Study on Selenium Assimilation and Transformation in Radish Sprouts Cultivated Using Maillard Reaction Products
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Synthesis of Se-MRPs
2.3. Purification of Se-MRPs
2.4. Sprout Germination and Cultivation
2.5. Extraction of Organic Selenoamino Acids
2.6. Sample Analysis
2.6.1. Preparation of Calibration Curves
2.6.2. Analysis of Organic SeAAs
2.6.3. Analysis of Se-MRPs
2.7. Statistical Analysis
3. Results and Discussions
3.1. Preparation of Se-MRPs
3.2. Molecular Recognition of Se-MRPs
3.3. Assimilation and Transformation of Se-MRPs by Radish Sprouts
3.3.1. Total Se Content
3.3.2. SeAAs Content
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fairweather-Tait, S.J.; Bao, Y.; Broadley, M.R.; Collings, R.; Ford, D.; Hesketh, J.E.; Hurst, R. Selenium in Human Health and Disease. Antioxid. Redox Signal. 2011, 14, 1337–1383. [Google Scholar] [CrossRef] [PubMed]
- Ying, H.; Zhang, Y. Systems Biology of Selenium and Complex Disease. Biol. Trace Elem. Res. 2019, 192, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Qazi, I.H.; Pan, B.; Angel, C.; Guo, S.; Yang, J.; Zhang, Y.; Ming, Z.; Zeng, C.; Meng, Q.; et al. Dietary Selenium Supplementation Ameliorates Female Reproductive Efficiency in Aging Mice. Antioxidants 2019, 8, 634. [Google Scholar] [CrossRef]
- Rayman, M.P. The Importance of Selenium to Human Health. Lancet 2000, 356, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Schiavon, M.; Berto, C.; Malagoli, M.; Trentin, A.; Sambo, P.; Dall’Acqua, S.; Pilon-Smits, E.A.H. Selenium Biofortification in Radish Enhances Nutritional Quality Via Accumulation of Methyl-Selenocysteine and Promotion of Transcripts and Metabolites Related to Glucosinolates, Phenolics, and Amino Acids. Front. Plant Sci. 2016, 7, 1371. [Google Scholar] [CrossRef]
- Thiry, C.; Ruttens, A.; De Temmerman, L.; Schneider, Y.-J.; Pussemier, L. Current Knowledge in Species-Related Bioavailability of Selenium in Food. Food Chem. 2012, 130, 767–784. [Google Scholar] [CrossRef]
- Alfthan, G.; Eurola, M.; Ekholm, P.; Venäläinen, E.-R.; Root, T.; Korkalainen, K.; Hartikainen, H.; Salminen, P.; Hietaniemi, V.; Aspila, P.; et al. Effects of Nationwide Addition of Selenium to Fertilizers on Foods, and Animal and Human Health in Finland: From Deficiency to Optimal Selenium Status of the Population. J. Trace Elem. Med. Biol. 2015, 31, 142–147. [Google Scholar] [CrossRef]
- Kieliszek, M.; Sandoval, S.N.S. The Importance of Selenium in Food Enrichment Processes. A Comprehensive Review. J. Trace Elem. Med. Biol. 2023, 79, 127260. [Google Scholar] [CrossRef]
- Dinh, Q.T.; Cui, Z.; Huang, J.; Tran, T.A.T.; Wang, D.; Yang, W.; Zhou, F.; Wang, M.; Yu, D.; Liang, D. Selenium Distribution in the Chinese Environment and Its Relationship with Human Health: A Review. Environ. Int. 2018, 112, 294–309. [Google Scholar] [CrossRef]
- Funes-Collado, V.; Morell-Garcia, A.; Rubio, R.; López-Sánchez, J.F. Study of Selenocompounds from Selenium-Enriched Culture of Edible Sprouts. Food Chem. 2013, 141, 3738–3743. [Google Scholar] [CrossRef]
- Jyske, T.; Järvenpää, E.; Kunnas, S.; Sarjala, T.; Raitanen, J.-E.; Mäki, M.; Pastell, H.; Korpinen, R.; Kaseva, J.; Tupasela, T. Sprouts and Needles of Norway Spruce (Picea abies (L.) Karst.) as Nordic Specialty—Consumer Acceptance, Stability of Nutrients, and Bioactivities during Storage. Molecules 2020, 25, 4187. [Google Scholar] [CrossRef]
- Sugihara, S.; Kondô, M.; Chihara, Y.; Yûji, M.; Hattori, H.; Yoshida, M. Preparation of Selenium-Enriched Sprouts and Identification of Their Selenium Species by High-Performance Liquid Chromatography-Inductively Coupled Plasma Mass Spectrometry. Biosci. Biotechnol. Biochem. 2004, 68, 193–199. [Google Scholar] [CrossRef]
- Zou, X.; Wang, Y.; Sun, R.; Wang, J. An Analysis of the Content Changes in Free and Combinative Forms of Organic Selenium in Radish Sprouts Cultivated with Solutions of Selenoamino Acids. Food Res. Int. 2022, 158, 111558. [Google Scholar] [CrossRef]
- Kikkert, J.; Berkelaar, E. Plant Uptake and Translocation of Inorganic and Organic Forms of Selenium. Arch. Environ. Contam. Toxicol. 2013, 65, 458–465. [Google Scholar] [CrossRef]
- Singh, K.; Tripathi, S.; Chandra, R. Maillard Reaction Product and Its Complexation with Environmental Pollutants: A Comprehensive Review of Their Synthesis and Impact. Bioresour. Technol. Rep. 2021, 15, 100779. [Google Scholar] [CrossRef]
- Wang, J.; Lu, Y.; Liu, B.; He, H. Electrospray Positive Ionization Tandem Mass Spectrometry of Amadori Compounds. J. Mass Spectrom. 2008, 43, 262–264. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Sun, L.; Chen, M.; Wang, J. An Analysis of the Changes on Intermediate Products during the Thermal Processing of Black Garlic. Food Chem. 2018, 239, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Shen, K.; Wang, C.; Wang, J. Molecular Recognition and Quantitative Analysis of Free and Combinative Selenium Speciation in Selenium-Enriched Millets Using Hplc-Esi-Ms/Ms. J. Food Compos. Anal. 2022, 106, 104333. [Google Scholar] [CrossRef]
- Yuan, H.; Sun, L.; Chen, M.; Wang, J. The Simultaneous Analysis of Amadori and Heyns Compounds in Dried Fruits by High Performance Liquid Chromatography Tandem Mass Spectrometry. Food Anal. Methods 2017, 10, 1097–1105. [Google Scholar] [CrossRef]
- Tsai, J.H.; Hiserodt, R.D.; Ho, C.-T.; Hartman, T.G.; Rosen, R.T. Determination of Volatile Organic Selenium Compounds from the Maillard Reaction in a Selenomethionine—Glucose Model System. J. Agric. Food Chem. 1998, 46, 2541–2545. [Google Scholar] [CrossRef]
- Martins, S.I.; Jongen, W.M.; van Boekel, M.A. A Review of Maillard Reaction in Food and Implications to Kinetic Modelling. Trends Food Sci. Technol. 2000, 11, 364–373. [Google Scholar] [CrossRef]
- van Boekel, M. Formation of Flavour Compounds in the Maillard Reaction. Biotechnol. Adv. 2006, 24, 230–233. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Alvarenga, M.; Martinez-Rodriguez, E.; Garcia-Amezquita, L.; Olivas, G.; Zamudio-Flores, P.; Acosta-Muniz, C.; Sepulveda, D. Effect of Maillard Reaction Conditions on the Degree of Glycation and Functional Properties of Whey Protein Isolate–Maltodextrin Conjugates. Food Hydrocoll. 2014, 38, 110–118. [Google Scholar] [CrossRef]
- Khanam, A.; Platel, K. Bioaccessibility of Selenium, Selenomethionine and Selenocysteine from Foods and Influence of Heat Processing on the Same. Food Chem. 2016, 194, 1293–1299. [Google Scholar] [CrossRef]
- Bodnar, M.; Konieczka, P. Evaluation of Candidate Reference Material Obtained from Selenium-Enriched Sprouts for the Purpose of Selenium Speciation Analysis. LWT 2016, 70, 286–295. [Google Scholar] [CrossRef]
- Zagrodzki, P.; Paśko, P.; Domínguez-Álvarez, E.; Salardón-Jiménez, N.; Sevilla-Hernández, C.; Sanmartín, C.; Bierła, K.; Łobiński, R.; Szpunar, J.; Handzlik, J.; et al. Synthesis of Novel Organic Selenium Compounds and Speciation of Their Metabolites in Biofortified Kale Sprouts. Microchem. J. 2022, 172, 106962. [Google Scholar] [CrossRef]
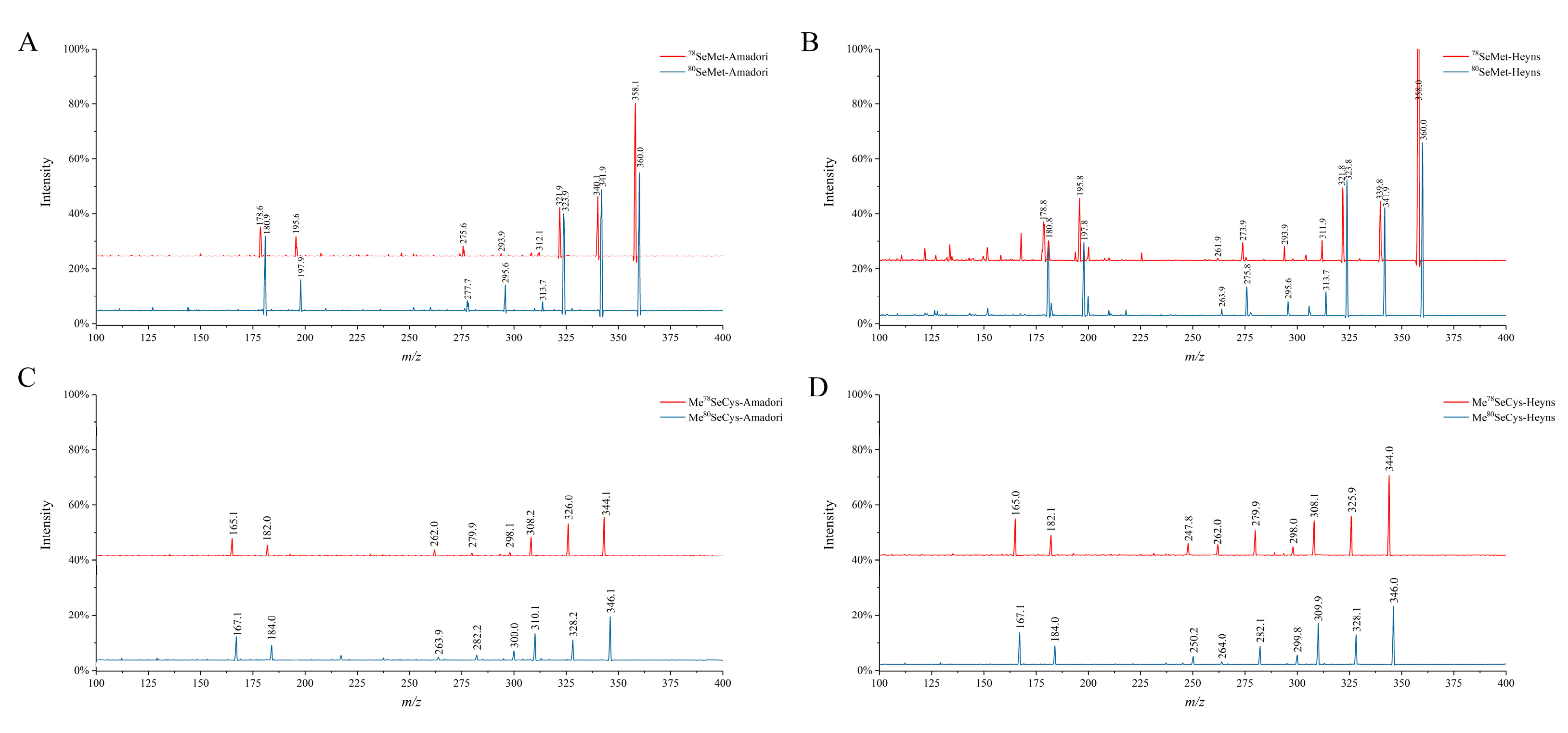
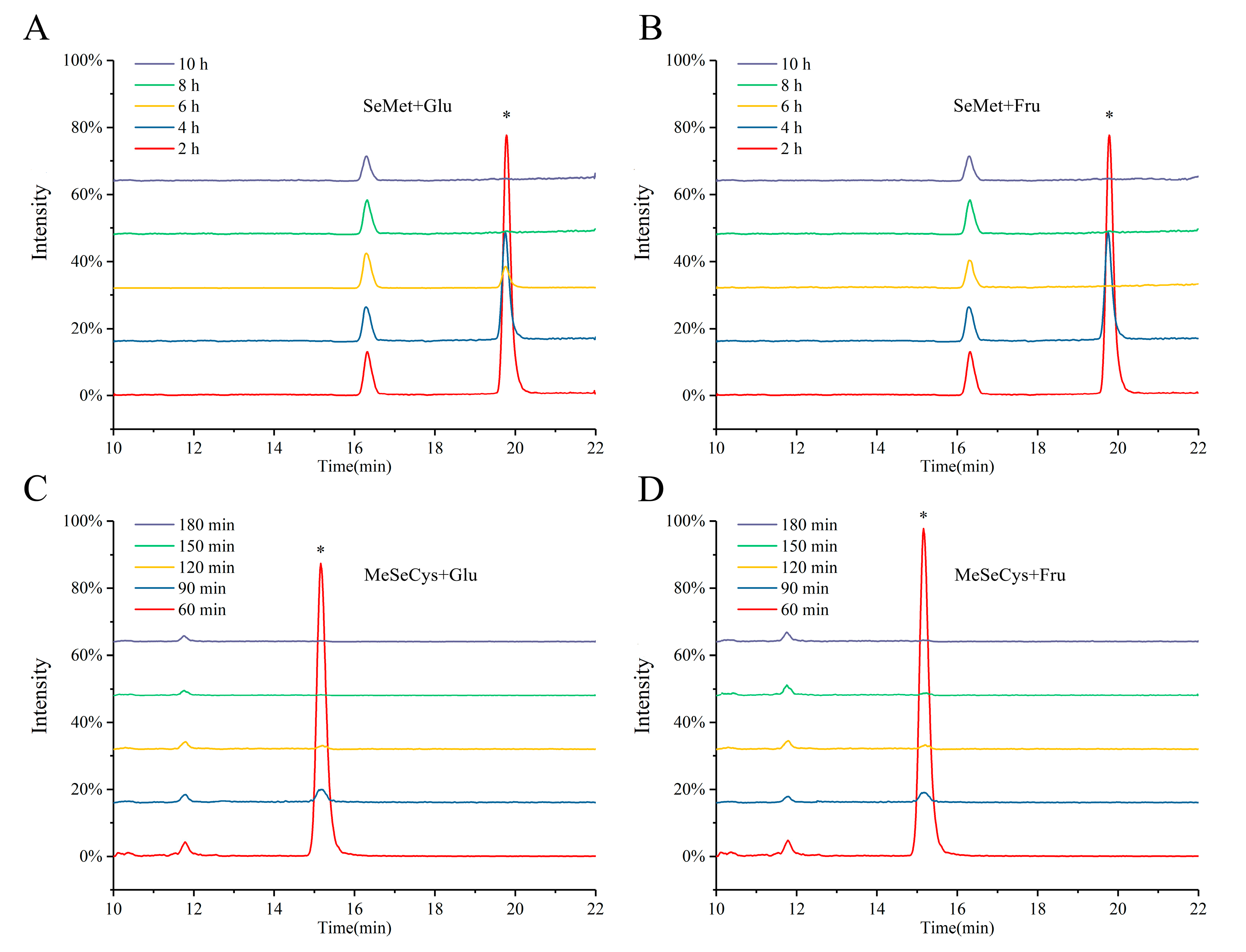
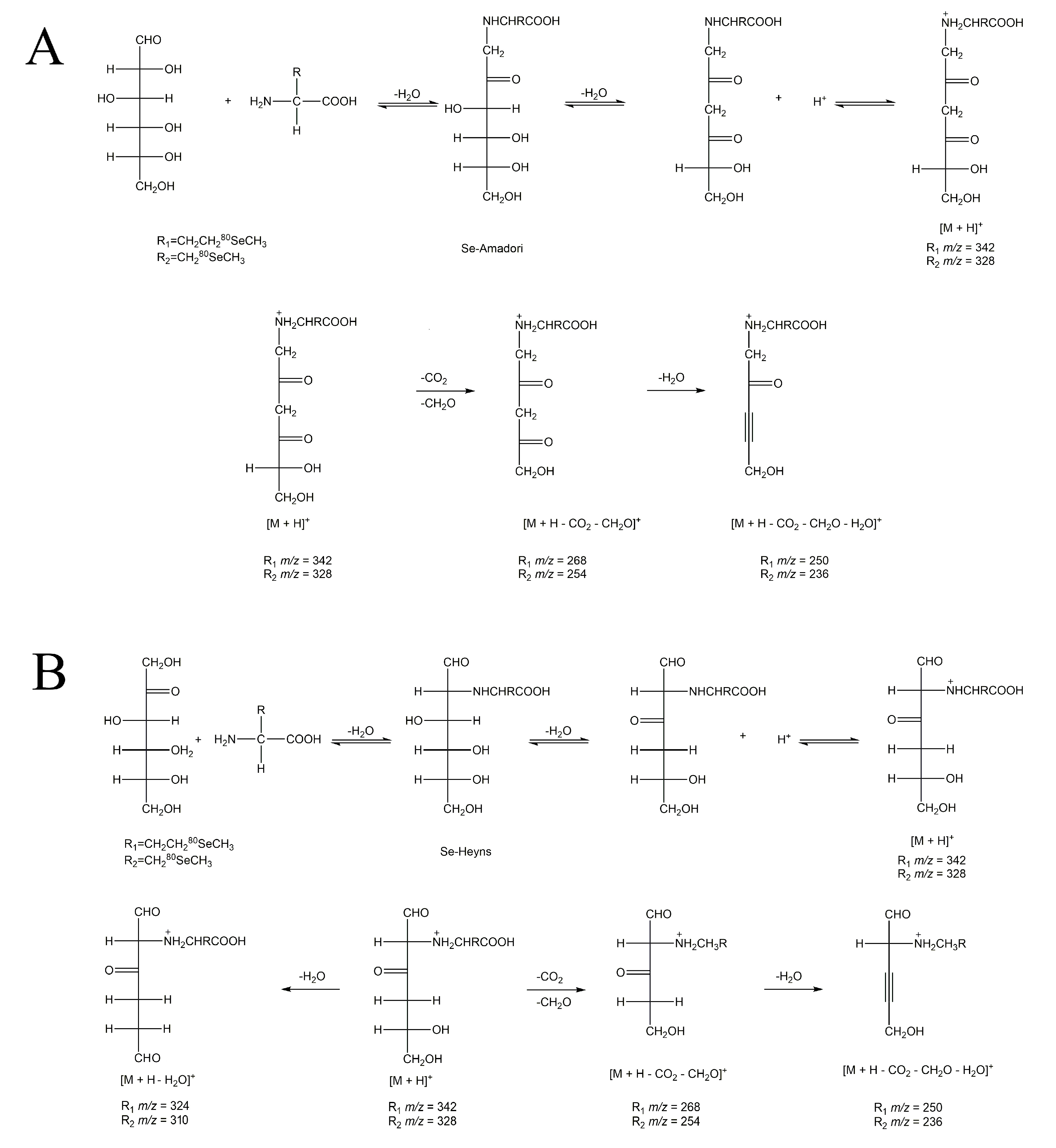
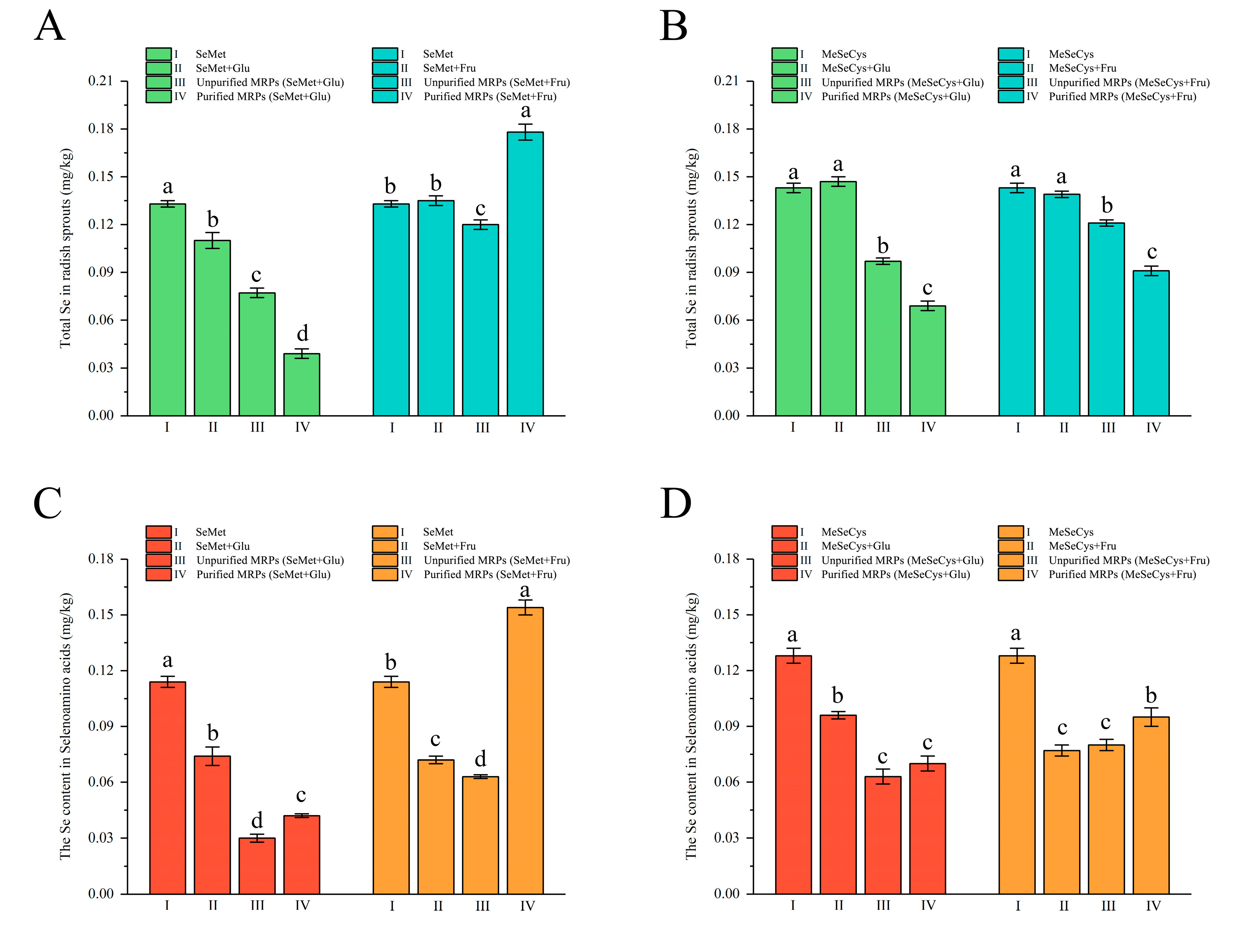
| Analytes | Precursor Ion (m/z) | Product Ion (m/z) |
|---|---|---|
| MRPs (80SeMet+Glu) | 342 | 268, 250 |
| MRPs (78SeMet+Glu) | 340 | 266, 248 |
| MRPs (80SeMet+Fru) | 342 | 324, 268, 250 |
| MRPs (78SeMet+Fru) | 340 | 322, 266, 248 |
| MRPs (Me80SeCys+Glu) | 328 | 254, 236 |
| MRPs (Me78SeCys+Glu) | 326 | 254, 234 |
| MRPs (Me80SeCys+Fru) | 328 | 310, 254, 236 |
| MRPs (Me78SeCys+Fru) | 326 | 308, 254, 234 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, X.; Sun, R.; Wang, C.; Wang, J. Study on Selenium Assimilation and Transformation in Radish Sprouts Cultivated Using Maillard Reaction Products. Foods 2024, 13, 2761. https://doi.org/10.3390/foods13172761
Zou X, Sun R, Wang C, Wang J. Study on Selenium Assimilation and Transformation in Radish Sprouts Cultivated Using Maillard Reaction Products. Foods. 2024; 13(17):2761. https://doi.org/10.3390/foods13172761
Chicago/Turabian StyleZou, Xiaoshuang, Ruiqi Sun, Can Wang, and Jun Wang. 2024. "Study on Selenium Assimilation and Transformation in Radish Sprouts Cultivated Using Maillard Reaction Products" Foods 13, no. 17: 2761. https://doi.org/10.3390/foods13172761







