Bioengineered Anthocyanin-Enriched Tomatoes: A Novel Approach to Colorectal Cancer Prevention
Abstract
:1. Introduction
2. Anthocyanins
2.1. Natural Sources of Anthocyanin
2.2. Factors That Affect the Yield of Anthocyanin
2.2.1. Extraction Techniques
2.2.2. Extraction Solvents
2.2.3. Extraction pH
2.2.4. Cultivar and Genetic Factors
2.3. Health Benefits and Functional Properties of Anthocyanins
2.3.1. Circulatory-Related Health Issues
2.3.2. Chronic Liver Disease
2.3.3. Immunity Responses
2.3.4. Cancers
2.4. Bioaccessibility and Absorption of Anthocyanin
3. Effect of Anthocyanin on Modulation of the Signaling Pathways in CRC
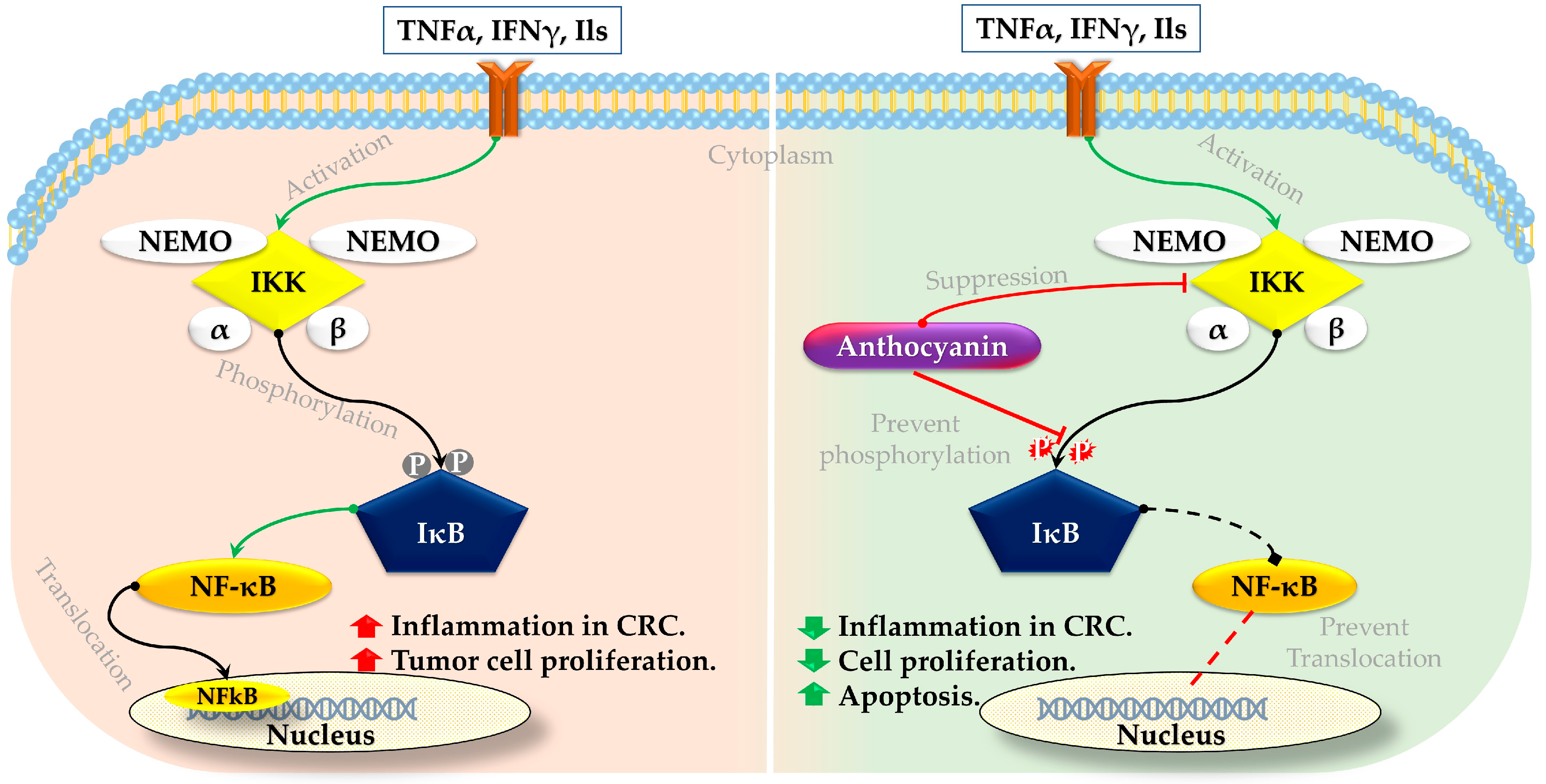
3.1. Anthocyanin on NFκB Signaling Pathway
3.2. Anthocyanin on Wnt/β-Catenin Signaling Pathway
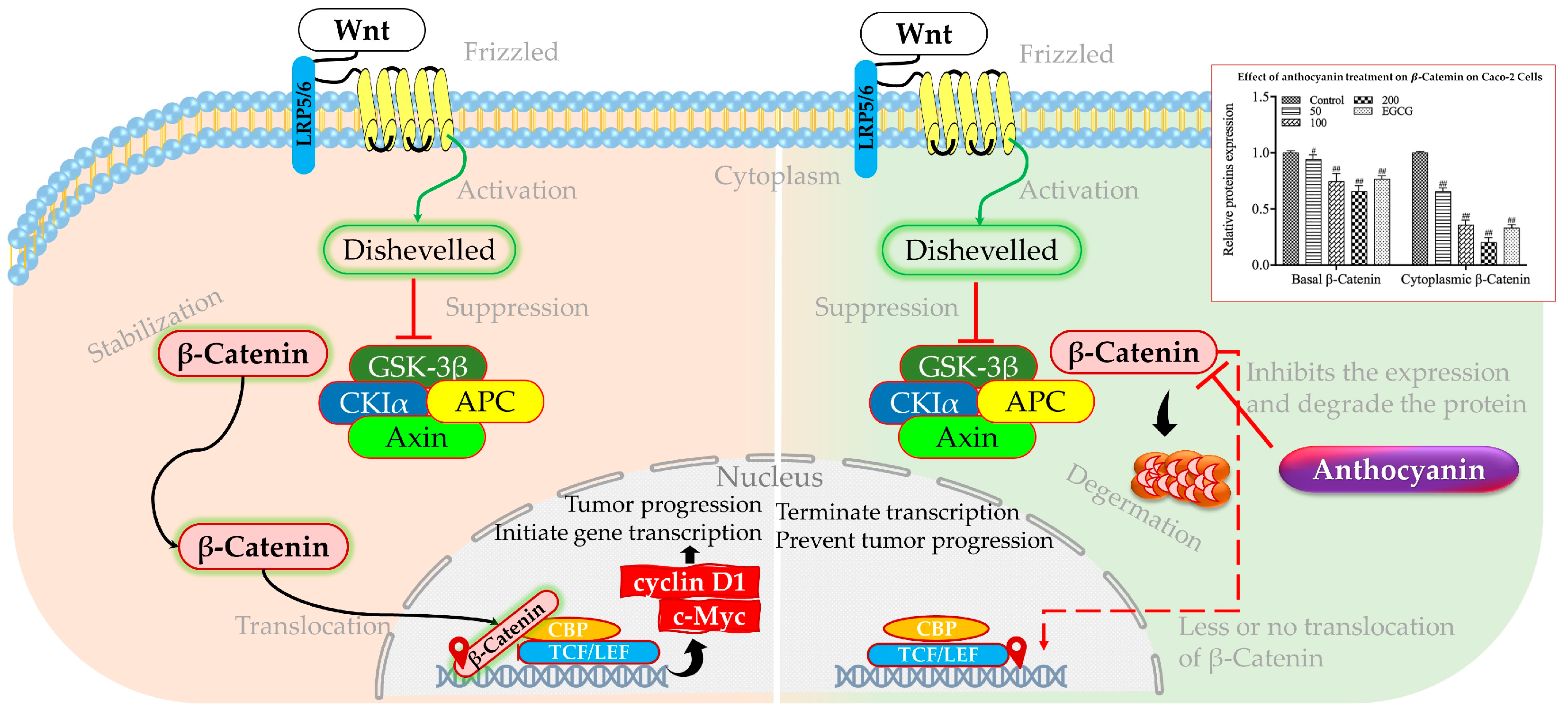
3.3. Anthocyanin on JAK/STAT Signaling Pathway
3.4. Anthocyanin on p53 Signaling Pathway
3.5. Anthocyanin on mTOR Signaling Pathway
3.6. Anthocyanin on PI3K-AKT Signaling Pathway
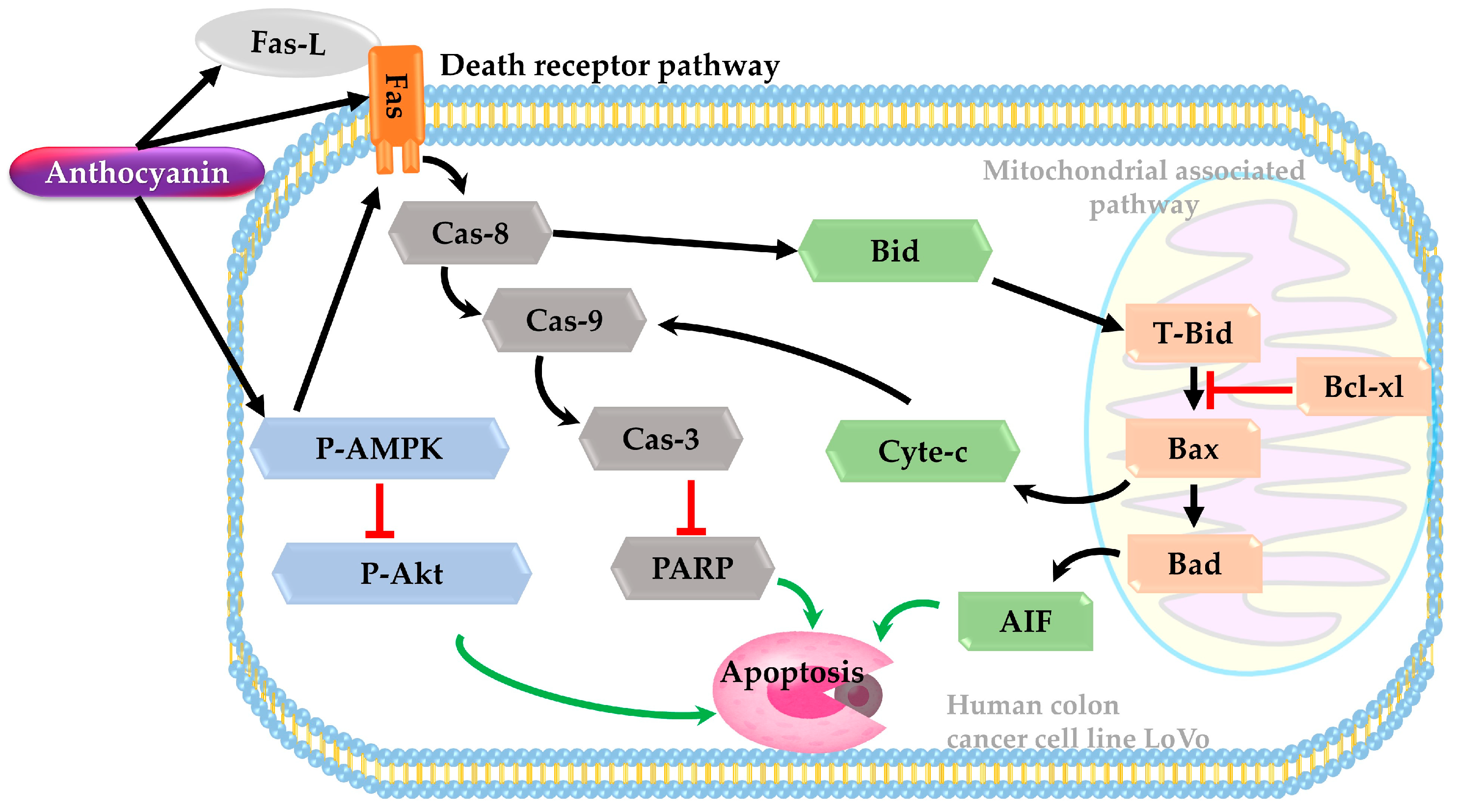
3.7. Death Receptor Pathway
3.8. Anthocyanin on TGF-β/BMPs Signaling Pathway
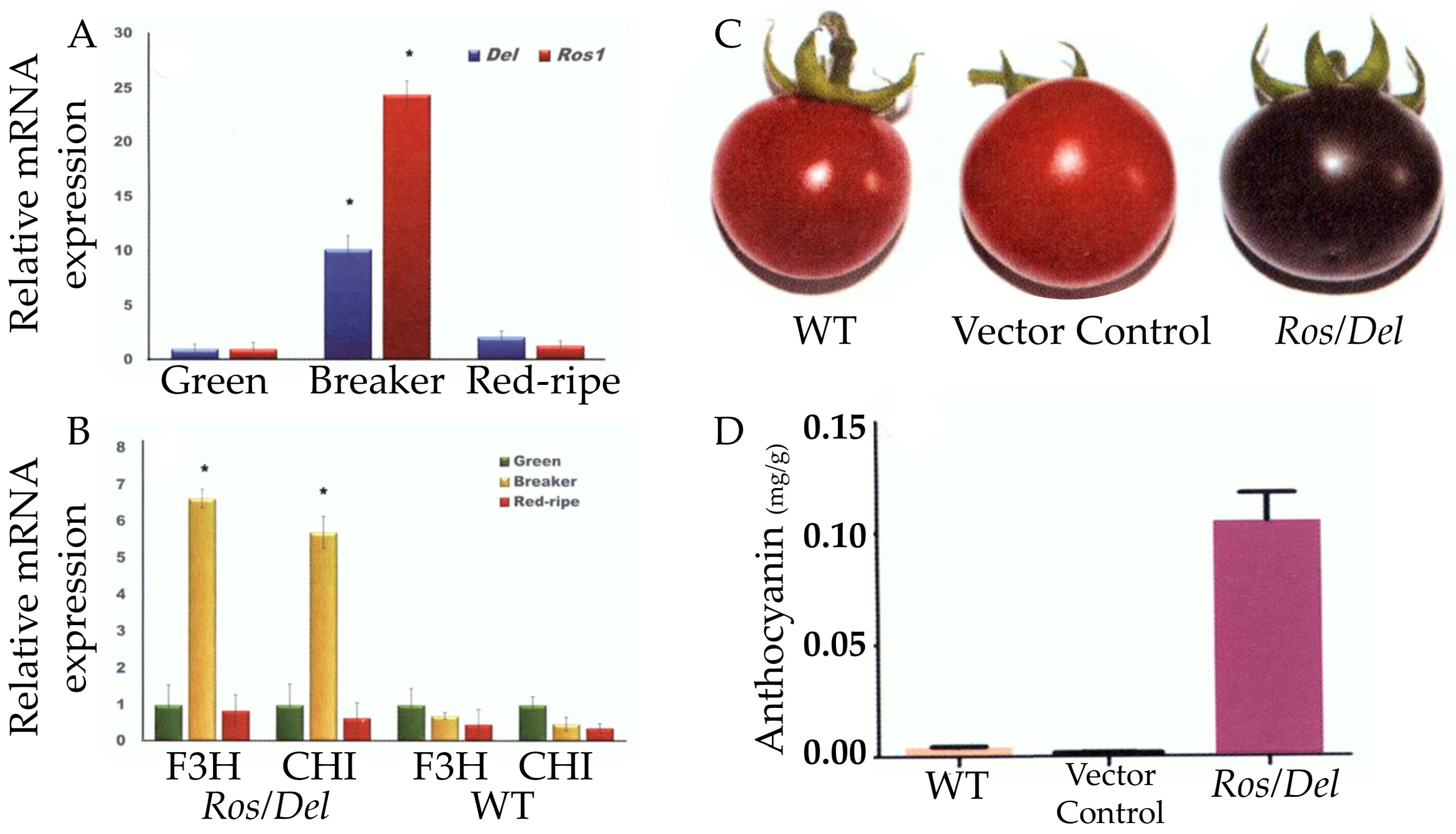
4. Potential of Bioengineering to Enrich Anthocyanin in Tomatoes
4.1. Introduce Delila (Del) and Rosea1 (Ros1) Genes in Tomato
4.2. Introduce the SlMYB75 Gene in Tomato
5. Evidence of Anthocyanin-Enriched Tomato Extract as a Therapeutic Agent in CRC
6. Implications, Limitations, and Future Research
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, H.; Wang, Z.; Yu, Y.; Qiu, M.; Yang, L.; Meng, W.; Wang, C.; Li, Y.; Li, L.; Xu, H.; et al. Global trend of colorectal cancer, prevention, and control in China: Challenges and strategies. Sci. Sin. Vitae 2022, 52, 1612–1625. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, C.; Wang, Q.; Wu, K.; Sun, Z.; Tang, Z.; Zhang, B. Temporal Trends in the Disease Burden of Colorectal Cancer with Its Risk Factors at the Global and National Level from 1990 to 2019, and Projections Until 2044. Clin. Epidemiol. 2023, 15, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Abbasi-Kangevari, M.; Abd-Rabu, R.; Abidi, H.; Abu-Gharbieh, E.; Acuna, J.; Adhikari, S.; Advani, S.; Afzal, M.; Aghaie Meybodi, M.; et al. Global, regional, and national burden of colorectal cancer and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Gastroenterol. Hepatol. 2022, 7, 627–647. [Google Scholar] [CrossRef] [PubMed]
- Tudosie, M.S.; Pauna, A.; Stefani, C.; Staicu, I.M. Diet and Food chemicals increasing the risk of colorectal cancer–literature review. J. Mind Med. Sci. 2022, 9, 118–124. [Google Scholar] [CrossRef]
- Dariya, B.; Chalikonda, G.; Nagaraju, G.P. Epidemiology of Colorectal Cancer. In Colon Cancer Diagnosis and Therapy; Nagaraju, G.P., Shukla, D., Vishvakarma, N.K., Eds.; Springer International Publishing: Cham, Switzerland, 2021; Volume 1, pp. 1–13. [Google Scholar] [CrossRef]
- Debela, D.T.; Muzazu, S.G.; Heraro, K.D.; Ndalama, M.T.; Mesele, B.W.; Haile, D.C.; Kitui, S.K.; Manyazewal, T. New approaches and procedures for cancer treatment: Current perspectives. SAGE Open Med. 2021, 9, 20503121211034366. [Google Scholar] [CrossRef]
- Heber, D.; Li, Z. Nutrition Intervention in Cancer. Med. Clin. N. Am. 2016, 100, 1329–1340. [Google Scholar] [CrossRef]
- Mori, T.; Kido, A.; Kawahara, I.; Nuaga, S.; Miyagawa, Y.; Goto, K.; Mori, S.; Kishi, S.; Fujii, K.; Fujiwara-Tani, R. Nutritional intervention for cancer sarcopenia. Ann. Musculoskelet. Med. 2021, 5, 001–004. [Google Scholar]
- Pak, H.; Maghsoudi, L.H.; Soltanian, A.; Gholami, F. Surgical complications in colorectal cancer patients. Ann. Med. Surg. 2020, 55, 13–18. [Google Scholar] [CrossRef]
- Birgisson, H.; Påhlman, L.; Gunnarsson, U.; Glimelius, B. Late adverse effects of radiation therapy for rectal cancer—A systematic overview. Acta Oncol. 2007, 46, 504–516. [Google Scholar] [CrossRef]
- Esmeeta, A.; Adhikary, S.; Dharshnaa, V.; Swarnamughi, P.; Ummul Maqsummiya, Z.; Banerjee, A.; Pathak, S.; Duttaroy, A.K. Plant-derived bioactive compounds in colon cancer treatment: An updated review. Biomed. Pharmacother. 2022, 153, 113384. [Google Scholar] [CrossRef]
- Colobatiu, L.; Gavrilas, L.; Mocan, A. Chapter 10—Natural compounds as chemosensitizers: A lesson from plants. In pH-Interfering Agents as Chemosensitizers in Cancer Therapy; Supuran, C.T., Carradori, S., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 147–165. [Google Scholar]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [PubMed]
- Hudlikar, R.; Wu, R.; Cheng, D.; Kuo, D.H.-C.; Wang, L.; Peter, R.; Yin, R.; Li, S.; Kong, A.-N. Anthocyanins and Cancer Prevention. In Natural Products for Cancer Chemoprevention: Single Compounds and Combinations; Pezzuto, J.M., Vang, O., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 351–373. [Google Scholar] [CrossRef]
- Thomasset, S.; Berry, D.P.; Cai, H.; West, K.; Marczylo, T.H.; Marsden, D.; Brown, K.; Dennison, A.; Garcea, G.; Miller, A.; et al. Pilot study of oral anthocyanins for colorectal cancer chemoprevention. Cancer Prev. Res. 2009, 2, 625–633. [Google Scholar] [CrossRef]
- Lim, S.; Xu, J.; Kim, J.; Chen, T.Y.; Su, X.; Standard, J.; Carey, E.; Griffin, J.; Herndon, B.; Katz, B.; et al. Role of anthocyanin-enriched purple-fleshed Sweetpotato P40 in colorectal cancer prevention. Mol. Nutr. Food Res. 2013, 57, 1908–1917. [Google Scholar] [CrossRef] [PubMed]
- Medic, N.; Tramer, F.; Passamonti, S. Anthocyanins in Colorectal Cancer Prevention. A Systematic Review of the Literature in Search of Molecular Oncotargets. Front. Pharmacol. 2019, 10, 675. [Google Scholar] [CrossRef]
- Butelli, E.; Titta, L.; Giorgio, M.; Mock, H.-P.; Matros, A.; Peterek, S.; Schijlen, E.G.W.M.; Hall, R.D.; Bovy, A.G.; Luo, J.; et al. Enrichment of tomato fruit with health-promoting anthocyanins by expression of select transcription factors. Nat. Biotechnol. 2008, 26, 1301–1308. [Google Scholar] [CrossRef]
- Naing, A.H.; Kim, C.K. Roles of R2R3-MYB transcription factors in transcriptional regulation of anthocyanin biosynthesis in horticultural plants. Plant Mol. Biol. 2018, 98, 1–18. [Google Scholar] [CrossRef]
- Thilmony, R.; Dasgupta, K.; Shao, M.; Harris, D.; Hartman, J.; Harden, L.A.; Chan, R.; Thomson, J.G. Tissue-specific expression of Ruby in Mexican lime (C. aurantifolia) confers anthocyanin accumulation in fruit. Front. Plant Sci. 2022, 13, 945738. [Google Scholar] [CrossRef]
- Menconi, J.; Perata, P.; Gonzali, S. In pursuit of purple: Anthocyanin biosynthesis in fruits of the tomato clade. Trends Plant Sci. 2024, 29, 589–604. [Google Scholar] [CrossRef]
- Cammareri, M.; Frary, A.; Frary, A.; Grandillo, S. Genetic and Biotechnological Approaches to Improve Fruit Bioactive Content: A Focus on Eggplant and Tomato Anthocyanins. Int. J. Mol. Sci. 2024, 25, 6811. [Google Scholar] [CrossRef]
- Choo, W.S.; Saik, A.Y.H. Chapter 4—Valorization of fruit and vegetable waste for bioactive pigments: Extraction and utilization. In Valorization of Agri-Food Wastes and By-Products; Bhat, R., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 61–81. [Google Scholar] [CrossRef]
- Alappat, B.; Alappat, J. Anthocyanin Pigments: Beyond Aesthetics. Molecules 2020, 25, 5500. [Google Scholar] [CrossRef]
- Dong, W.; Yang, X.; Zhang, N.; Chen, P.; Sun, J.; Harnly, J.M.; Zhang, M. Study of UV–Vis molar absorptivity variation and quantitation of anthocyanins using molar relative response factor. Food Chem. 2024, 444, 138653. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Ali, M.M.; Ke, M.; Lu, Y.; Zheng, Y.; Cai, X.; Fang, S.; Wu, J.; Lin, Z.; Chen, F. Novel R2R3-MYB Transcription Factor LhMYB1 Promotes Anthocyanin Accumulation in Lilium concolor var. pulchellum. Horticulturae 2024, 10, 509. [Google Scholar] [CrossRef]
- Wallace, T.C.; Giusti, M.M. Anthocyanins—Nature’s Bold, Beautiful, and Health-Promoting Colors. Foods 2019, 8, 550. [Google Scholar] [CrossRef] [PubMed]
- Goulas, V.; Vicente, A.; Manganaris, G. Structural diversity of anthocyanins in fruits. In Anthocyanins: Structure, Biosynthesis and Health Benefits; Nova Sciences: Hauppauge, NY, USA, 2012; pp. 225–250. [Google Scholar]
- Kumar, S.; Mitharwal, S.; Kumar, S.; Bashir, K.; Jan, K.; Kaushik, A. Commercial Production of Anthocyanins from Subtropical Fruits. In Anthocyanins in Subtropical Fruits; CRC Press: Boca Raton, FL, USA, 2023; pp. 71–90. [Google Scholar]
- Romualdo, G.R.; Fragoso, M.F.; Borguini, R.G.; de Araújo Santiago, M.C.P.; Fernandes, A.A.H.; Barbisan, L.F. Protective effects of spray-dried açaí (Euterpe oleracea Mart) fruit pulp against initiation step of colon carcinogenesis. Food Res. Int. 2015, 77, 432–440. [Google Scholar] [CrossRef]
- da Costa, D.S.; Bragotto, A.P.A.; de Carvalho, L.M.; Amado, L.L.; Lima, R.R.; Rogez, H. Analysis of polyphenols, anthocyanins and toxic elements in Açaí Juice (Euterpe oleracea Mart.): Quantification and in vivo assessment of the antioxidant capacity of clarified Açaí juice. Meas. Food 2024, 14, 100149. [Google Scholar] [CrossRef]
- Šimerdová, B.; Bobríková, M.; Lhotská, I.; Kaplan, J.; Křenová, A.; Šatínský, D. Evaluation of Anthocyanin Profiles in Various Blackcurrant Cultivars over a Three-Year Period Using a Fast HPLC-DAD Method. Foods 2021, 10, 1745. [Google Scholar] [CrossRef]
- Tian, Y.; Karhu, S.; Virtanen, M.; Linderborg, K.M.; Yang, B.; Laaksonen, O. Variation of chemical and sensory profiles of blackcurrant (Ribes nigrum) juices produced from different cultivars of European origins. LWT 2023, 173, 114353. [Google Scholar] [CrossRef]
- Johnson, J.B.; Collins, T.; Mani, J.S.; Naiker, M. Nutritional Quality and Bioactive Constituents of Six Australian Plum Varieties. Int. J. Fruit Sci. 2021, 21, 115–132. [Google Scholar] [CrossRef]
- Kodagoda, G.; Hong, H.T.; O’Hare, T.J.; Sultanbawa, Y.; Topp, B.; Netzel, M.E. Effect of Storage on the Nutritional Quality of Queen Garnet Plum. Foods 2021, 10, 352. [Google Scholar] [CrossRef]
- Wang, S.; Wang, B.; Dong, K.; Li, J.; Li, Y.; Sun, H. Identification and quantification of anthocyanins of 62 blueberry cultivars via UPLC-MS. Biotechnol. Biotechnol. Equip. 2022, 36, 587–597. [Google Scholar] [CrossRef]
- Yang, L.-C.; Hsu, S.-H.; Meng, Y.-Y.; Chen, S.-F. Quantification of anthocyanins in blueberries (Vaccinium spp.) by modified QuEChERS and liquid chromatography-mass spectrometry. J. Chin. Chem. Soc. 2022, 69, 1070–1078. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, S.; Deng, G.; Xu, K.; Xu, H.; Liu, J. Extracting Total Anthocyanin from Purple Sweet Potato Using an Effective Ultrasound-Assisted Compound Enzymatic Extraction Technology. Molecules 2022, 27, 4344. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhao, Y.; Zhou, Q.; Luo, C.-L.; Deng, A.-P.; Zhang, Z.-C.; Zhang, J.-L. Characterization and hepatoprotective activity of anthocyanins from purple sweet potato (Ipomoea batatas L. cultivar Eshu No. 8). J. Food Drug Anal. 2017, 25, 607–618. [Google Scholar] [CrossRef] [PubMed]
- Szot, I.; Łysiak, G.P.; Sosnowska, B. The Beneficial Effects of Anthocyanins from Cornelian Cherry (Cornus mas L.) Fruits and Their Possible Uses: A Review. Agriculture 2024, 14, 52. [Google Scholar] [CrossRef]
- Toshima, S.; Hirano, T.; Kunitake, H. Comparison of anthocyanins, polyphenols, and antioxidant capacities among raspberry, blackberry, and Japanese wild Rubus species. Sci. Hortic. 2021, 285, 110204. [Google Scholar] [CrossRef]
- Teng, H.; Fang, T.; Lin, Q.; Song, H.; Liu, B.; Chen, L. Red raspberry and its anthocyanins: Bioactivity beyond antioxidant capacity. Trends Food Sci. Tech. 2017, 66, 153–165. [Google Scholar] [CrossRef]
- Anirban, A.; Hong, H.T.; O’Hare, T.J. Profiling and Quantification of Anthocyanins in Purple-Pericarp Sweetcorn and Purple-Pericarp Maize. Molecules 2023, 28, 2665. [Google Scholar] [CrossRef]
- Zhao, X.; Corrales, M.; Zhang, C.; Hu, X.; Ma, Y.; Tauscher, B. Composition and Thermal Stability of Anthocyanins from Chinese Purple Corn (Zea mays L.). J. Agric. Food Chem. 2008, 56, 10761–10766. [Google Scholar] [CrossRef]
- Araújo, A.C.d.; Gomes, J.P.; Silva, F.B.d.; Nunes, J.S.; Santos, F.S.d.; Silva, W.P.d.; Ferreira, J.P.d.L.; Queiroz, A.J.d.M.; Figueirêdo, R.M.F.d.; Lima, G.S.d.; et al. Optimization of Extraction Method of Anthocyanins from Red Cabbage. Molecules 2023, 28, 3549. [Google Scholar] [CrossRef]
- Wiczkowski, W.; Szawara-Nowak, D.; Topolska, J. Red cabbage anthocyanins: Profile, isolation, identification, and antioxidant activity. Food Res. Int. 2013, 51, 303–309. [Google Scholar] [CrossRef]
- Blando, F.; Berland, H.; Maiorano, G.; Durante, M.; Mazzucato, A.; Picarella, M.E.; Nicoletti, I.; Gerardi, C.; Mita, G.; Andersen, Ø.M. Nutraceutical Characterization of Anthocyanin-Rich Fruits Produced by “Sun Black” Tomato Line. Front. Nutr. 2019, 6, 133. [Google Scholar] [CrossRef] [PubMed]
- Jian, W.; Ou, X.; Sun, L.; Chen, Y.; Liu, S.; Lu, W.; Yang, X.; Zhao, Z.; Li, Z. Characterization of anthocyanin accumulation, nutritional properties, and postharvest attributes of transgenic purple tomato. Food Chem. 2023, 408, 135181. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Shi, C.; Shen, D.; Han, T.; Wu, W.; Lyu, L.; Li, W. Composition and Antioxidant Activity of Anthocyanins and Non-Anthocyanin Flavonoids in Blackberry from Different Growth Stages. Foods 2022, 11, 2902. [Google Scholar] [CrossRef]
- Taghavi, T.; Patel, H.; Rafie, R. Extraction Solvents Affect Anthocyanin Yield, Color, and Profile of Strawberries. Plants 2023, 12, 1833. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.; Suzauddula, M.; Akter, K.; Hossen, M.; Islam, M.N. Green Technology for Fungal Protein Extraction—A Review. Separations 2024, 11, 186. [Google Scholar] [CrossRef]
- Taghavi, T.; Patel, H.; Rafie, R. Anthocyanin Extraction Method and Sample Preparation Affect Anthocyanin Yield of Strawberries. Nat. Prod. Commun. 2022, 17, 1934578X221099970. [Google Scholar] [CrossRef]
- Ngoc Nhon, H.T.; Diem My, N.T.; Tuong Vi, V.N.; Kim Lien, P.T.; Thao Minh, N.T.; Doan Duy, L.N.; Hong Anh, L.T.; Anh Dao, D.T. Enhancement of extraction effectiveness and stability of anthocyanin from Hibiscus sabdariffa L. J. Agric. Food Res. 2022, 10, 100408. [Google Scholar] [CrossRef]
- Nunes Mattos, G.; Pessanha de Araújo Santiago, M.C.; Sampaio Doria Chaves, A.C.; Rosenthal, A.; Valeriano Tonon, R.; Correa Cabral, L.M. Anthocyanin Extraction from Jaboticaba Skin (Myrciaria cauliflora Berg.) Using Conventional and Non-Conventional Methods. Foods 2022, 11, 885. [Google Scholar] [CrossRef]
- Ahmed, T.; Rana, M.R.; Hossain, M.A.; Ullah, S.; Suzauddula, M. Optimization of ultrasound-assisted extraction using response surface methodology for total anthocyanin content, total phenolic content, and antioxidant activities of Roselle (Hibiscus sabdariffa L.) calyces and comparison with conventional Soxhlet extraction. Biomass Convers. Biorefinery 2023, 14, 17127–17148. [Google Scholar] [CrossRef]
- Sri Raghavi, R.; Visalakshi, M.; Karthikeyan, S.; Amutha Selvi, G.; Thamaraiselvi, S.; Gurusamy, K. Standardisation of anthocyanin extraction techniques from hibiscus (Hibiscus rosa-sinensis) petals for biocolour utilisation. J. Pharm. Innov. 2022, 11, 303–309. [Google Scholar]
- Thornton, D.; Barton, L.; Hsu, L. The development of an automated countercurrent chromatography process for isolation of anthocyanins. J. Chromatogr. A 2018, 1575, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Ma, Y.; Xu, Z.; Liao, X.; Chen, A.; Yang, S. Isolation of strawberry anthocyanins using high-speed counter-current chromatography and the copigmentation with catechin or epicatechin by high pressure processing. Food Chem. 2018, 247, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Harahap, A.A.; Letare, S.K.; Hendrianie, N. The effect of solvents and extraction time on anthocyanin extraction from butterfly pea (Clitoria ternatea L.). AIP. Conf. Proc. 2023, 2667, 020003. [Google Scholar] [CrossRef]
- Han, X.; Huang, J.; Cai, J.; Yu, C.; Jiang, S.; Ye, Q.; Yang, S.; Pan, W.; Zhang, Q.; Wang, Y. Microwave-ultrasonic technique development coupled with natural deep eutectic solvents in anthocyanin extraction from perilla leaves (Perilla frutescens var. Acuta). J. Sci. Food Agric. 2023, 103, 3006–3016. [Google Scholar] [CrossRef]
- Jovanović, M.; Krgović, N.; Radan, M.; Ćujić-Nikolić, N.; Mudrić, J.; Drinić, Z.; Šavikin, K. Extraction of chokeberry anthocyanins using natural deep eutectic solvents. Planta Med. 2022, 88, P-314. [Google Scholar] [CrossRef]
- Vannuchi, N.; Braga, A.R.C.; De Rosso, V.V. High-Performance Extraction Process of Anthocyanins from Jussara (Euterpe edulis) Using Deep Eutectic Solvents. Processes 2022, 10, 615. [Google Scholar] [CrossRef]
- Jusoh, Y.M.M.; Idris, A.A.; Khairuddin, N.; Zaidel, D.N.A.; Hashim, Z.; Mahmooda, N.A.N.; Zakaria, Z.Y.; Muhamad, I.I. Effect of solvent pH, microwave power and extraction time on microwave-assisted extraction of Hibiscus rosa-sinensis. Chem. Eng. Trans. 2018, 63, 541–546. [Google Scholar]
- Adhikari, B.; Shrestha, O.K. Effect of Processing Variables on Anthocyanin and Total Polyphenol Extraction from Water Caltrop (Trapa bispinosa) Hull. Himal. J. Sci. Technol. 2018, 2, 76–83. [Google Scholar] [CrossRef]
- Chen, W.; Karangwa, E.; Yu, J.; Duhoranimana, E.; Xia, S.; Feng, B.; Zhang, X.; Jia, C. Coupling effects of preheating time and extraction medium pH on red radish anthocyanin yield, glucosinolate degradation and off-odour removal. Int. J. Food Sci. Technol. 2018, 53, 709–718. [Google Scholar] [CrossRef]
- Zhao, Y.; Gao, L.; Wang, J.; Xue, Z.; Zhang, M.; Ma, X.; Wang, G.; Lv, S. Preparation and Application of pH-Sensitive Film Containing Anthocyanins Extracted from Lycium ruthenicum Murr. Materials 2023, 16, 3828. [Google Scholar] [CrossRef]
- Nguyen, H.M.; Putterill, J.; Dare, A.P.; Plunkett, B.J.; Cooney, J.; Peng, Y.; Souleyre, E.J.F.; Albert, N.W.; Espley, R.V.; Günther, C.S. Two genes, ANS and UFGT2, from Vaccinium spp. are key steps for modulating anthocyanin production. Front. Plant Sci. 2023, 14, 1082246. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M.; Muto, A.; Bruno, L.; Muzzalupo, I.; Chiappetta, A. Modulation of Anthocyanin Biosynthesis-Related Genes during the Ripening of Olea europaea L. cvs Carolea and Tondina Drupes in Relation to Environmental Factors. Int. J. Mol. Sci. 2023, 24, 8770. [Google Scholar] [CrossRef] [PubMed]
- Massa, S.; Pagliarello, R.; Cemmi, A.; Di Sarcina, I.; Bombarely, A.; Demurtas, O.C.; Diretto, G.; Paolini, F.; Petzold, H.E.; Bliek, M.; et al. Modifying Anthocyanins Biosynthesis in Tomato Hairy Roots: A Test Bed for Plant Resistance to Ionizing Radiation and Antioxidant Properties in Space. Front. Plant Sci. 2022, 13, 830931. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.-I.; Rahim, M.A.; Afrin, K.S.; Jung, H.-J.; Kim, H.-T.; Park, J.-I.; Nou, I.-S. Expression of anthocyanin biosynthesis-related genes reflects the peel color in purple tomato. Horticult. Environ. Biotechnol. 2018, 59, 435–445. [Google Scholar] [CrossRef]
- Lin-Wang, K.; McGhie, T.K.; Wang, M.; Liu, Y.; Warren, B.; Storey, R.; Espley, R.V.; Allan, A.C. Engineering the anthocyanin regulatory complex of strawberry (Fragaria vesca). Front. Plant Sci. 2014, 5, 651. [Google Scholar] [CrossRef]
- Zhang, Y.; Chu, G.; Hu, Z.; Gao, Q.; Cui, B.; Tian, S.; Wang, B.; Chen, G. Genetically engineered anthocyanin pathway for high health-promoting pigment production in eggplant. Mol. Breed 2016, 36, 54. [Google Scholar] [CrossRef]
- Xie, R. Anthocyanin biosynthesis in fruit tree crops: Genes and their regulation. Afr. J. Biotechnol. 2011, 10, 19890–19897. [Google Scholar] [CrossRef]
- Gao, X.; Chen, Q.; Yang, Y.; Zhu, B.; Xiao, J. Transcriptomic analysis reveals anthocyanin biosynthesis regulation in blueberry (Vaccinium ashei) fruit. Can. J. Plant Sci. 2022, 102, 195–206. [Google Scholar] [CrossRef]
- Feng, S.; Wang, Y.; Yang, S.; Xu, Y.; Chen, X. Anthocyanin biosynthesis in pears is regulated by a R2R3-MYB transcription factor PyMYB10. Planta 2010, 232, 245–255. [Google Scholar] [CrossRef]
- Tiwari, V.; Sharma, S.; Tiwari, A.; Sheoran, B.; Kaur, S.; Sharma, A.; Yadav, M.; Bhatnagar, A.; Garg, M. Effect of dietary anthocyanins on biomarkers of type 2 diabetes and related obesity: A systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2023, 64, 7517–7534. [Google Scholar] [CrossRef]
- Ugwu, P.; Ubom, R.; Madueke, P.; Okorie, P.; Nwachukwu, D. Anti-Hypertensive Effects of Anthocyanins from Hibiscus sabdarifa Calyx on the Renin-Angiotensin-Aldoslestrone System in Wistar Rats. Niger. J. Physiolog. Sci. 2022, 37, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Zhu, J.; Gong, G.; Guo, L.; Zhang, Y.; Zhang, Z.; Ma, C. Anthocyanin attenuates high salt-induced hypertension via inhibiting the hyperactivity of the sympathetic nervous system. Clin. Exp. Hypertens. 2023, 45, 2233717. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Choi, M.; Lee, M. Effects of anthocyanin supplementation on reduction of obesity criteria: A systematic review and meta-analysis of randomized controlled trials. Nutrients 2021, 13, 2121. [Google Scholar] [CrossRef] [PubMed]
- Xiao, T.; Luo, Z.; Guo, Z.; Wang, X.; Ding, M.; Wang, W.; Shen, X.; Zhao, Y. Multiple roles of black raspberry anthocyanins protecting against alcoholic liver disease. Molecules 2021, 26, 2313. [Google Scholar] [CrossRef] [PubMed]
- Trushina, E.; Mustafina, O.; Aksenov, I.; Tutelyan, V. Anthocyanins as a factor in the alimentary restoration of cellular immunity in diet induced obesity in rats. Med. Immunol. 2023, 25, 703–708. [Google Scholar] [CrossRef]
- Fan, M.J.; Yeh, P.H.; Lin, J.P.; Huang, A.C.; Lien, J.C.; Lin, H.Y.; Chung, J.G. Anthocyanins from black rice (Oryza sativa) promote immune responses in leukemia through enhancing phagocytosis of macrophages in vivo. Exp. Ther. Med. 2017, 14, 59–64. [Google Scholar] [CrossRef]
- Tan, S.; Pang, S.; Zhu, S.; Wei, W.; Sun, D. Protective Effect of Anthocyanins from Blueberry on Fluoride-Induced Immune System Injury in Wistar Rats. Fluoride 2023, 56, 217–243. [Google Scholar]
- Lin, B.W.; Gong, C.C.; Song, H.F.; Cui, Y.Y. Effects of anthocyanins on the prevention and treatment of cancer. Br. J. Pharmacol. 2017, 174, 1226–1243. [Google Scholar] [CrossRef]
- Usha, S.; Murugan, K.; Krishnan, R. Antimetastatic potential of anthocyanins from Cordyline australis (G. Forst.) Endl. Red star variety on MCF onco cell lines. J. Appl. Nat. Sci. 2022, 14, 777–783. [Google Scholar]
- Shi, N.; Chen, X.; Chen, T. Anthocyanins in Colorectal Cancer Prevention Review. Antioxidants 2021, 10, 1600. [Google Scholar] [CrossRef]
- Pan, D.; Huang, B.; Gan, Y.; Gao, C.; Liu, Y.; Tang, Z. Phycocyanin ameliorates colitis-associated colorectal cancer by regulating the gut microbiota and the IL-17 signaling pathway. Mar. Drugs 2022, 20, 260. [Google Scholar] [CrossRef] [PubMed]
- Kay, C.D.; Pereira-Caro, G.; Ludwig, I.A.; Clifford, M.N.; Crozier, A. Anthocyanins and Flavanones Are More Bioavailable than Previously Perceived: A Review of Recent Evidence. Annu. Rev. Food Sci. Technol. 2017, 8, 155–180. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, Y.; Donlao, N.; Thuengtung, S.; Tian, J.; Cai, Y.; Reginio, F.C.; Ketnawa, S.; Yamamoto, N.; Tamura, M. Impact of food structure and cell matrix on digestibility of plant-based food. Curr. Opin. Food Sci. 2018, 19, 36–41. [Google Scholar] [CrossRef]
- Zhang, H.; Hassan, Y.I.; Renaud, J.; Liu, R.; Yang, C.; Sun, Y.; Tsao, R. Bioaccessibility, bioavailability, and anti-inflammatory effects of anthocyanins from purple root vegetables using mono- and co-culture cell models. Mol. Nutr. Food Res. 2017, 61, 1600928. [Google Scholar] [CrossRef]
- Li, H.; Deng, Z.; Liu, R.; Loewen, S.; Tsao, R. Bioaccessibility, in vitro antioxidant activities and in vivo anti-inflammatory activities of a purple tomato (Solanum lycopersicum L.). Food Chem. 2014, 159, 353–360. [Google Scholar] [CrossRef]
- Milbury, P.E.; Cao, G.; Prior, R.L.; Blumberg, J. Bioavailablility of elderberry anthocyanins. Mech. Ageing Dev. 2002, 123, 997–1006. [Google Scholar] [CrossRef]
- Mallery, S.R.; Budendorf, D.E.; Larsen, M.P.; Pei, P.; Tong, M.; Holpuch, A.S.; Larsen, P.E.; Stoner, G.D.; Fields, H.W.; Chan, K.K.; et al. Effects of human oral mucosal tissue, saliva, and oral microflora on intraoral metabolism and bioactivation of black raspberry anthocyanins. Cancer Prev. Res. 2011, 4, 1209–1221. [Google Scholar] [CrossRef]
- Gonzali, S.; Perata, P. Anthocyanins from Purple Tomatoes as Novel Antioxidants to Promote Human Health. Antioxidants 2020, 9, 1017. [Google Scholar] [CrossRef]
- Wanyo, P.; Chamsai, T.; Toontom, N.; Nghiep, L.K.; Tudpor, K. Differential Effects of In Vitro Simulated Digestion on Antioxidant Activity and Bioaccessibility of Phenolic Compounds in Purple Rice Bran Extracts. Molecules 2024, 29, 2994. [Google Scholar] [CrossRef]
- McDougall, G.J.; Dobson, P.; Smith, P.; Blake, A.; Stewart, D. Assessing Potential Bioavailability of Raspberry Anthocyanins Using an in Vitro Digestion System. J. Agric. Food Chem. 2005, 53, 5896–5904. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Jiang, Y.; Liu, C.; Zhang, W.; Chen, W.; Tian, L.; Sun, J.; Lai, C.; Bai, W. Microencapsulation with fructooligosaccharides and whey protein enhances the antioxidant activity of anthocyanins and their ability to modulate gut microbiota in vitro. Food Res. Int. 2024, 181, 114082. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, X.; Ma, X.; Li, H.; Liu, J.; Zeng, Y.; Cai, D.; Xu, Q.; Chen, G.; Tian, L.; et al. Microencapsulation of anthocyanins extracted from black soybean peels by whey protein/fructo-oligosaccharide contributes to improved stability, bioavailability, and ability to regulate glycolipid metabolism. Food Front. 2024, 5, 570–583. [Google Scholar] [CrossRef]
- Dobre, M.; Trandafir, B.; Milanesi, E.; Salvi, A.; Bucuroiu, I.A.; Vasilescu, C.; Niculae, A.M.; Herlea, V.; Hinescu, M.E.; Constantinescu, G. Molecular profile of the NF-κB signalling pathway in human colorectal cancer. J. Cell. Mol. Med. 2022, 26, 5966–5975. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Li, X. Advances of Wnt signalling pathway in colorectal cancer. Cells 2023, 12, 447. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Gan, W.J. Wnt/β-Catenin Signaling Pathway in the Development and Progression of Colorectal Cancer. Cancer Manag. Res. 2023, 15, 435–448. [Google Scholar] [CrossRef] [PubMed]
- Fleming-de-Moraes, C.D.; Rocha, M.R.; Tessmann, J.W.; de Araujo, W.M.; Morgado-Diaz, J.A. Crosstalk between PI3K/Akt and Wnt/β-catenin pathways promote colorectal cancer progression regardless of mutational status. Cancer Biol. Ther. 2022, 23, 1–13. [Google Scholar] [CrossRef]
- Arqués, O.; Chicote, I.; Puig, I.; Tenbaum, S.P.; Argilés, G.; Dienstmann, R.; Fernandez, N.; Caratu, G.; Matito, J.; Silberschmidt, D. Tankyrase inhibition blocks Wnt/β-catenin pathway and reverts resistance to PI3K and AKT inhibitors in the treatment of colorectal cancer. Clin. Cancer Res. 2016, 22, 644–656. [Google Scholar] [CrossRef]
- Silva, V.R.; Santos, L.d.S.; Dias, R.B.; Quadros, C.A.; Bezerra, D.P. Emerging agents that target signaling pathways to eradicate colorectal cancer stem cells. Cancer Commun. 2021, 41, 1275–1313. [Google Scholar] [CrossRef]
- Zhong, J.; Ding, S.; Zhang, X.; Di, W.; Wang, X.; Zhang, H.; Chen, Y.; Zhang, Y.; Hu, Y. To investigate the occurrence and development of colorectal cancer based on the PI3K/AKT/mTOR signaling pathway. Front. Biosci.-Landmark 2023, 28, 37. [Google Scholar] [CrossRef]
- Ghobashi, A.H.; Vuong, T.T.; Kimani, J.W.; Ladaika, C.A.; Hollenhorst, P.C.; O’Hagan, H.M. Activation of AKT induces EZH2-mediated β-catenin trimethylation in colorectal cancer. Iscience 2023, 26, 107630. [Google Scholar] [CrossRef]
- Jeong, J.-W.; Lee, W.S.; Shin, S.C.; Kim, G.-Y.; Choi, B.T.; Choi, Y.H. Anthocyanins Downregulate Lipopolysaccharide-Induced Inflammatory Responses in BV2 Microglial Cells by Suppressing the NF-κB and Akt/MAPKs Signaling Pathways. Int. J. Mol. Sci. 2013, 14, 1502–1515. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.L.; Yu, H.G.; Yu, J.P.; Luo, H.S.; Xu, X.M.; Li, J.H. Nuclear factor-kappaB p65 (RelA) transcription factor is constitutively activated in human colorectal carcinoma tissue. World J. Gastroenterol. 2004, 10, 3255–3260. [Google Scholar] [CrossRef] [PubMed]
- Gambhir, S.; Vyas, D.; Hollis, M.; Aekka, A.; Vyas, A. Nuclear factor kappa B role in inflammation associated gastrointestinal malignancies. World J. Gastroenterol. 2015, 21, 3174–3183. [Google Scholar] [CrossRef] [PubMed]
- Sunami, Y.; Wirth, T. Intestinal carcinogenesis: IKK can go all the way. J. Clin. Investig. 2011, 121, 2551–2553. [Google Scholar] [CrossRef]
- Zhang, Z.; Pan, Y.; Zhao, Y.; Ren, M.; Li, Y.; Lu, G.; Wu, K.; He, S. Delphinidin modulates JAK/STAT3 and MAPKinase signaling to induce apoptosis in HCT116 cells. Environ. Toxicol. 2021, 36, 1557–1566. [Google Scholar] [CrossRef]
- Corvinus, F.M.; Orth, C.; Moriggl, R.; Tsareva, S.A.; Wagner, S.; Pfitzner, E.B.; Baus, D.; Kaufman, R.; Huber, L.A.; Zatloukal, K. Persistent STAT3 activation in colon cancer is associated with enhanced cell proliferation and tumor growth. Neoplasia 2005, 7, 545–555. [Google Scholar] [CrossRef]
- Takayama, T.; Miyanishi, K.; Hayashi, T.; Sato, Y.; Niitsu, Y. Colorectal cancer: Genetics of development and metastasis. J. Gastroenterol. 2006, 41, 185–192. [Google Scholar] [CrossRef]
- Iacopetta, B. TP53 mutation in colorectal cancer. Hum. Mutat. 2003, 21, 271–276. [Google Scholar] [CrossRef]
- Ha, U.S.; Bae, W.J.; Kim, S.J.; Yoon, B.I.; Hong, S.H.; Lee, J.Y.; Hwang, T.K.; Hwang, S.Y.; Wang, Z.; Kim, S.W. Anthocyanin induces apoptosis of DU-145 cells in vitro and inhibits xenograft growth of prostate cancer. Yonsei Med. J. 2015, 56, 16–23. [Google Scholar] [CrossRef]
- Cooks, T.; Pateras, I.S.; Tarcic, O.; Solomon, H.; Schetter, A.J.; Wilder, S.; Lozano, G.; Pikarsky, E.; Forshew, T.; Rosenfeld, N.; et al. Mutant p53 prolongs NF-κB activation and promotes chronic inflammation and inflammation-associated colorectal cancer. Cancer Cell 2013, 23, 634–646. [Google Scholar] [CrossRef]
- Haas, M.J.; Onstead-Haas, L.; Naem, E.; Arnold, A.; Rohrbaugh, N.; Flowers, M.; Mooradian, A.D. The effect of black seed (Nigella sativa) extract on FOXO3 expression in HepG2 cells. Phytother. Res. 2014, 28, 873–879. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.M.; Yoon, H.; Lee, S.; Ko, H.C.; Shin, M.J.; Lee, M.C.; Hur, O.S.; Ro, N.Y.; Desta, K.T. Isoflavones, anthocyanins, phenolic content, and antioxidant activities of black soybeans (Glycine max (L.) Merrill) as affected by seed weight. Sci. Rep. 2020, 10, 19960. [Google Scholar] [CrossRef] [PubMed]
- Khaleghpour, K.; Li, Y.; Banville, D.; Yu, Z.; Shen, S.H. Involvement of the PI 3-kinase signaling pathway in progression of colon adenocarcinoma. Carcinogenesis 2004, 25, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Itoh, N.; Semba, S.; Ito, M.; Takeda, H.; Kawata, S.; Yamakawa, M. Phosphorylation of Akt/PKB is required for suppression of cancer cell apoptosis and tumor progression in human colorectal carcinoma. Cancer 2002, 94, 3127–3134. [Google Scholar] [CrossRef]
- Chen, D.; Yuan, M.; Ye, Q.; Wang, X.; Xu, J.; Shi, G.; Hu, Z. Cyanidin-3-O-glucoside inhibits epithelial-to-mesenchymal transition, and migration and invasion of breast cancer cells by upregulating KLF4. Food Nutr. Res. 2020, 64. [Google Scholar] [CrossRef]
- Zhao, W.; Hisamuddin, I.M.; Nandan, M.O.; Babbin, B.A.; Lamb, N.E.; Yang, V.W. Identification of Krüppel-like factor 4 as a potential tumor suppressor gene in colorectal cancer. Oncogene 2004, 23, 395–402. [Google Scholar] [CrossRef]
- Xu, J.; Lü, B.; Xu, F.; Gu, H.; Fang, Y.; Huang, Q.; Lai, M. Dynamic down-regulation of Krüppel-like factor 4 in colorectal adenoma-carcinoma sequence. J. Cancer Res. Clin. Oncol. 2008, 134, 891–898. [Google Scholar] [CrossRef]
- Imamura, Y.; Hibi, K.; Koike, M.; Fujiwara, M.; Kodera, Y.; Ito, K.; Nakao, A. RUNX3 promoter region is specifically methylated in poorly-differentiated colorectal cancer. Anticancer Res. 2005, 25, 2627–2630. [Google Scholar]
- Paramanantham, A.; Kim, M.J.; Jung, E.J.; Nagappan, A.; Yun, J.W.; Kim, H.J.; Shin, S.C.; Kim, G.S.; Lee, W.S. Pretreatment of Anthocyanin from the Fruit of Vitis coignetiae Pulliat Acts as a Potent Inhibitor of TNF-α Effect by Inhibiting NF-κB-Regulated Genes in Human Breast Cancer Cells. Molecules 2020, 25, 2396. [Google Scholar] [CrossRef]
- Lee, D.-Y.; Yun, S.-M.; Song, M.-Y.; Jung, K.; Kim, E.-H. Cyanidin Chloride Induces Apoptosis by Inhibiting NF-κB Signaling through Activation of Nrf2 in Colorectal Cancer Cells. Antioxidants 2020, 9, 285. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Gaynor, R.B. Therapeutic potential of inhibition of the NF-kappaB pathway in the treatment of inflammation and cancer. J. Clin. Investig. 2001, 107, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Horgan, P.G.; McMillan, D.C.; Edwards, J. NF-κB pathways in the development and progression of colorectal cancer. Transl. Res. 2018, 197, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.-M.; Afaq, F.; Khan, N.; Mukhtar, H. Delphinidin, an anthocyanidin in pigmented fruits and vegetables, induces apoptosis and cell cycle arrest in human colon cancer HCT116 cells. Mol. Carcinog. 2009, 48, 260–270. [Google Scholar] [CrossRef] [PubMed]
- He, W.-L.; Weng, X.-T.; Wang, J.-L.; Lin, Y.-K.; Liu, T.-W.; Zhou, Q.-Y.; Hu, Y.; Pan, Y.; Chen, X.-L. Association Between c-Myc and Colorectal Cancer Prognosis: A Meta-Analysis. Front. Physiol. 2018, 9, 1549. [Google Scholar] [CrossRef] [PubMed]
- de Sousa Moraes, L.F.; Sun, X.; Peluzio, M.; Zhu, M.J. Anthocyanins/anthocyanidins and colorectal cancer: What is behind the scenes? Crit. Rev. Food Sci. Nutr. 2019, 59, 59–71. [Google Scholar] [CrossRef]
- Tian, Y.; Qi, P.; Hu, X. Downregulated FOXO3a associates with poor prognosis and promotes cell invasion and migration via WNT/β-catenin signaling in cervical carcinoma. Front. Oncol. 2020, 10, 903. [Google Scholar] [CrossRef]
- Sun, L.; Liu, J.; Bao, D.; Hu, C.; Zhao, Y.; Chen, S. Progress in the study of FOXO3a interacting with microRNA to regulate tumourigenesis development. Front. Oncol. 2023, 13, 1293968. [Google Scholar] [CrossRef]
- Lee, C.W.L.; Ito, K.; Ito, Y. Role of RUNX3 in bone morphogenetic protein signaling in colorectal cancer. Cancer Res. 2010, 70, 4243–4252. [Google Scholar] [CrossRef]
- Jin, Q.; Qu, H.; Quan, C. New insights into the regulation of METTL3 and its role in tumors. Cell Commun Signal. 2023, 21, 334. [Google Scholar] [CrossRef]
- Wei, J.; Yu, W.; Hao, R.; Fan, J.; Gao, J. Anthocyanins from Aronia melanocarpa Induce Apoptosis in Caco-2 Cells through Wnt/β-Catenin Signaling Pathway. Chem. Biodivers. 2020, 17, e2000654. [Google Scholar] [CrossRef]
- Dharmawansa, K.V.S.; Hoskin, D.W.; Rupasinghe, H.P.V. Chemopreventive Effect of Dietary Anthocyanins against Gastrointestinal Cancers: A Review of Recent Advances and Perspectives. Int. J. Mol. Sci. 2020, 21, 6555. [Google Scholar] [CrossRef] [PubMed]
- Charepalli, V.; Reddivari, L.; Radhakrishnan, S.; Vadde, R.; Agarwal, R.; Vanamala, J.K. Anthocyanin-containing purple-fleshed potatoes suppress colon tumorigenesis via elimination of colon cancer stem cells. J. Nutr. Biochem. 2015, 26, 1641–1649. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, X. Targeting the Wnt/β-catenin signaling pathway in cancer. J. Hematol. Oncol. 2020, 13, 165. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Sun, Y.; Wu, M.; Zhou, L.; Zheng, Y.; Ren, T.; Li, M.; Zhao, W. Hawthorn Proanthocyanidin Extract Inhibits Colorectal Carcinoma Metastasis by Targeting the Epithelial-Mesenchymal Transition Process and Wnt/β-Catenin Signaling Pathway. Foods 2024, 13, 1171. [Google Scholar] [CrossRef]
- Ahmed, H.H.; El-Abhar, H.S.; Hassanin, E.A.K.; Abdelkader, N.F.; Shalaby, M.B. Punica granatum suppresses colon cancer through downregulation of Wnt/β-Catenin in rat model. Rev. Bras. Farmacogn. 2017, 27, 627–635. [Google Scholar] [CrossRef]
- Xue, C.; Yao, Q.; Gu, X.; Shi, Q.; Yuan, X.; Chu, Q.; Bao, Z.; Lu, J.; Li, L. Evolving cognition of the JAK-STAT signaling pathway: Autoimmune disorders and cancer. Signal Transduct. Target. Ther. 2023, 8, 204. [Google Scholar] [CrossRef]
- Xiong, H.; Zhang, Z.G.; Tian, X.Q.; Sun, D.F.; Liang, Q.C.; Zhang, Y.J.; Lu, R.; Chen, Y.X.; Fang, J.Y. Inhibition of JAK1, 2/STAT3 signaling induces apoptosis, cell cycle arrest, and reduces tumor cell invasion in colorectal cancer cells. Neoplasia 2008, 10, 287–297. [Google Scholar] [CrossRef]
- Pourshahidi, S.; Davari, M. Anthocyanins: Promising Natural Compounds for Prevention and Treatment of Oral Squamous Cell Carcinoma. Middle East J. Rehabil. Health Stud. 2020, 7, e105844. [Google Scholar] [CrossRef]
- Luo, L.N.; Yang, P.; Huang, W. The effect and mechanism of anthocyanin on hepatic ischemia reperfusion injury in rats. J. Xi’an Jiaotong Univ. 2016, 37, 594–598. [Google Scholar] [CrossRef]
- Liebl, M.C.; Hofmann, T.G. The role of p53 signaling in colorectal cancer. Cancers 2021, 13, 2125. [Google Scholar] [CrossRef]
- Zhang, S.; Zhou, L.; Hong, B.; van den Heuvel, A.P.J.; Prabhu, V.V.; Warfel, N.A.; Kline, C.L.B.; Dicker, D.T.; Kopelovich, L.; El-Deiry, W.S. Small-molecule NSC59984 restores p53 pathway signaling and antitumor effects against colorectal cancer via p73 activation and degradation of mutant p53. Cancer Res. 2015, 75, 3842–3852. [Google Scholar] [CrossRef] [PubMed]
- Tomicic, M.T.; Dawood, M.; Efferth, T. Epigenetic alterations upstream and downstream of p53 signaling in colorectal carcinoma. Cancers 2021, 13, 4072. [Google Scholar] [CrossRef]
- Ghaleb, A.M.; Yang, V.W. Krüppel-like factor 4 (KLF4): What we currently know. Gene 2017, 611, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.J.; Cho, Y.G.; Song, J.W.; Kim, C.J.; Kim, S.Y.; Nam, S.W.; Yoo, N.J.; Lee, J.Y.; Park, W.S. Altered expression of the KLF4 in colorectal cancers. Pathol. Res. Pract. 2006, 202, 585–589. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; He, J.; Xie, K. KLF4 transcription factor in tumorigenesis. Cell Death Discov. 2023, 9, 118. [Google Scholar] [CrossRef] [PubMed]
- Selvamuthukumaran, M. Natural Anthocyanins from Subtropical Fruits for Cancer Prevention. In Anthocyanins in Subtropical Fruits; CRC Press: Boca Raton, FL, USA, 2023; pp. 97–103. [Google Scholar]
- Lee, E.; Cheung, J.; Bialkowska, A.B. Krüppel-like Factors 4 and 5 in Colorectal Tumorigenesis. Cancers 2023, 15, 2430. [Google Scholar] [CrossRef]
- Wangensteen, H.; Bräunlich, M.; Nikolic, V.; Malterud, K.E.; Slimestad, R.; Barsett, H. Anthocyanins, proanthocyanidins and total phenolics in four cultivars of aronia: Antioxidant and enzyme inhibitory effects. J. Funct. Foods 2014, 7, 746–752. [Google Scholar] [CrossRef]
- Dobros, N.; Zielińska, A.; Siudem, P.; Zawada, K.D.; Paradowska, K. Profile of Bioactive Components and Antioxidant Activity of Aronia melanocarpa Fruits at Various Stages of Their Growth, Using Chemometric Methods. Antioxidants 2024, 13, 462. [Google Scholar] [CrossRef]
- Malik, M.; Zhao, C.; Schoene, N.; Guisti, M.M.; Moyer, M.P.; Magnuson, B.A. Anthocyanin-rich extract from Aronia meloncarpa E induces a cell cycle block in colon cancer but not normal colonic cells. Nutr. Cancer 2003, 46, 186–196. [Google Scholar] [CrossRef]
- Asahi, Y.; Okuno, K.; Xu, C.; Taketomi, A.; Goel, A. Abstract 3822: Novel evidence for the role of the p53 signaling pathway in mediating the anticancer effects of aronia berry extract in colorectal cancer cells. Cancer Res. 2023, 83, 3822. [Google Scholar] [CrossRef]
- Ghaleb, A.M.; Elkarim, E.A.; Bialkowska, A.B.; Yang, V.W. KLF4 Suppresses Tumor Formation in Genetic and Pharmacological Mouse Models of Colonic Tumorigenesis. Mol. Cancer Res. 2016, 14, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Yun-Kyoung, L.; Park, S.Y.; Kim, Y.-M.; Lee, W.S.; Park, O.J. Anthocyanins target AMPK/mTOR and AMPK/Wnt pathways in exerting anti-tumor effects in colon cancer or hepatocarcinoma cells. FASEB J. 2010, 24, lb259. [Google Scholar] [CrossRef]
- Fan, H.; Ji, Y.; Wang, Y.; Liu, D.; Wei, T.; Cao, X.; Yang, M.; Bai, C.; Wang, Z. Anthocyanins from Lycium ruthenicum Murray Inhibit HepG2 Cells Growth, Metastasis and Promote Apoptosis and G2/M Phase Cycle Arrest by Activating the AMPK/mTOR Autophagy Pathway. Evid. Based Complement. Altern. Med. 2022, 2022, 9609596. [Google Scholar] [CrossRef] [PubMed]
- Rascio, F.; Spadaccino, F.; Rocchetti, M.T.; Castellano, G.; Stallone, G.; Netti, G.S.; Ranieri, E. The Pathogenic Role of PI3K/AKT Pathway in Cancer Onset and Drug Resistance: An Updated Review. Cancers 2021, 13, 3949. [Google Scholar] [CrossRef]
- Hornsveld, M.; Dansen, T.B.; Derksen, P.W.; Burgering, B.M.T. Re-evaluating the role of FOXOs in cancer. Semin. Cancer Biol. 2018, 50, 90–100. [Google Scholar] [CrossRef]
- Zhao, X.; Feng, P.; He, W.; Du, X.; Chen, C.; Suo, L.; Liang, M.; Zhang, N.; Na, A.; Zhang, Y. The prevention and inhibition effect of anthocyanins on colorectal cancer. Curr. Pharm. Des. 2019, 25, 4919–4927. [Google Scholar] [CrossRef]
- Anwar, S.; Fratantonio, D.; Ferrari, D.; Saija, A.; Cimino, F.; Speciale, A. Berry anthocyanins reduce proliferation of human colorectal carcinoma cells by inducing caspase-3 activation and p21 upregulation. Mol. Med. Rep. 2016, 14, 1397–1403. [Google Scholar] [CrossRef]
- Tsai, M.C.; Chen, C.C.; Tseng, T.H.; Chang, Y.C.; Lin, Y.J.; Tsai, I.N.; Wang, C.C.; Wang, C.J. Hibiscus Anthocyanins Extracts Induce Apoptosis by Activating AMP-Activated Protein Kinase in Human Colorectal Cancer Cells. Nutrients 2023, 15, 3972. [Google Scholar] [CrossRef]
- Li, X.; Wu, Y.; Tian, T. TGF-β Signaling in Metastatic Colorectal Cancer (mCRC): From Underlying Mechanism to Potential Applications in Clinical Development. Int. J. Mol. Sci. 2022, 23, 14436. [Google Scholar] [CrossRef]
- Rashmita, S.; Govinda, B.; Arvind, S. A study on marketing behaviour of tomato growers in Shivpuri District MP, India. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 331–334. [Google Scholar] [CrossRef]
- Kaboré, K.; Konaté, K.; Sanou, A.; Dakuyo, R.; Sama, H.; Santara, B.; Compaoré, E.W.R.; Dicko, M.H. Tomato By-Products, a Source of Nutrients for the Prevention and Reduction of Malnutrition. Nutrients 2022, 14, 2871. [Google Scholar] [CrossRef] [PubMed]
- Mattoo, A.K.; Dwivedi, S.L.; Dutt, S.; Singh, B.; Garg, M.; Ortiz, R. Anthocyanin-rich vegetables for human consumption—Focus on potato, sweetpotato and tomato. Int. J. Mol. Sci. 2022, 23, 2634. [Google Scholar] [CrossRef] [PubMed]
- Menconi, J.; Perata, P.; Gonzali, S. Novel R2R3 MYB transcription factors regulate anthocyanin synthesis in Aubergine tomato plants. BMC Plant Biol. 2023, 23, 148. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wu, H.; Ding, Q.; Li, H.; Li, Z.; Ding, J.; Li, Y. The heterologous expression of Arabidopsis PAP2 induces anthocyanin accumulation and inhibits plant growth in tomato. Funct. Integr. Genom. 2018, 18, 341–353. [Google Scholar] [CrossRef]
- Lim, W.; Li, J. Synergetic effect of the Onion CHI gene on the PAP1 regulatory gene for enhancing the flavonoid profile of tomato skin. Sci. Rep. 2017, 7, 12377. [Google Scholar] [CrossRef]
- Maligeppagol, M.; Chandra, G.S.; Navale, P.M.; Deepa, H.; Rajeev, P.R.; Asokan, R.; Babu, K.P.; Babu, C.S.B.; Rao, V.K.; Kumar, N.K.K. Anthocyanin enrichment of tomato (Solanum lycopersicum L.) fruit by metabolic engineering. Curr. Sci. 2013, 105, 72–80. [Google Scholar]
- Lim, W.; Miller, R.; Park, J.; Park, S. Consumer Sensory Analysis of High Flavonoid Transgenic Tomatoes. J. Food Sci. 2014, 79, S1212–S1217. [Google Scholar] [CrossRef]
- Jian, W.; Cao, H.; Yuan, S.; Liu, Y.; Lu, J.; Lu, W.; Li, N.; Wang, J.; Zou, J.; Tang, N.; et al. SlMYB75, an MYB-type transcription factor, promotes anthocyanin accumulation and enhances volatile aroma production in tomato fruits. Horticult. Res. 2019, 6, 22. [Google Scholar] [CrossRef]
- Su, X.; Xu, J.; Rhodes, D.; Shen, Y.; Song, W.; Katz, B.; Tomich, J.; Wang, W. Identification and quantification of anthocyanins in transgenic purple tomato. Food Chem. 2016, 202, 184–188. [Google Scholar] [CrossRef]
- Hassanin, A.A.; Saad, A.M.; Bardisi, E.A.; Salama, A.; Sitohy, M.Z. Transfer of Anthocyanin Accumulating Delila and Rosea1 Genes from the Transgenic Tomato Micro-Tom Cultivar to Moneymaker Cultivar by Conventional Breeding. J. Agric. Food Chem. 2020, 68, 10741–10749. [Google Scholar] [CrossRef]
- Naing, A.H.; Ai, T.N.; Lim, K.B.; Lee, I.J.; Kim, C.K. Overexpression of Rosea1 from Snapdragon Enhances Anthocyanin Accumulation and Abiotic Stress Tolerance in Transgenic Tobacco. Front. Plant Sci. 2018, 9, 1070. [Google Scholar] [CrossRef] [PubMed]
- Naing, A.H.; Park, K.I.; Ai, T.N.; Chung, M.Y.; Han, J.S.; Kang, Y.-W.; Lim, K.B.; Kim, C.K. Overexpression of snapdragon Delila (Del) gene in tobacco enhances anthocyanin accumulation and abiotic stress tolerance. BMC Plant Biol. 2017, 17, 65. [Google Scholar] [CrossRef]
- Lim, W.; Li, J. Co-expression of onion chalcone isomerase in Del/Ros1-expressing tomato enhances anthocyanin and flavonol production. Plant Cell Tissue Organ Cult. 2017, 128, 113–124. [Google Scholar] [CrossRef]
- Zhu, J.; Zhao, W.; Li, R.; Guo, D.; Li, H.; Wang, Y.; Mei, W.; Peng, S. Identification and Characterization of Chalcone Isomerase Genes Involved in Flavonoid Production in Dracaena cambodiana. Front. Plant Sci. 2021, 12, 616396. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Huang, K.; Liu, Y.; Jiao, T.; Ma, G.; Qian, Y.; Wang, P.; Dai, X.; Gao, L.; Xia, T. Functional Analysis of Two Flavanone-3-Hydroxylase Genes from Camellia sinensis: A Critical Role in Flavonoid Accumulation. Genes 2017, 8, 300. [Google Scholar] [CrossRef] [PubMed]
- Zuluaga, D.L.; Gonzali, S.; Loreti, E.; Pucciariello, C.; Degl’Innocenti, E.; Guidi, L.; Alpi, A.; Perata, P. Arabidopsis thaliana MYB75/PAP1 transcription factor induces anthocyanin production in transgenic tomato plants. Funct. Plant Biol. 2008, 35, 606–618. [Google Scholar] [CrossRef]
- Zhang, Y.; Butelli, E.; Alseekh, S.; Tohge, T.; Rallapalli, G.; Luo, J.; Kawar, P.G.; Hill, L.; Santino, A.; Fernie, A.R.; et al. Multi-level engineering facilitates the production of phenylpropanoid compounds in tomato. Nat. Commun. 2015, 6, 8635. [Google Scholar] [CrossRef]
- Meng, X.; Yang, D.; Li, X.; Zhao, S.; Sui, N.; Meng, Q. Physiological changes in fruit ripening caused by overexpression of tomato SlAN2, an R2R3-MYB factor. Plant Physiol. Biochem. 2015, 89, 24–30. [Google Scholar] [CrossRef]
- Mazzucato, A.; Willems, D.; Bernini, R.; Picarella, M.E.; Santangelo, E.; Ruiu, F.; Tilesi, F.; Soressi, G.P. Novel phenotypes related to the breeding of purple-fruited tomatoes and effect of peel extracts on human cancer cell proliferation. Plant Physiol. Biochem. 2013, 72, 125–133. [Google Scholar] [CrossRef]
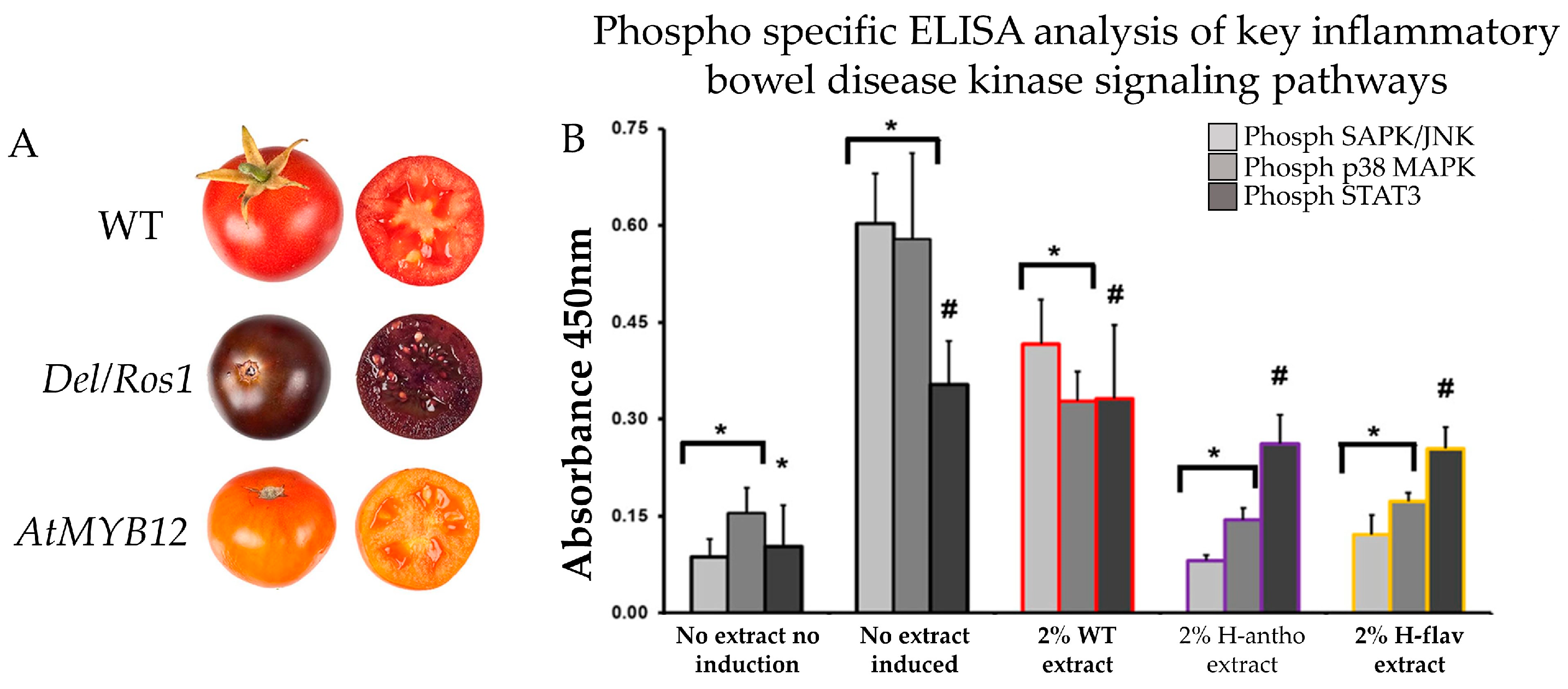
- Tomlinson, M.L.; Butelli, E.; Martin, C.; Carding, S.R. Flavonoids from Engineered Tomatoes Inhibit Gut Barrier Pro-inflammatory Cytokines and Chemokines, via SAPK/JNK and p38 MAPK Pathways. Front. Nutr. 2017, 4, 61. [Google Scholar] [CrossRef]



| Scheme | Subsets of Anthocyanin | References | ||
|---|---|---|---|---|
| Fruits | TAC | Name of the Compounds | Concentration | |
| Açaí | 732 mg/100 g | Cyanidin-3-O-glucoside | 133.25 mg/100 g DW | [30,31] |
| Cyanidin-3-O-rutinoside | 225.61 mg/100 g DW | |||
| Blackcurrant | 294.38 mg/100 g | Delphinidin-3-O-glucoisde | 8.58 mg/100 g FW | [32,33] |
| Delphinidin-3-O-rutinoside | 42.73 mg/100 g FW | |||
| Cyanidin-3-O-glucoside | 2.99 mg/100 g FW | |||
| Cyanidin-3-O-rutinoside | 30.11 mg/100 g FW | |||
| Queen Garnet plum | 277 mg/100 g | Cyanidin-3-O-glucoside | 17.00 mg/100 g FW | [34,35] |
| Blueberry | 275.86 mg/100 g | Cyanidin-3-O-glucoside | 0.01 mg/100 g | [36,37] |
| Sweet potato | 223 mg/100 g | Cyanidin 3-p-hydroxybenzoyl sophoroside-5-glucoside | 85.80 mg/100 g | [38,39] |
| Cyanidin 3-(6‴-caffeoyl sophoroside)-5-glucoside | 33.90 mg/100 g | |||
| Peonidin 3-p-hydroxybenzoyl sophoroside-5-glucoside | 710.00 mg/100 g | |||
| Peonidin 3-(6‴-caffeoyl sophoroside)-5-glucoside | 229.00 mg/100 g | |||
| Cyanidin 3-feruloyl sophoroside-5-glucoside | 204.00 mg/100 g | |||
| Peonidin 3-feruloyl sophoroside-5-glucoside | 712.00 mg/100 g | |||
| Cyanidin 3-caffeoyl sophoroside-5-glucoside | 1310.00 mg/100 g | |||
| Cyanidin 3-sophoroside-5-glucoside | 444.00 mg/100 g | |||
| Cyanidin 3-dicaffeoyl sophoroside-5-glucoside | 1220.00 mg/100 g | |||
| Cyanidin 3-caffeoyl-p-gydroxybenzoyl sophoroside-5-glucoside | 1480.00 mg/100 g | |||
| Peonidin-3-caffeoyl sophoroside-5-glucoside | 3250.00 mg/100 g | |||
| Cyanidin 3-caffeoyl-feruloyl sophoroside-5-glucoside | 1620.00 mg/100 g | |||
| Peonidin 3-dicaffeoyl sophoroside-5-glucoside | 5790.00 mg/100 g | |||
| Peonidin 3-caffeoyl-p-hydroxybenzoyl sophoroside-5-glucoside | 7570.00 mg/100 g | |||
| Peonidin 3-caffeoyl-feruloyl sophoroside-5-glucoside | 6920.00 mg/100 g | |||
| Peonidin 3-caffeoyl-p-coumaryl sophoroside-5-glucoside | 559.00 mg/100 g | |||
| Peonidin 3-feruloyl-p-hydroxybenzoyl sophoroside-5-glucoside | 581.00 mg/100 g | |||
| Peonidin 3-coumaryl-p-hydroxybenzoyl sophoroside-5-glucoside | 181.00 mg/100 g | |||
| Peonidin 3-(6″, 6‴-diferuloyl sophoroside)-5-glucoside | 243.00 mg/100 g | |||
| Cherry | 223 mg/100 g | Cyanidin 3-O-galactoside | 22.62 mg/100 g DW | [40] |
| Raspberries | 211.3 mg/100 g | Cyanidin-3-O-sophoroside | 25.40 mg/100 g | [41,42] |
| Purple corn | 194.47 mg/100 g | Cyanidin-3-O-glucoside | 41.45 mg/100 g DW | [43,44] |
| Red cabbage | 191.37 mg/100 g | Cyanidin-3-diglucoside-5-glucoside | 58.00 mg/100 g DW | [45,46] |
| Cyanidin-3-(sinapoyl)(sinapoyl)-diglucoside-5-glucoside | 26.00 mg/100 g DW | |||
| Cyanidin-3-(feruloyl)(sinapoyl)-diglucoside-5-glucoside | 18.00 mg/100 g DW | |||
| Cyanidin-3-(feruloyl)(feruloyl)-diglucoside-5-glucoside | 17.00 mg/100 g DW | |||
| Cyanidin-3-(sinapoyl)-diglucoside-5-glucoside | 18.00 mg/100 g DW | |||
| Cyanidin-3-(p-coumaroyl)-diglucoside-5-glucoside | 19.00 mg/100 g DW | |||
| Tomato | 120 mg/100 g | Cyanidin-3-O-galactoside | 0.03 mg/100 g FW | [47,48] |
| Cyanidin-3-O-rutinoside | 0.11 mg/100 g FW | |||
| Cyanidin-3-(6-caffeoyl)-glucoside | 0.09 mg/100 g FW | |||
| Delphinidin-3-O-glucoside | 2.00 mg/100 g FW | |||
| Delphinidin-3-rutinoside-5-glucoside | 0.10 mg/100 g FW | |||
| Delphinidin-3,5-O-diglucoside | 0.20 mg/100 g FW | |||
| Delphinidin-3-O-rutinoside | 7.50 mg/100 g FW | |||
| Peonidin-3-O-rutinoside | 0.07 mg/100 g FW | |||
| Peonidin-3-O-(6-O-p-counmaryl)-glucoside | 0.07 mg/100 g FW | |||
| Petunidin-3-O-glucoside | 0.19 mg/100 g FW | |||
| Petunidin-3-O-rutinoside | 0.51 mg/100 g FW | |||
| Malvidin-3-)-glucoside | 0.00 mg/100 g FW | |||
| Malvidin-3-O-rutinoside | 0.09 mg/100 g FW | |||
| Cyanidin-3-(sinapoyl)-diglucoside-5-glucoside | 12.00 mg/100 g DW | |||
| Cyanidin-3-(feruloyl)-diglucoside-5-glucoside | 14.00 mg/100 g DW | |||
| Blackberry | 102.7 mg/100 g | Cyanidin-3-O-glucoside | 40.43 mg/100 g | [49] |
| Cyanidin-3-O-sophoroside | 42.30 mg/100 g | |||
| Cyanidin-3-O-xyloside | 0.11 mg/100 g | |||
| Pelargonidin-3-O-glucoside | 0.79 mg/100 g | |||
| Petunidin-3-O-glucoside | 0.01 mg/100 g | |||
| Cyanidin-3-O-rutinoside | 18.63 mg/100 g | |||
| Peonidin-3-O-galactoside | 0.07 mg/100 g | |||
| Peonidin-3-O-glucoside | 0.36 mg/100 g | |||
| Pathways in CRC | Transcription Factor | Effect of Anthocyanin on the Transcription Factors | References |
|---|---|---|---|
| NFκB signaling pathway | NFκB |
| [107,108,109,110] |
| Wnt/β-catenin signaling pathway | NFκB | ||
| JAK/STAT signaling pathway | STAT3 |
| [111,112] |
| p53 signaling pathway | p53 |
| [113,114,115,116] |
| NF-κB signaling pathway | p53 | ||
| PI3K-AKT signaling pathway | FOXO3a |
| [117,118,119,120] |
| Wnt/β-catenin signaling pathway | FOXO3a | ||
| mTOR signaling pathway | KLF4 |
| [121,122,123] |
| Wnt/β-catenin signaling pathway | KLF4 | ||
| p53 signaling pathway | KLF4 | ||
| TGF-β/BMPs signaling pathway | RUNX3 | - | [124] |
| Wnt/β-catenin signaling pathway | RUNX3 | - |
| Transgenic Lines | Maximum Anthocyanin Contents | Other Function Enhanced | References |
|---|---|---|---|
| Delila and Rosea1 transgenic Solanum lycopersicum cv. MicroTom | Fruits 2.83 ± 0.46 mg/g FW |
| [18] |
| AftAft/atvatv purple line (SB) | Whole Mature fruit 1.2 mg/g DW and 7.1 mg/100 g FW. |
| [47] |
| MYB90/PAP2 transgenic tomato cv. Micro-Tom | Leaves 0.21. units/g FW. Flower > 0.3 unit/g FW. |
| [171] |
| CHI × PAP1 transgenic Solanum lycopersicum L. cv. Rubion | Fruits skin: 48.11 µg/g |
| [172] |
| Del and Ros1 tomato cv. Arka Vikas | Fruits 0.01 mg/g FW |
| [173] |
| CHI, Delila and Rosea1 transgenic Solanum lycopersicum L. | Peel 0.5–0.9 mg/g Flesh 0.03–0.08 mg/g |
| [174] |
| SlMYB75-OE Solanum lycopersicum cv. Micro-Tom | Fruits 2.0 mg/g FW |
| [175] |
| Del/Ros1 transgenic purple tomatoes | Fruit 5.2 g/kg DW, Peel 5.1 g/kg DW, and Flesh 5.8 g/kg DW. |
| [176] |
| Del/Ros1 bred Moneymaker tomato | Fruits 0.7–0.8 g/kg DW (green) and Fruits 1.3–3.0 g/kg DW (mature) |
| [177] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suzauddula, M.; Kobayashi, K.; Park, S.; Sun, X.S.; Wang, W. Bioengineered Anthocyanin-Enriched Tomatoes: A Novel Approach to Colorectal Cancer Prevention. Foods 2024, 13, 2991. https://doi.org/10.3390/foods13182991
Suzauddula M, Kobayashi K, Park S, Sun XS, Wang W. Bioengineered Anthocyanin-Enriched Tomatoes: A Novel Approach to Colorectal Cancer Prevention. Foods. 2024; 13(18):2991. https://doi.org/10.3390/foods13182991
Chicago/Turabian StyleSuzauddula, Md, Kaori Kobayashi, Sunghun Park, Xiuzhi Susan Sun, and Weiqun Wang. 2024. "Bioengineered Anthocyanin-Enriched Tomatoes: A Novel Approach to Colorectal Cancer Prevention" Foods 13, no. 18: 2991. https://doi.org/10.3390/foods13182991








