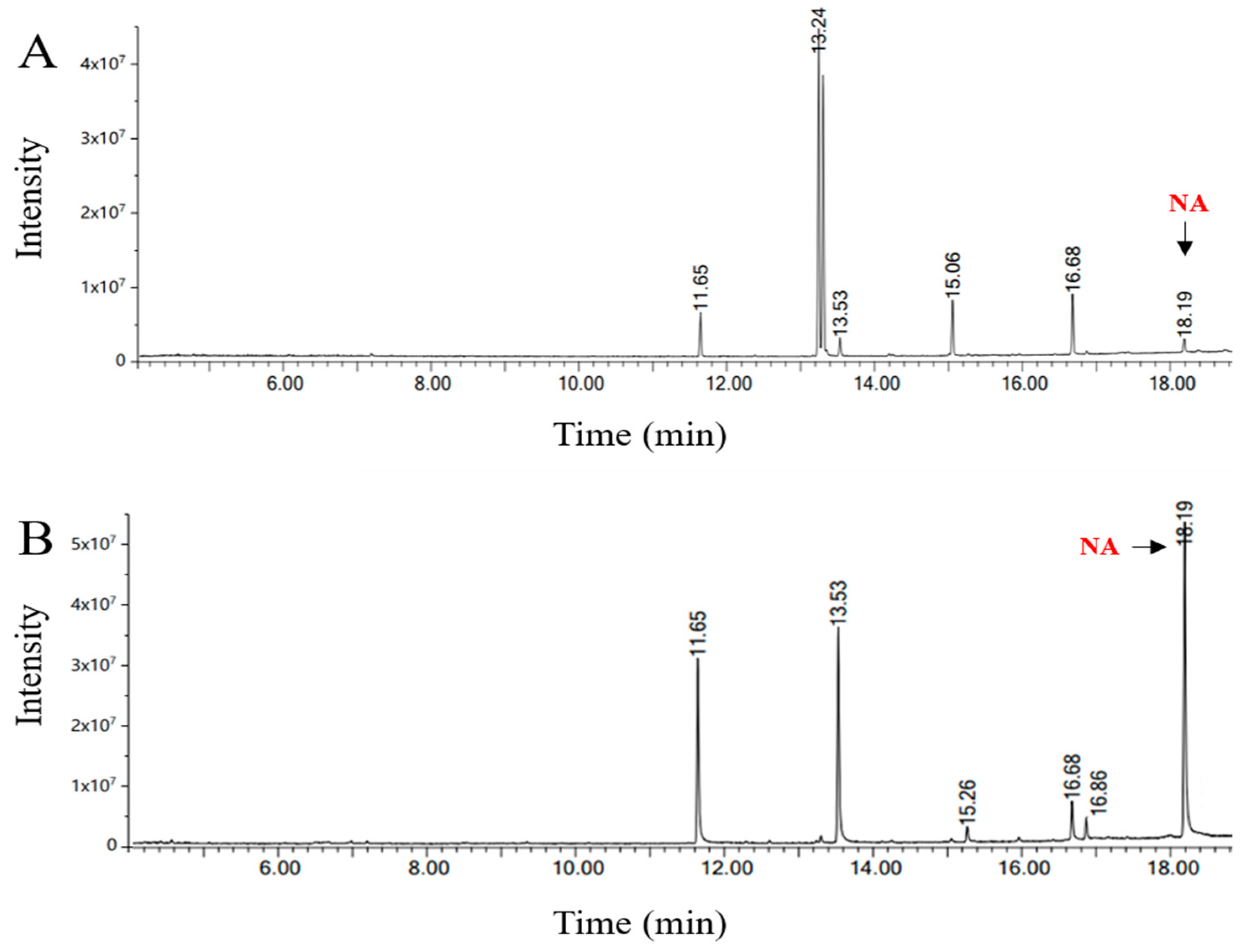
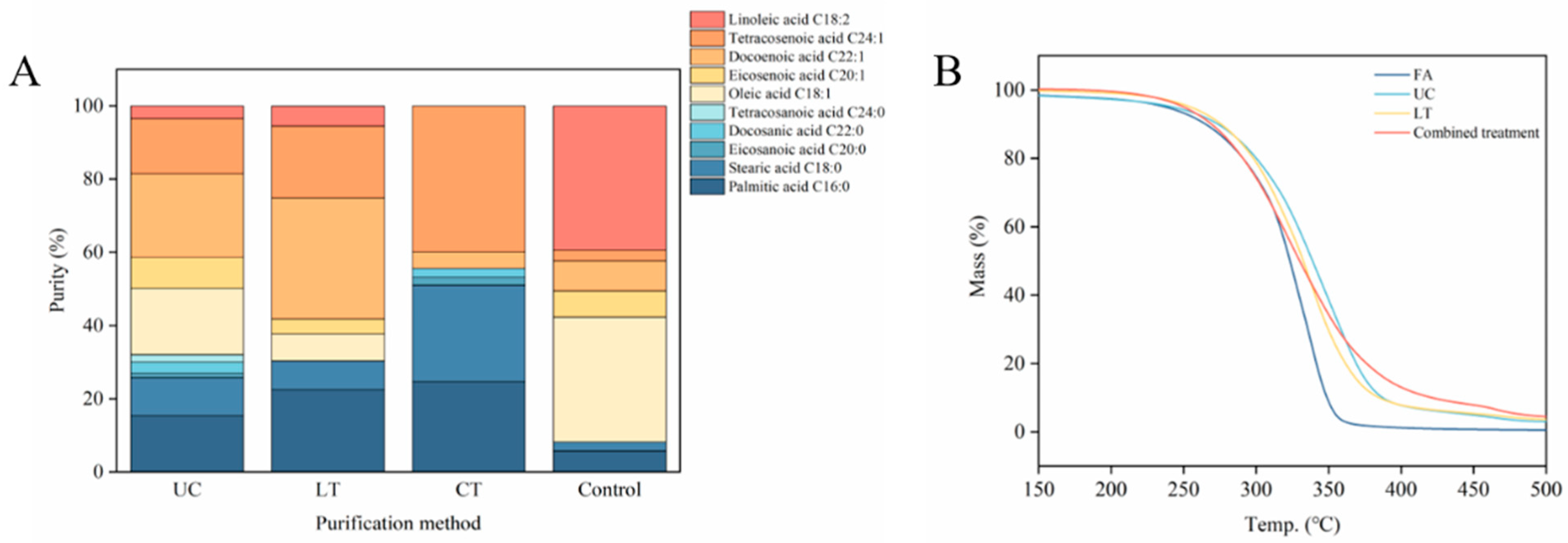
3.4. Combined Treatment with Urea Complexation and Low-Temperature Solvent Crystallization
3.5. Thermogravimetric Behavior Analysis
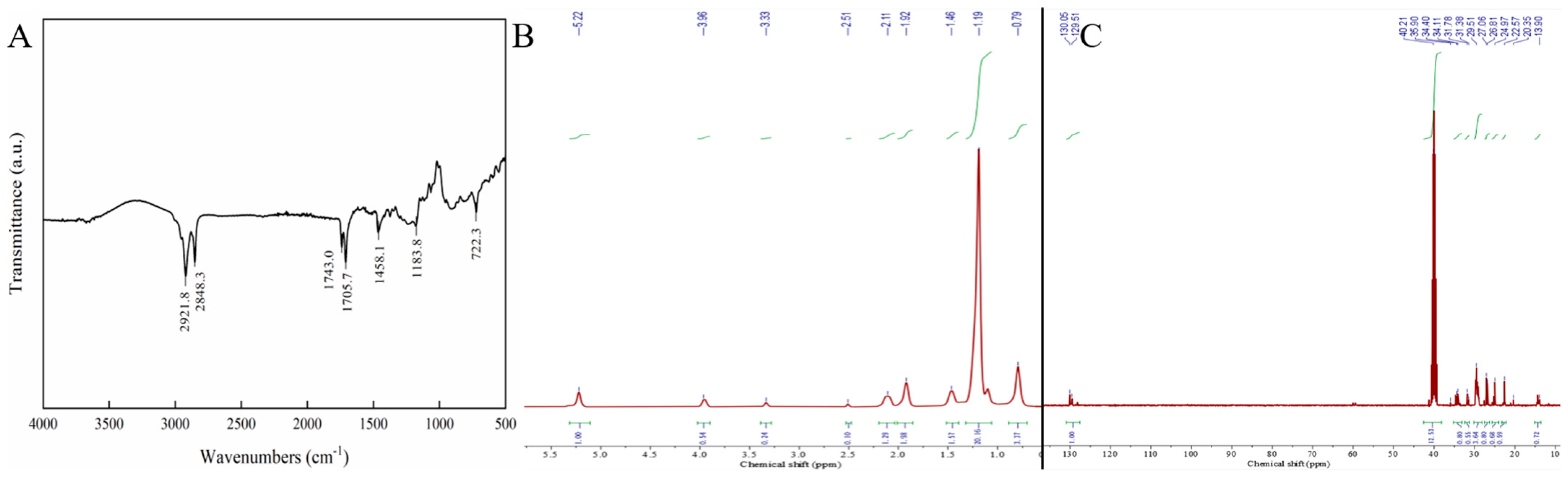
3.6. FTIR Analysis
3.7. NMR Analysis
3.7.1. 1H-NMR Analysis
3.7.2. 13C-NMR Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, Q.Y.; Liu, G.S. The embryology of Xanthoceras and its phylogenetic implications. Plant Syst. Evol. 2012, 298, 457–468. [Google Scholar] [CrossRef]
- Sha, H.; Li, S.; Li, J.; Zhao, J.; Su, D. Widely Targeted Metabolomics and Network Pharmacology Reveal the Nutritional Potential of Yellowhorn (Xanthoceras sorbifolium Bunge) Leaves and Flowers. Foods 2024, 13, 1274. [Google Scholar] [CrossRef]
- Yao, Z.-Y.; Qi, J.-H.; Yin, L.-M. Biodiesel production from Xanthoceras sorbifolia in China: Opportunities and challenges. Renew. Sustain. Energy Rev. 2013, 24, 57–65. [Google Scholar] [CrossRef]
- Xiao, J.; Sun, L.; Pan, Y.; Bai, X.; Chen, G.; Zhang, X.; Chen, X.; Li, N. Multipurpose Research from a Native Woody Oil Plant Xanthoceras sorbifolia in China. Forests 2023, 14, 86. [Google Scholar] [CrossRef]
- Zishuo, Z.; Yi, Z.; Yan, A.; Michael Ray, S.; Xu, W. Diversity of seed and seed oil physicochemical traits of Xanthoceras sorbifolium Bunge. J. Food Compos. Anal. 2021, 96, 103705. [Google Scholar] [CrossRef]
- Haiyan, Y.; Siqi, F.; Quanxin, B.; Shaoxiong, W.; Xiaoyu, H.; Mengyuan, C.; Libing, W. Seed morphology, oil content and fatty acid composition variability assessment in yellow horn (Xanthoceras sorbifolium Bunge) germplasm for optimum biodiesel production. Ind. Crop. Prod. 2017, 97, 425–430. [Google Scholar] [CrossRef]
- Gao, H.; Liu, M.; Zheng, L.; Zhang, T.; Chang, X.; Liu, H.; Zhou, S.; Zhang, Z.; Li, S.; Sun, J. Comparative Analysis of Key Odorants and Aroma Characteristics in Hot-Pressed Yellow Horn (Xanthoceras sorbifolia bunge) Seed Oil Via Gas Chromatography-Ion Mobility Spectrometry and Gas Chromatography-Olfactory-Mass Spectrometry. Foods 2023, 12, 3174. [Google Scholar] [CrossRef]
- Tu, X.; Wan, J.; Xie, Y.; Wei, F.; Quek, S.; Lv, X.; Du, L.; Chen, H. Lipid analysis of three special nervonic acid resources in China. Oil Crop Sci. 2020, 5, 180–186. [Google Scholar] [CrossRef]
- Liu, F.; Wu, R.; Ma, X.; Su, E. The Advancements and Prospects of Nervonic Acid Production. J. Agric. Food Chem. 2022, 70, 12772–12783. [Google Scholar] [CrossRef]
- Qian, Q.; Xue, W.; Hongjian, R.; Kai, A.; Zhen, F.; Tiantian, C.; Zhongkui, S. Oil Content and Nervonic Acid Content of Acer truncatum Seeds from 14 Regions in China. Hortic. Plant J. 2019, 5, 24–30. [Google Scholar] [CrossRef]
- Phung, N.V.; Rong, F.; Xia, W.Y.; Fan, Y.; Li, X.Y.; Wang, S.A.; Li, F.L. Nervonic acid and its sphingolipids: Biological functions and potential food applications. Crit. Rev. Food Sci. Nutr. 2023, 1–20. [Google Scholar] [CrossRef]
- Li, Q.; Chen, J.; Yu, X.; Gao, J.-M. A mini review of nervonic acid: Source, production, and biological functions. Food Chem. 2019, 301, 125286. [Google Scholar] [CrossRef]
- Song, W.; Zhang, K.; Xue, T.; Han, J.; Peng, F.; Ding, C.; Lin, F.; Li, J.; Sze, F.T.A.; Gan, J.; et al. Cognitive improvement effect of nervonic acid and essential fatty acids on rats ingesting Acer truncatum Bunge seed oil revealed by lipidomics approach. Food Funct. 2022, 13, 2475–2490. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Q.; Hu, K.; Wu, C.; Wang, J.; Liu, Q.; Tao, J.; Liu, Y.; Lu, Y.; Feng, Z. Acer triflorum as a new nervonic acid-containing oilseed resource for the future: Fruit-and-seed weight, oil content and fatty acid composition. Ind. Crop. Prod. 2022, 179, 114684. [Google Scholar] [CrossRef]
- Graciela, E.D.; Bernhard, K.K.; Stefan, L.; Winfried, M.; Clemens; Marcus, E.K. Individual omega-9 monounsaturated fatty acids and mortality—The Ludwigshafen Risk and Cardiovascular Health Study. J. Clin. Lipidol. 2017, 11, 126–135.e125. [Google Scholar] [CrossRef]
- Gregory, C.S.; Juan, J.C.; Olof, H.; Peter, B.; Peter, S. Plasma Fatty Acids in Chronic Kidney Disease: Nervonic Acid Predicts Mortality. J. Ren. Nutr. 2012, 22, 277–283. [Google Scholar] [CrossRef]
- Fan, Y.; Meng, H.-M.; Hu, G.-R.; Li, F.-L. Biosynthesis of nervonic acid and perspectives for its production by microalgae and other microorganisms. Appl. Microbiol. Biotechnol. 2018, 102, 3027–3035. [Google Scholar] [CrossRef] [PubMed]
- Mudgal, S.; Ran-Ressler, R.R.; Liu, L.; Brenna, J.T.; Rizvi, S.S.H. Branched chain fatty acids concentrate prepared from butter oil via urea adduction. Eur. J. Lipid Sci. Technol. 2015, 118, 669–674. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Z.-J.; Li, H.-Z.; Hou, T.-Y.; Zhao, Y.-N.; Li, H. Urea complexation combined with rapid preparative reversed-phase liquid chromatography to separate α-linolenic acid from perilla seed oil: Purity, yield, and oxidation stability. Ind. Crop. Prod. 2022, 187, 115473. [Google Scholar] [CrossRef]
- Yingxi, G.; Jiayi, S.; Jie, W.; Fan, Z.; Shichao, T.; Zhiyong, Z.; Zhongqi, R. Enrichment of nervonic acid in Acer truncatum Bunge oil by combination of two-stage molecular distillation, one-stage urea complexation and five-stage solvent crystallization. Chin. J. Chem. Eng. 2023, 59, 61–71. [Google Scholar] [CrossRef]
- Umemoto, H.; Yasugi, S.; Tsuda, S.; Yoda, M.; Ishiguro, T.; Kaba, N.; Itoh, T. Protective Effect of Nervonic Acid Against 6-Hydroxydopamine-Induced Oxidative Stress in PC-12 Cells. J. Oleo Sci. 2021, 70, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yang, J.; Yin, Z.Q.; Xu, J.; Hu, N.; Svir, I.; Wang, M.; Li, Y.Y.; Zhan, L.; Wu, S.; et al. Study of the inhibitory effect of fatty acids on the interaction between DNA and polymerase beta. Biochem. Biokhimiia 2009, 74, 813–818. [Google Scholar] [CrossRef]
- Mu, J.; Wu, G.; Chen, Z.; Brennan, C.S.; Tran, K.; Dilrukshi, H.N.N.; Shi, C.; Zhen, H.; Hui, X. Identification of the fatty acids profiles in supercritical CO2 fluid and Soxhlet extraction of Samara oil from different cultivars of Elaeagnus mollis Diels seeds. J. Food Compos. Anal. 2021, 101, 103982. [Google Scholar] [CrossRef]
- Nima, M.; Nikoo, O.; Daniel, G. Pyrus glabra seed oil as a new source of mono and polyunsaturated fatty acids: Composition, thermal, and FTIR spectroscopic characterization. Lwt 2023, 181, 114790. [Google Scholar] [CrossRef]
- Lydia, V.; Maroula, G.K.; Christos, S.P. GC-MS, FTIR and Raman spectroscopic analysis of fatty acids of Pistacia vera (Greek variety “Aegina”) oils from two consecutive harvest periods and chemometric differentiation of oils quality. Food Res. Int. 2021, 148, 110590. [Google Scholar] [CrossRef]
- Qian, J.; Zhao, C.; Zhu, H.; Tong, J.; Zhao, X.; Yang, H.; Guo, H. NMR detection of fatty acids content in walnut oil and compared with liquid chromatography. J. Food Meas. Charact. 2021, 15, 2716–2726. [Google Scholar] [CrossRef]
- Hama, J.R.; Fitzsimmons-Thoss, V. Determination of Unsaturated Fatty Acids Composition in Walnut (Juglans regia L.) Oil Using NMR Spectroscopy. Food Anal. Methods 2022, 15, 1226–1236. [Google Scholar] [CrossRef]
- Ying, L.; Jianjun, T.; Tingting, Z.; Liuping, F. Effects of frying temperature and pore profile on the oil absorption behavior of fried potato chips. Food Chem. 2021, 345, 128832. [Google Scholar] [CrossRef]
- Hurtado-Benavides, A.; Dorado, A.D.; Sánchez-Camargo, A.D.P. Study of the fatty acid profile and the aroma composition of oil obtained from roasted Colombian coffee beans by supercritical fluid extraction. J. Supercrit. Fluids 2016, 113, 44–52. [Google Scholar] [CrossRef]
- Mann, J.; Reznik, E.; Santer, M.; Fongheiser, M.A.; Smith, N.; Hirschhorn, T.; Zandkarimi, F.; Soni, R.K.; Dafré, A.L.; Miranda-Vizuete, A.; et al. Ferroptosis inhibition by oleic acid mitigates iron-overload-induced injury. Cell Chem. Biol. 2023. [Google Scholar] [CrossRef]
- Vanessa, S.; Davy Willian Hidalgo, C.; Ormindo Domingues, G.; Alexandra Christine Helena Frankland, S.; Geni Rodrigues, S.; Rosane Nora, C.; Elizabeth Aparecida Ferraz da Silva, T.; Tatiana, S. Effect of aroeira (Schinus terebinthifolius Raddi) fruit against polyunsaturated fatty acids and cholesterol thermo-oxidation in model systems containing sardine oil (Sardinella brasiliensis). Food Res. Int. 2020, 132, 109091. [Google Scholar] [CrossRef]
- Leal-Castañeda, E.J.; Hernández-Becerra, J.A.; Rodríguez-Estrada, M.T.; García, H.S. Formation of cholesterol oxides in lipid medium during microwave heating. Eur. J. Lipid Sci. Technol. 2017, 119, 1500597. [Google Scholar] [CrossRef]
- Luis, V.; Isabel, M.P.; Guillermo, R.; Carlos, F.T. Identification and quantification of ethyl carbamate occurring in urea complexation processes commonly utilized for polyunsaturated fatty acid concentration. Food Chem. 2017, 229, 28–34. [Google Scholar] [CrossRef]
- Zheng, Z.; Dai, Z.; Cao, Y. Isolation, Purification of DPAn-3 from the Seal Oil Ethyl Ester. Eur. J. Lipid Sci. Technol. 2018, 120, 1800225. [Google Scholar] [CrossRef]
- María José, G.-F.; Dmitri, F.; Svetlana, L.; Francisca, F.-C.; José Luis, G.-G. Highly concentrated very long-chain PUFA obtainment by Urea complexation methodology. Environ. Technol. Innov. 2020, 18, 100736. [Google Scholar] [CrossRef]
- Hongyan Mu, X.L.Q.J.; Qingjie, S. Preparation of highly purified ω-3 docosapentaenoic acid from seal oil via urea complexation combined with preparative high performance liquid chromatography. Sep. Sci. Technol. 2021, 56, 1769–1778. [Google Scholar] [CrossRef]
- Nirmala, N.; Dawn, S.S. Optimization of Chlorella variabilis. MK039712.1 lipid transesterification using Response Surface Methodology and analytical characterization of biodiesel. Renew. Energy 2021, 179, 1663–1673. [Google Scholar] [CrossRef]
- Chen, X.; Li, Z.; Smith, S.A.; Chen, M.; Liu, H.; Zhang, J.; Tang, L.; Li, J.; Liu, Q.; Wu, X. Optimization of Supercritical CO2 Extraction of Moringa oleifera Seed Oil Using Response Surface Methodological Approach and Its Antioxidant Activity. Front. Nutr. 2022, 8, 829146. [Google Scholar] [CrossRef]
- Muhammad, G.; Potchamyou Ngatcha, A.D.; Lv, Y.; Xiong, W.; El-Badry, Y.A.; Asmatulu, E.; Xu, J.; Alam, M.A. Enhanced biodiesel production from wet microalgae biomass optimized via response surface methodology and artificial neural network. Renew. Energy 2022, 184, 753–764. [Google Scholar] [CrossRef]
- Cheng, X.; Huang, Y.; Yang, Z.; Wang, T.; Wang, X. Enrichment of Palmitoleic Acid by a Combination of Two-step Solvent Crystallization and Molecular Distillation. J. Oleo Sci. 2021, 70, 599–606. [Google Scholar] [CrossRef]
- Luis, V.; Casimir, C.A. Enrichment of stearidonic acid in modified soybean oil by low temperature crystallisation. Food Chem. 2012, 130, 147–155. [Google Scholar] [CrossRef]
- Bodkowski, R.; Czyż, K.; Sokoła-Wysoczańska, E.; Janczak, M.; Cholewińska, P.; Wyrostek, A. The Effect of Low-Temperature Crystallization of Fish Oil on the Chemical Composition, Fatty Acid Profile, and Functional Properties of Cow’s Milk. Animals 2020, 10, 1834. [Google Scholar] [CrossRef] [PubMed]
- Santos, O.V.; Lorenzo, N.D.; Souza, A.L.G.; Costa, C.E.F.; Conceição, L.R.V.; Lannes, S.C.d.S.; Teixeira-Costa, B.E. CO2 supercritical fluid extraction of pulp and nut oils from Terminalia catappa fruits: Thermogravimetric behavior, spectroscopic and fatty acid profiles. Food Res. Int. 2021, 139, 109814. [Google Scholar] [CrossRef] [PubMed]
- Janporn, S.; Ho, C.T.; Chavasit, V.; Pan, M.H.; Chittrakorn, S.; Ruttarattanamongkol, K.; Weerawatanakorn, M. Physicochemical properties of Terminalia catappa seed oil as a novel dietary lipid source. J. Food Drug Anal. 2015, 23, 201–209. [Google Scholar] [CrossRef]
- Hassan Elsayed, E.; Takuya, M.; Satoshi, H.; Tomonari, M.; Masayuki, N.; Masaru, T. Crystallization and melting properties studied by DSC and FTIR spectroscopy of goldenberry (Physalis peruviana) oil. Food Chem. 2022, 366, 130645. [Google Scholar] [CrossRef]
| Fatty Acids | Temperature (°C) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 50 °C | 60 °C | 70 °C | 80 °C | 90 °C | 100 °C | 110 °C | 120 °C | 130 °C | 140 °C | 150 °C | 160 °C | 170 °C | |
| Saturated fatty acids | |||||||||||||
| Palmitic acid C16:0 | 5.93 ± 0.87 ef | 8.26 ± 0.14 b | 5.78 ± 0.08 f | 8.97 ± 0.17 a | 8.29 ± 0.08 b | 6.23 ± 0.25 def | 5.98 ± 0.04 ef | 6.71 ± 0.28 cd | 6.46 ± 0.06 de | 6.70 ± 0.15 cd | 7.25 ± 0.08 c | 7.12 ± 0.11 c | 7.31 ± 0.10 c |
| Stearic acid C18:0 | 3.94 ± 0.66 a | 2.8 ± 0.19 bc | 2.45 ± 0.12 c | 3.96 ± 0.08 a | 4.4 ± 0.62 a | 2.81 ± 0.11 bc | 2.89 ± 0.06 bc | 2.93 ± 0.06 bc | 2.93 ± 0.12 bc | 2.75 ± 0.14 bc | 3.18 ± 0.07 b | 2.89 ± 0.12 bc | 3.04 ± 0.11 bc |
| Monounsaturated fatty acids | |||||||||||||
| Oleic acid C18:1 | 36.62 ± 1.25 b | 34.83 ± 0.55 c | 34.10 ± 0.16 cd | 32.74 ± 1.19 de | 31.48 ± 1.66 e | 37.09 ± 0.16 b | 34.16 ± 0.01 cd | 34.33 ± 0.31 c | 37.43 ± 0.07 b | 36.58 ± 0.12 b | 36.54 ± 0.09 b | 39.19 ± 0.11 a | 37.27 ± 0.18 b |
| Eicosenoic acid C20:1 | 5.97 ± 0.14 e | 5.51 ± 0.15 f | 7.15 ± 0.07 cd | 6.99 ± 0.37 d | 7.51 ± 0.44 bc | 7.33 ± 0.11 cd | 7.79 ± 0.03 b | 7.49 ± 0.2 bc | 6.98 ± 0.12 d | 6.95 ± 0.14 d | 7.52 ± 0.11 bc | 5.88 ± 0.07 ef | 8.95 ± 0.14 a |
| Docosenoic acid C22:1 | 7.07 ± 1.42 bc | 7.84 ± 0.74 bc | 8.23 ± 0.09 bc | 8.06 ± 1.04 bc | 10.52 ± 1.82 a | 8.23 ± 0.13 bc | 8.66 ± 0.07 b | 7.55 ± 0.39 bc | 7.11 ± 0.09 bc | 7.70 ± 0.11 bc | 6.52 ± 0.12 cd | 4.91 ± 0.10 e | 5.45 ± 0.11 de |
| Tetracosenoic acid C24:1 | 2.33 ± 0.12 abc | 2.74 ± 0.22 ab | 2.95 ± 0.14 a | 2.78 ± 0.21 a | 2.38 ± 0.21 abc | 2.29 ± 0.11 abc | 2.26 ± 0.12 abc | 2.06 ± 0.27 bcd | 2.02 ± 0.07 cd | 1.82 ± 0.02 cde | 1.53 ± 0.07 def | 1.29 ± 0.04 ef | 0.99 ± 0.09 f |
| Polyunsaturated fatty acids | |||||||||||||
| Linoleic acid C18:2 | 38.14 ± 0.76 bcde | 38.02 ± 0.28 cde | 39.33 ± 0.12 a | 36.5 ± 0.66 f | 35.42 ± 1.35 g | 37.09 ± 0.16 ef | 38.26 ± 0.19 abcd | 38.93 ± 0.2 abc | 37.43 ± 0.08 def | 36.58 ± 0.13 f | 36.54 ± 0.10 f | 39.19 ± 0.11 ab | 37.27 ± 0.09 def |
| Total | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 |
| Saturated fatty acids (SFAs) | 9.87 ± 0.21 | 11.06 ± 0.33 | 8.23 ± 0.19 | 12.93 ± 0.25 | 12.69 ± 0.54 | 9.04 ± 0.26 | 8.87 ± 0.1 | 9.64 ± 0.34 | 9.38 ± 0.15 | 9.45 ± 0.02 | 10.43 ± 0.15 | 5.01 ± 0.10 | 10.35 ± 0.16 |
| Unsaturated fatty acids (UFAs) | 90.13 ± 0.21 | 88.94 ± 1.88 | 91.76 ± 0.30 | 87.07 ± 0.24 | 87.31 ± 0.54 | 92.02 ± 0.45 | 91.13 ± 0.36 | 90.36 ± 0.34 | 90.97 ± 0.07 | 89.63 ± 0.32 | 88.65 ± 0.22 | 90.47 ± 0.36 | 89.92 ± 0.32 |
| Independent Variables | Symbols | Coded Levels | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| Temperature (°C) | A | −20 | −10 | 0 |
| Ethanol (mL) | B | 80.00 | 105.00 | 130.00 |
| Urea (g) | C | 4.00 | 6.00 | 8.00 |
| Source | SS | df | MS | F-Value | p-Value | Remark |
|---|---|---|---|---|---|---|
| Model | 39.16 | 9 | 4.35 | 92.62 | <0.0001 | significant |
| A | 2.31 | 1 | 2.31 | 49.20 | 0.0002 | |
| B | 1.27 | 1 | 1.27 | 27.08 | 0.0012 | |
| C | 0.78 | 1 | 0.78 | 16.50 | 0.0048 | |
| AB | 2.54 | 1 | 2.54 | 54.16 | 0.0002 | |
| AC | 2.81 | 1 | 2.81 | 59.73 | 0.0001 | |
| BC | 3.69 | 1 | 3.69 | 78.48 | <0.0001 | |
| A2 | 1.40 | 1 | 1.40 | 29.89 | 0.0009 | |
| B2 | 11.32 | 1 | 11.32 | 241.08 | <0.0001 | |
| C2 | 10.78 | 1 | 10.78 | 229.46 | <0.0001 | |
| Residual | 0.33 | 7 | 0.05 | |||
| Lack of Fit | 0.27 | 3 | 0.09 | 6.02 | 0.058 | not significant |
| Pure Error | 0.06 | 4 | 0.02 | |||
| Cor Total | 39.48 | 16 |
| Run | Independent Variables | Purity (%) | |||
|---|---|---|---|---|---|
| A: Temperature (°C) | B: Ethanol (mL) | C: Urea (g) | Experimental | Predicted | |
| 1 | −10 | 105 | 6 | 13.51 | 13.22 |
| 2 | −10 | 130 | 8 | 8.91 | 8.31 |
| 3 | −10 | 105 | 6 | 13.47 | 13.22 |
| 4 | 0 | 80 | 6 | 11.53 | 11.66 |
| 5 | 0 | 105 | 4 | 10.01 | 9.98 |
| 6 | 0 | 105 | 8 | 10.59 | 11.03 |
| 7 | 0 | 130 | 6 | 9.31 | 9.27 |
| 8 | −20 | 130 | 6 | 11.77 | 11.94 |
| 9 | −10 | 80 | 4 | 9.43 | 9.73 |
| 10 | −10 | 80 | 8 | 11.10 | 11.03 |
| 11 | −10 | 105 | 6 | 13.31 | 13.22 |
| 12 | −10 | 105 | 6 | 13.42 | 13.22 |
| 13 | −20 | 80 | 6 | 11.10 | 11.14 |
| 14 | −10 | 105 | 6 | 13.38 | 13.22 |
| 15 | −20 | 105 | 8 | 10.47 | 10.43 |
| 16 | −10 | 130 | 4 | 10.78 | 10.85 |
| 17 | −20 | 105 | 4 | 12.97 | 12.73 |
| Fatty Acids | Composition (%) | |||
|---|---|---|---|---|
| −20 °C-One-Stage | −20 °C-Two-Stage | −60 °C-One-Stage | −60 °C-Two-Stage | |
| Saturated fatty acids | ||||
| Palmitic acid C16:0 | 11.51 ± 0.11 c | 10.83 ± 0.09 d | 25.48 ± 0.13 a | 22.52 ± 0.08 b |
| Stearic acid C18:0 | 4.94 ± 0.14 d | 22.86 ± 0.12 b | 23.64 ± 0.23 a | 7.85 ± 0.14 c |
| Monounsaturated fatty acids | ||||
| Oleic acid C18:1 | 44.69 ± 0.05 a | 8.70 ± 0.19 c | 16.32 ± 0.11 b | 7.41 ± 0.17 d |
| Eicosenoic acid C20:1 | 11.39 ± 0.09 a | 8.95 ± 0.21 b | 3.88 ± 0.16 c | 4.05 ± 0.28 c |
| Docoenoic acid C22:1 | 13.75 ± 0.17 c | 26.01 ± 0.08 b | 11.95 ± 0.14 c | 33.04 ± 0.22 a |
| Tetracosenoic acid C24:1 | 3.76 ± 0.06 d | 18.34 ± 0.07 b | 6.91 ± 0.15 c | 19.66 ± 0.17 a |
| Polyunsaturated fatty acids | ||||
| Linoleic acid C18:2 | 9.96 ± 0.09 b | 4.31 ± 0.09 d | 11.82 ± 0.24 a | 5.47 ± 0.12 c |
| Total | 7 | 7 | 7 | 7 |
| Saturated fatty acids (SFAs) | 16.45 ± 0.03 | 33.69 ± 0.12 | 49.12 ± 0.26 | 30.37 ± 0.21 |
| Unsaturated fatty acids (UFAs) | 83.55 ± 0.23 | 66.31 ± 0.60 | 50.88 ± 0.55 | 69.63 ± 0.23 |
| Fatty Acids | Composition (%) | RT (min) | ||
|---|---|---|---|---|
| Urea Complexation | Low-Temperature Solvent Crystallization | Combined Treatment | ||
| Saturated fatty acids | ||||
| Palmitic acid C16:0 | 15.43 ± 0.03 c | 22.52 ± 0.05 b | 24.66 ± 0.14 a | 11.65 |
| Stearic acid C18:0 | 10.41 ± 0.09 b | 7.85 ± 0.18 c | 26.40 ± 0.18 a | 13.54 |
| Eicosanoic acid C20:0 | 1.17 ± 0.10 b | - | 2.15 ± 0.08 a | 15.26 |
| Docosanoic acid C22:0 | 3.05 ± 0.24 a | - | 2.40 ± 0.08 b | 16.87 |
| Tetracosanoic acid C24:0 | 2.09 ± 0.24 a | - | - | |
| Monounsaturated fatty acids | ||||
| Oleic acid C18:1 | 18.51 ± 0.15 a | 7.41 ± 0.15 b | - | 13.31 |
| Eicosenoic acid C20:1 | 8.42 ± 0.12 a | 4.05 ± 0.15 b | - | 15.06 |
| Docoenoic acid C22:1 | 22.93 ± 0.23 b | 33.04 ± 0.18 a | 4.22 ± 0.07 c | 16.70 |
| Tetracosenoic acid C24:1 | 14.07 ± 0.10 c | 19.66 ± 0.06 b | 40.17 ± 0.23 a | 18.20 |
| Polyunsaturated fatty acids | ||||
| Linoleic acid C18:2 | 3.92 ± 0.11 b | 5.47 ± 0.10 a | - | 13.25 |
| Total | 10 | 7 | 6 | |
| Saturated fatty acids (SFAs) | 32.15 ± 0.41 | 30.37 ± 0.21 | 55.61 ± 0.18 | |
| Unsaturated fatty acids (UFAs) | 67.85 ± 0.39 | 69.63 ± 0.01 | 44.39 ± 0.28 | |
| Yield (%) | 77.13 | 75.82 | 76.85 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, H.; Sun, J.; Guo, X.; Zhang, Z.; Liu, H.; Zhang, Z.; Liu, M.; Zhou, S.; Li, S.; Zhang, T. Study on the Extraction of Nervonic Acid from the Oil of Xanthoceras sorbifolium Bunge Seeds. Foods 2024, 13, 2757. https://doi.org/10.3390/foods13172757
Gao H, Sun J, Guo X, Zhang Z, Liu H, Zhang Z, Liu M, Zhou S, Li S, Zhang T. Study on the Extraction of Nervonic Acid from the Oil of Xanthoceras sorbifolium Bunge Seeds. Foods. 2024; 13(17):2757. https://doi.org/10.3390/foods13172757
Chicago/Turabian StyleGao, Hui, Jie Sun, Xuan Guo, Ziyan Zhang, He Liu, Zhiran Zhang, Mengkai Liu, Sen Zhou, Shengxin Li, and Tingting Zhang. 2024. "Study on the Extraction of Nervonic Acid from the Oil of Xanthoceras sorbifolium Bunge Seeds" Foods 13, no. 17: 2757. https://doi.org/10.3390/foods13172757





