Inhibitory Effects of Lactobionic Acid on Biofilm Formation and Virulence of Staphylococcus aureus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents, Bacterial Strains, and Culture Conditions
2.2. Minimum Inhibitory Concentration (MIC)
2.3. Crystal Violet Assay
2.4. Viable Count Assay
2.5. Microscopic Visualization
2.5.1. Visualization by SEM
2.5.2. Visualization by CLSM
2.6. Determination of Extracellular Polymeric Matrix
2.6.1. EPS Content
2.6.2. eDNA Content
2.7. Biofilm Metabolic Activity
2.8. Hemolysis Measurement
2.9. RNA Isolation and qRT-PCR
2.10. Statistical Analysis
3. Results and Discussion
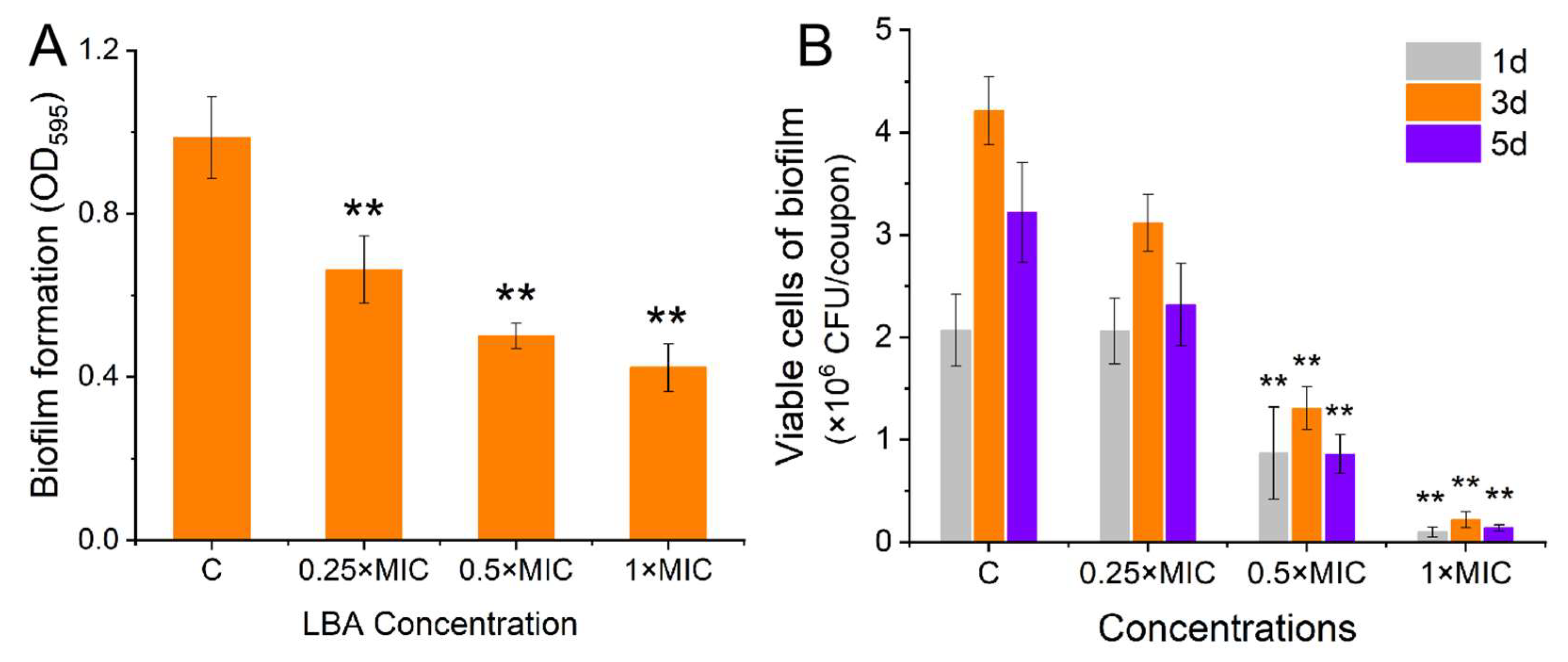
3.1. Effect of LBA on Biofilm Formation of S. aureus
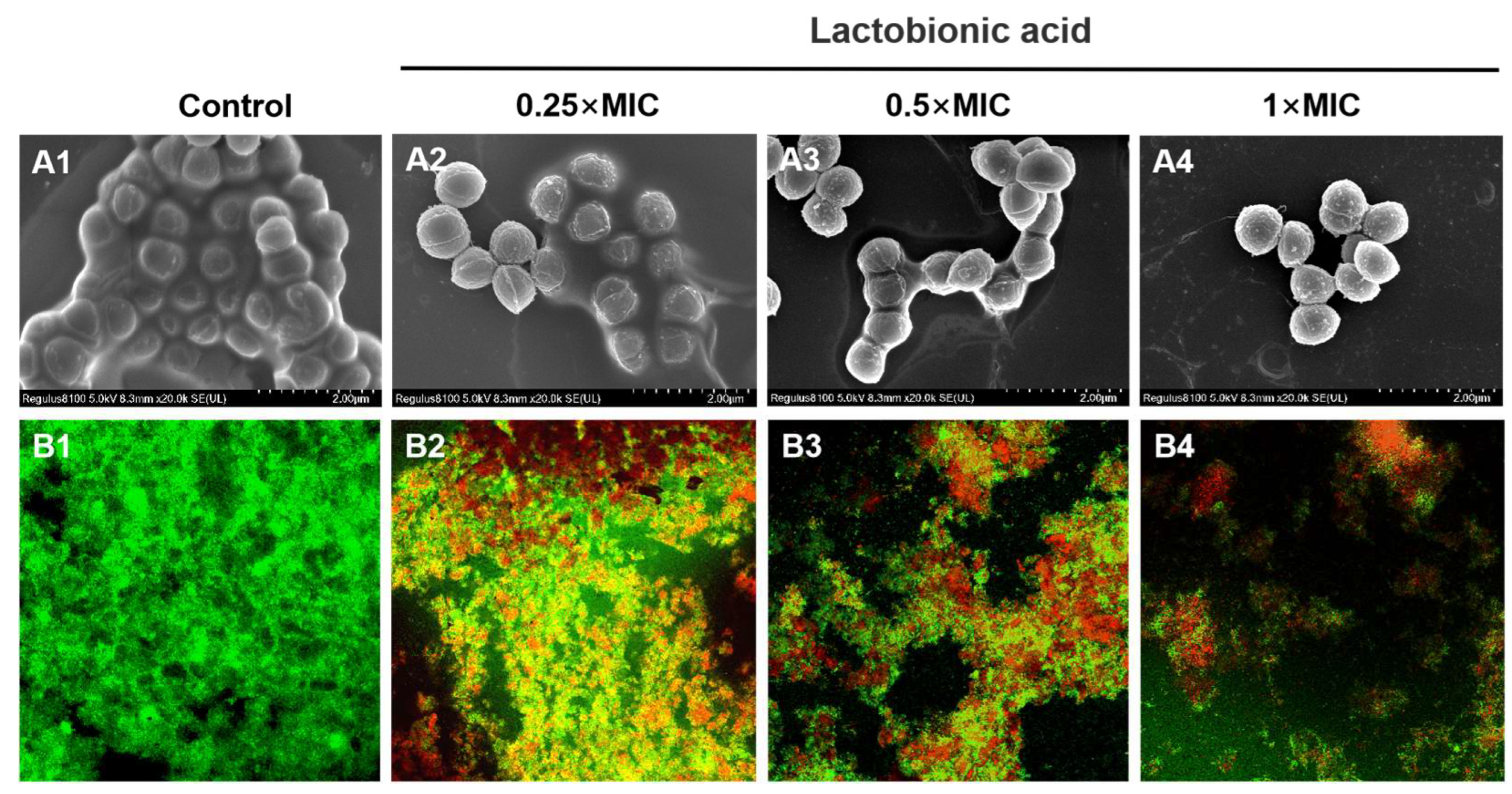
3.2. Micromorphological observation
3.3. Effect of LBA on the Releases of the Extracellular Polymeric Matrix in S. aureus Biofilm
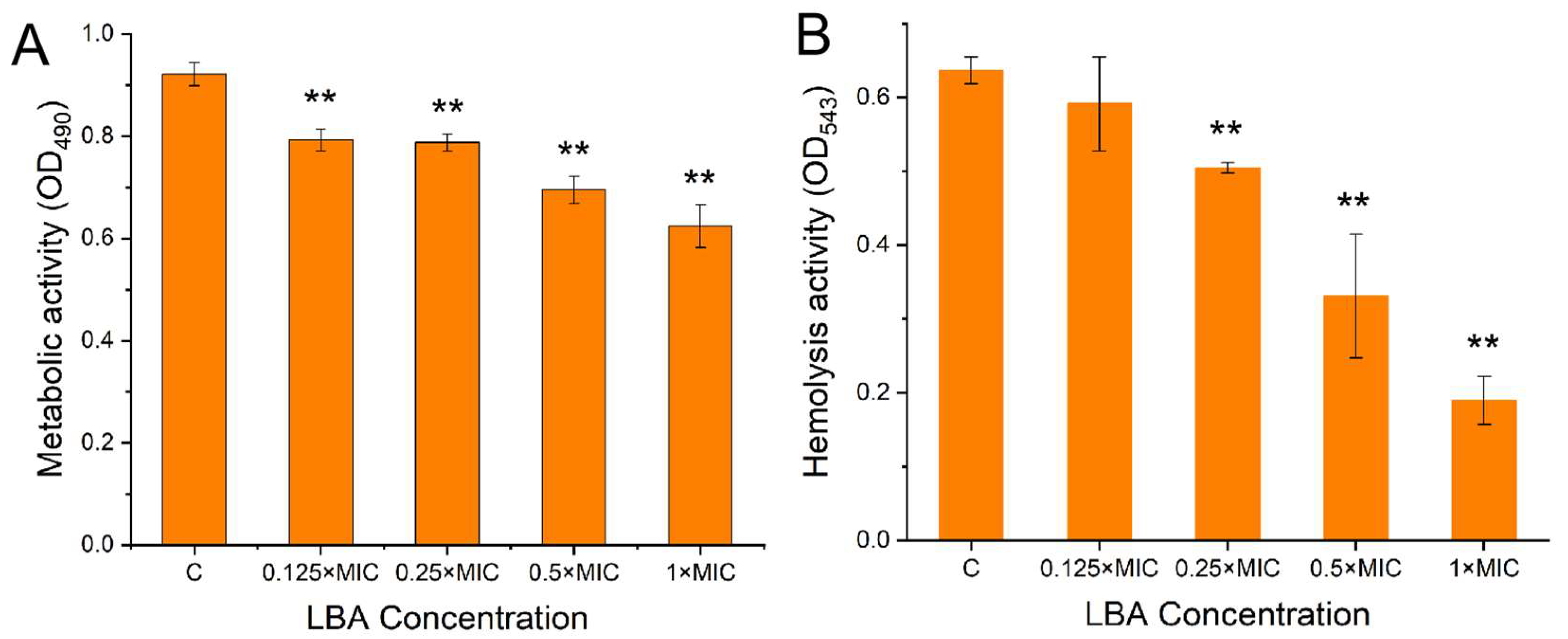
3.4. Effect of LBA on the Biofilm Metabolic Activity and Hemolysis Activity of S. aureus
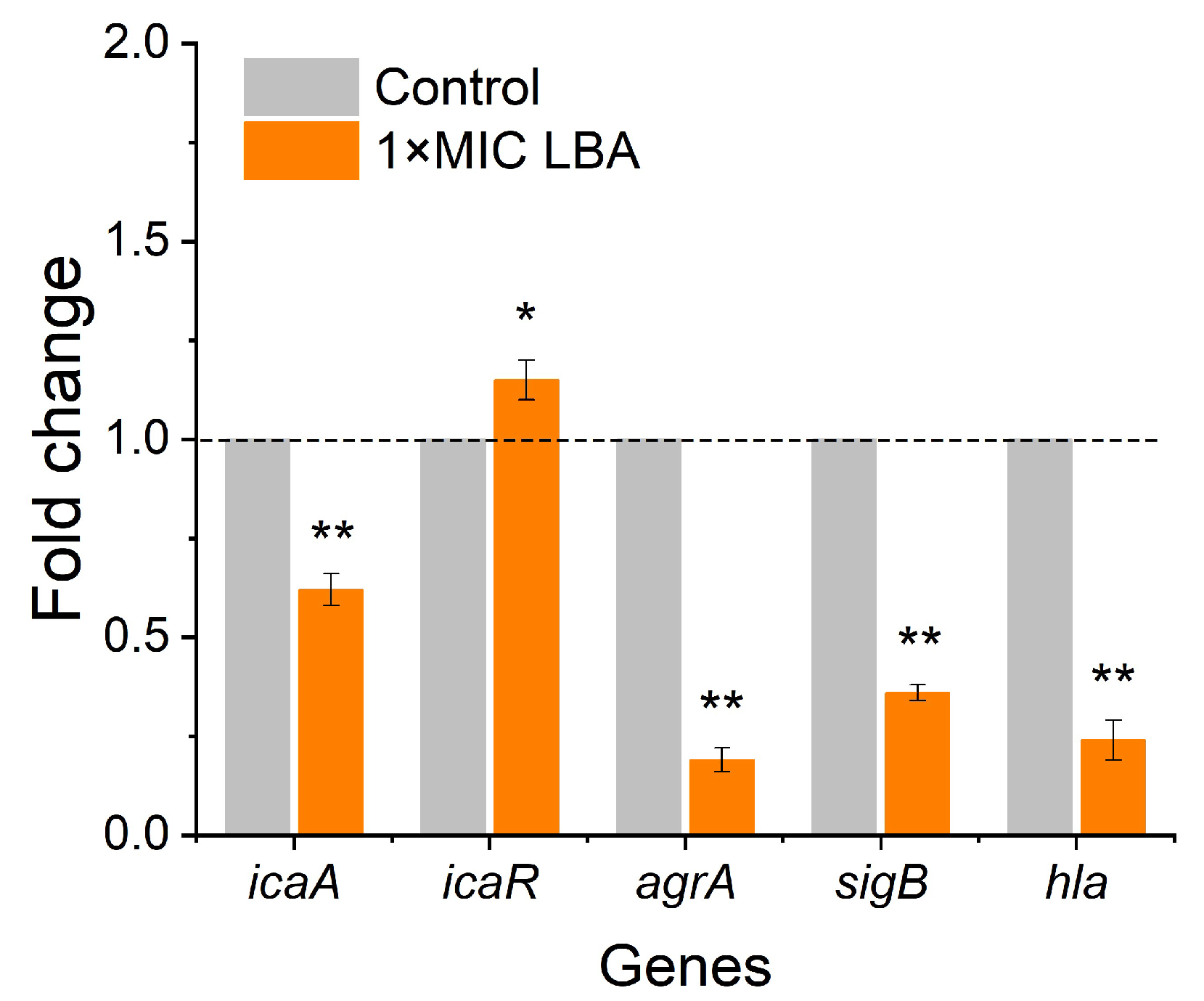
3.5. LBA Modulated Biofilm- and Virulence-Related Genes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pang, X.; Song, X.; Che, M.; Tian, S.; Lu, Z.; Sun, J.; Yuk, H. Combating biofilms of foodborne pathogens with bacteriocins by lactic acid bacteria in the food industry. Compr. Rev. Food Sci. Food Saf. 2022, 21, 1657–1676. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Estimates of the Global Burden of Foodborne Diseases: Foodborne Disease Burden Epidemiology Reference Group; World Health Organization: Geneva, Switzerland, 2015; Available online: https://apps.who.int/iris/handle/10665/199350 (accessed on 1 December 2015).
- Arunachalam, K.; Pandurangan, P.; Shi, C.L.; Lagoa, R. Regulation of Staphylococcus aureus Virulence and Application of Nanotherapeutics to Eradicate S. aureus Infection. Pharmaceutics 2023, 15, 310. [Google Scholar] [CrossRef]
- Suwal, N.; Subba, R.K.; Paudyal, P.; Khanal, D.P.; Koirala, N. Antimicrobial and antibiofilm potential of curcuma longa linn. rhizome extract against biofilm producing Staphylococcus aureus and Pseudomonas aeruginosa isolates. Cell. Mol. Biol. 2021, 67, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yao, H.; Zhao, X.; Ge, C. Biofilm Formation and Control of Foodborne Pathogenic Bacteria. Molecules 2023, 28, 2432. [Google Scholar] [CrossRef]
- Guo, A.; Li, Q.; Liu, L.; Zhang, X.; Yao, R. Formation of multi-species biofilms and their resistance to disinfectants in food processing environments: A review. J. Food Prot. 2021, 84, 2071–2083. [Google Scholar]
- Winkelstroter, L.; Teixeira, F.B.D.R.; Silva, E.P.; Alves, V.F.; De Martinis, E.C.P. Unraveling microbial biofilms of importance for food microbiology. Microb. Ecol. 2014, 68, 35–46. [Google Scholar] [CrossRef]
- Sun, J.; Wang, D.; Sun, Z.; Liu, F.; Du, L.; Wang, D. The combination of ultrasound and chlorogenic acid to inactivate Staphylococcus aureus under planktonic, biofilm, and food systems. Ultrason. Sonochem. 2021, 80, 105801. [Google Scholar] [CrossRef]
- Chan, W.C.; Coyle, B.J.; Williams, P. Virulence regulation and quorum sensing in staphylococcal infections: Competitive agrc antagonists as quorum sensing inhibitors. J. Med. Chem. 2004, 47, 4633–4641. [Google Scholar] [CrossRef]
- Faleye, O.O.; Faleye, O.S.; Lee, J.H. Antibacterial and antibiofilm activities of iodinated hydrocarbons against Vibrio parahaemolyticus and Staphylococcus aureus. Sci. Rep. 2024, 14, 9160. [Google Scholar] [CrossRef]
- Álvarez-Fernández, E.; Cancelo, A.; Díaz-Vega, C.; Capita, R.; Alonso-Calleja, C. Antimicrobial resistance in E. coli isolates from conventionally and organically reared poultry: A comparison of agar disc diffusion and Sensi Test Gram-negative methods. Food Cont. 2013, 30, 227–234. [Google Scholar] [CrossRef]
- Kiryu, T.; Kiso, T.; Nakano, H.; Ooe, K.; Kimura, T.; Murakami, H. Involvement of Acetobacter orientalis in the production of lactobionic acid in Caucasian yogurt (“Caspian Sea yogurt”) in Japan. J. Dairy Sci. 2009, 92, 25–34. [Google Scholar] [CrossRef]
- Alonso, S.; Rendueles, M.; Díaz, M. Bio-production of lactobionic acid: Current status, applications and future prospects. Biotechnol. Adv. 2013, 31, 1275–1291. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.M.; Kong, F.H.; Shi, X.Y.; Han, H.J.; Li, M.H.; Guan, B.Y.; Yang, M.; Cao, X.Y.; Tao, D.B.; Zheng, Y.; et al. Antibacterial activity and mechanism of lactobionic acid against Pseudomonas fluorescens and Methicillin-resistant Staphylococcus aureus and its application on whole milk. Food Control 2020, 108, 106876. [Google Scholar] [CrossRef]
- Kang, S.M.; Kong, F.H.; Liang, X.N.; Li, M.H.; Yang, N.; Cao, X.Y.; Yang, M.; Tao, D.B.; Yue, X.Q.; Zheng, Y. Label-free quantitative proteomics reveals the multitargeted antibacterial mechanisms of lactobionic acid against methicillin-resistant Staphylococcus aureus (MRSA) using SWATH-MS technology. J. Agric. Food Chem. 2019, 67, 12322–12332. [Google Scholar] [CrossRef]
- Hou, W.; Kang, S.; Chang, J.; Tian, X.; Shi, C. Correlation Analysis between GlpQ-Regulated Degradation of Wall Teichoic Acid and Biofilm Formation Triggered by Lactobionic Acid in Staphylococcus aureus. Foods 2022, 11, 3438. [Google Scholar] [CrossRef]
- Zhang, H.; Li, S.; Cheng, Y. Antibiofilm Activity of Allicin and Quercetin in Treating Biofilm-Associated Orthopaedics Infection. Appl. Biochem. Biotechnol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Shen, F.; Ge, C.; Yuan, P. Metabolomics Study Reveals Inhibition and Metabolic Dysregulation in Staphylococcus aureus Planktonic Cells and Biofilms Induced by Carnosol. Front. Microbiol. 2022, 11, 538572. [Google Scholar] [CrossRef] [PubMed]
- Lyu, X.; Li, C.; Zhang, J.; Wang, L.; Jiang, Q.; Shui, Y.; Chen, L.; Luo, Y.; Xu, X. A Novel Small Molecule, LCG-N25, Inhibits Oral Streptococcal Biofilm. Front. Microbiol. 2021, 12, 654692. [Google Scholar] [CrossRef]
- Rice, K.C.; Mann, E.E.; Endres, J.L. The cidA murein hydrolase regulator contributes to DNA release and biofilm development in Staphylococcus aureus. Proc. Natl. Acad. Sci. USA 2007, 104, 8113–8118. [Google Scholar] [CrossRef]
- Sivaranjani, M.; Gowrishankar, S.; Kamaladevi, A.; Pandian, S.K.; Balamurugan, K.; Ravi, A.V. Morin inhibits biofilm production and reduces the virulence of Listeria monocytogene–an in vitro and in vivo approach. Int. J. Food Microbiol. 2016, 237, 73–82. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, Y.G.; Lee, J. Inhibition of Staphylococcus aureus biofilm formation and virulence factor production by petroselinic acid and other unsaturated C18 fatty acids. Microbiol. Spectr. 2022, 10, e01330–e11322. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.M.; Shi, C.L.; Chang, J.; Kong, F.H.; Li, M.H.; Guan, B.Y.; Zhang, Z.H.; Shi, X.Y.; Zhao, H.W.; Peng, Y.Q.; et al. Label free-based proteomic analysis of the food spoiler Pseudomonas fluorescens response to lactobionic acid by SWATH-MS. Food Control 2021, 123, 107834. [Google Scholar] [CrossRef]
- Akbas, M.Y.; Kokumer, T. The prevention and removal of biofilm formation of Staphylococcus aureus strains isolated from raw milk samples by citric acid treatments. Int. J. Food Sci. Technol. 2015, 50, 1666–1672. [Google Scholar] [CrossRef]
- Raja, A.F.; Ali, F.; Khan, I.A.; Shawl, A.S.; Taneja, S.C. Antistaphylococcal and biofilm inhibitory activities of acetyl-11-keto-β-boswellic acid from Boswellia serrata. BMC Microbiol. 2011, 11, 54. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.R.; Zhong, K.; Wu, Y.P.; Elena, G.; Gao, H. Antibiofilm activity of shikimic acid against Staphylococcus aureus. Food Control 2019, 95, 327–333. [Google Scholar] [CrossRef]
- Liu, F.; Du, L.H.; Zhao, T.; Zhao, P.; Doyle, M.P. Effects of phenyllactic acid as sanitizing agent for inactivation of Listeria monocytogenes biofilms. Food Control 2017, 78, 72–78. [Google Scholar] [CrossRef]
- Fan, Q.X.; Yuan, Y.H.; Zhang, T.; Song, W.; Sheng, Q.L.; Yue, T.L. Inhibitory effects of lactobionic acid on Vibrio parahaemolyticus planktonic cells and biofilms. Food Microbiol. 2022, 103, 103963. [Google Scholar] [CrossRef]
- Sharma, S.; Mohler, J.; Mahajan, S.D.; Schwartz, S.A.; Bruggemann, L.; Aalinkeel, R. Microbial Biofilm: A Review on Formation, Infection, Antibiotic Resistance, Control Measures, and Innovative Treatment. Microorganisms 2023, 11, 1614. [Google Scholar] [CrossRef]
- Mahto, K.U.; Priyadarshanee, M.; Samantaray, D.P.; Das, S. Bacterial biofilm and extracellular polymeric substances in the treatment of environmental pollutants: Beyond the protective role in survivability. J. Clean. Prod. 2022, 379, 134759. [Google Scholar] [CrossRef]
- Fan, Q.X.; He, Q.; Zhang, T.; Song, W.; Sheng, Q.L.; Yuan, Y.H.; Yue, T.L. Antibiofilm potential of lactobionic acid against Salmonella Typhimurium. LWT 2022, 162, 113461. [Google Scholar] [CrossRef]
- Sivasubramanian, S.; Nizam, M.N.; Jeyaraj, G.P.; Shunmugiah, K.P.; Arumugam, V.R. In vitro and in vivo exploration of palmitic acid from Synechococcus elongatus as an antibiofilm agent on the survival of Artemia franciscana against virulent vibrios. J. Invertebr. Patholo. 2017, 150, 21–31. [Google Scholar]
- Carla, R.A.; Davide, C.; Pietro, S.; Lucio, M.; John, W.C. Biofilm formation in Staphylococcus implant infections. A review of molecular mechanisms and implications for biofilm-resistant materials. Biomaterials 2012, 33, 5967–5982. [Google Scholar]
- Lu, L.; Hu, W.; Tian, Z.; Yuan, D.; Yi, G.; Zhou, Y.; Cheng, Q.; Zhu, J.; Li, M. Developing natural products as potential anti-biofilm agents. Chin. Med. 2019, 14, 11. [Google Scholar] [CrossRef] [PubMed]
- Valliammai, A.; Sethupathy, S.; Priya, A.; Selvaraj, A.; Bhaskar, J.P.; Krishnan, V. 5-Dodecanolide interferes with biofilm formation and reduces the virulence of Methicillin-resistant Staphylococcus aureus (MRSA) through up regulation of agr system. Sci. Rep. 2019, 9, 13744. [Google Scholar] [CrossRef] [PubMed]
- Farha, A.; Yang, Q.; Kim, G. Inhibition of multidrug-resistant foodborne Staphylococcus aureus biofilms by a natural terpenoid (+)-nootkatone and related molecular mechanism. Food Control. 2020, 112, 107154. [Google Scholar] [CrossRef]
- Das, T.; Sharma, P.K.; Busscher, H.J.; Mei, H.C.; Krom, B.P. Role of extracellular DNA in initial bacterial adhesion and surface aggregation. Appl. Environ. Microbiol. 2010, 76, 3405–3408. [Google Scholar] [CrossRef]
- Das, T.; Sehar, S.; Koop, L. Influence of calcium in extracellular DNA mediated bacterial aggregation and biofilm formation. PLoS ONE 2014, 9, e91935. [Google Scholar] [CrossRef]
- Khan, S.N.; Khan, S.; Iqbal, J.; Khan, R.; Khan, A.U. Enhanced Killing and Antibiofilm Activity of Encapsulated Cinnamaldehyde against Candida albicans. Front Microbiol. 2017, 8, 1641. [Google Scholar] [CrossRef]
- Ariyanti, D.; Salasia, S.L.O.; Tato, S. “Characterization of Haemolysin of Staphylococcus Aureus Isolated from Food of Animal Origin”, lndones. J. Biotechnol. 2011, 16, 32–37. [Google Scholar]
- Song, L. Structure of staphylococcal α-hemolysin, a heptameric transmembrane pore. Science 1996, 274, 1859–1866. [Google Scholar] [CrossRef]
- Caiazza, N.C.; O’toole, G.A. Alpha-toxin is required for biofilm formation by Staphylococcus aureus. J. Bacteriol. 2003, 185, 3214–3217. [Google Scholar] [CrossRef] [PubMed]
- Mestre, M.B.; Fader, C.M.; Sola, C.; Colombo, M.I. Alpha-hemolysin is required for the activation of the autophagic pathway in Staphylococcus aureus-infected cells. Autophagy 2010, 6, 110–125. [Google Scholar] [CrossRef]
- Smith-Palmer, A.; Stewart, J.; Fyfe, L. Influence of subinhibitory concentrations of plant essential oils on the production of enterotoxins A and B and alpha-toxin by Staphylococcus aureus. J. Med. Microbiol. 2004, 53, 1023–1027. [Google Scholar] [CrossRef]
- O’Gara, J.P. ica and beyond: Biofilm mechanisms and regulation in Staphylococcus epidermidis and Staphylococcus aureus. FEMS Microbiol. Lett. 2007, 270, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Zhao, L.; Xue, T.; Sun, B. Staphylococcus aureus autoinducer-2 quorum sensing decreases biofilm formation in an icaR-dependent manner. BMC Microbiol. 2012, 12, 288. [Google Scholar] [CrossRef] [PubMed]
- Cue, D.; Lei, M.G.; Lee, C.Y. Genetic regulation of the intercellular adhesion locus in staphylococci. Front. Cell. Inf. Microbiol. 2012, 2, 38. [Google Scholar] [CrossRef]
- Ikonomidis, A.; Vasdeki, A.; Kristo, I.; Maniatis, A.N.; Tsakris, A.; Malizos, K.N. Association of biofilm formation and methicillin-resistance with accessory gene regulator (agr) loci in Greek Staphylococcus aureus clones. Microb. Pathog. 2009, 47, 341–344. [Google Scholar] [CrossRef]
- Ji, G.; Beavis, R.C.; Novick, R.P. Cell density control of staphylococcal virulence mediated by an octapeptide pheromone. Proc. Natl. Acad. Sci. USA 1995, 92, 12055–12059. [Google Scholar] [CrossRef]
- Mansson, M.; Nielsen, A.; Kjærulff, L.; Gotfredsen, C.H.; Wietz, M.; Ingmer, H.; Gram, L.; Larsen, T.O. Inhibition of Virulence Gene Expression in Staphylococcus aureus by Novel Depsipeptides from a Marine Photobacterium. Mar. Drugs 2011, 9, 2537–2552. [Google Scholar] [CrossRef]
- Mitchell, G.; Fugère, A.; Pépin Gaudreau, K.; Brouillette, E.; Frost, E.H.; Cantin, A.M.; Malouin, F. SigB is a dominant regulator of virulence in Staphylococcus aureus small-colony variants. PLoS ONE 2013, 8, e65018. [Google Scholar] [CrossRef]
- Cho, H.S.; Lee, J.H.; Cho, M.H.; Lee, J. Red wines and flavonoids diminish Staphylococcus aureus virulence with anti-biofilm and anti-hemolytic activities. Biofouling 2015, 31, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, Y.G.; Yong, R.S. Calcium-chelating alizarin and other anthraquinones inhibit biofilm formation and the hemolytic activity of Staphylococcus aureus. Sci. Rep. 2016, 6, 19267. [Google Scholar] [CrossRef] [PubMed]

| Genes | Primer Sequence (5’-3’) |
|---|---|
| icaA | forward: TTCCAGAAACATTGGGAGGTC reverse: CCTTTTCGTTTTCATTGTGCTA |
| icaR | forward: ACGCCTGAGGAATTTTCTGGA reverse: TTGCGAAAAGGATGCTTTCAA |
| agrA | forward: TCTCACAGACTCATTGCCCATT reverse: GGCGATTGACGACAAAGCT |
| hla | forward: GGTTTAGCCTGGCCTTCAGC reverse: ACCAGTAACATTACCGTTGAATCCA |
| sigB | forward: CTTTGAACGGAAGTTTGAAGCCT reverse: GCGGTTAGTTCATCGCTCACT |
| 16sRNA | forward: ACTGGGCGTAAAGAGYTCGT reverse: CGCATTTCACCGCTACAC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, S.; Yang, Y.; Hou, W.; Zheng, Y. Inhibitory Effects of Lactobionic Acid on Biofilm Formation and Virulence of Staphylococcus aureus. Foods 2024, 13, 2781. https://doi.org/10.3390/foods13172781
Kang S, Yang Y, Hou W, Zheng Y. Inhibitory Effects of Lactobionic Acid on Biofilm Formation and Virulence of Staphylococcus aureus. Foods. 2024; 13(17):2781. https://doi.org/10.3390/foods13172781
Chicago/Turabian StyleKang, Shimo, Yahui Yang, Wanwan Hou, and Yan Zheng. 2024. "Inhibitory Effects of Lactobionic Acid on Biofilm Formation and Virulence of Staphylococcus aureus" Foods 13, no. 17: 2781. https://doi.org/10.3390/foods13172781






