Qualitative Profiling, Antioxidant and Antimicrobial Activities of Polar and Nonpolar Basil Extracts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Extraction Procedure
2.3. HPLC–DAD/ESI–ToF-MS Analysis
2.4. Antioxidant Assay
2.5. Antimicrobial Assay
2.6. Statistical Analysis
3. Results
3.1. Phytochemical Profile
3.2. Antioxidant Potential
3.3. Antimicrobial Properties
4. Discussion
4.1. Phytochemical Profile
4.2. Antioxidant Potential
4.3. Antimicrobial Properties
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Venkatesan, P. Food is medicine: Clinical trials show the health benefits of dietary interventions. Nat. Med. 2024, 30, 916–919. [Google Scholar] [CrossRef] [PubMed]
- Guerriero, G.; Berni, R.; Muñoz-Sanchez, J.A.; Apone, F.; Abdel-Salam, E.M.; Qahtan, A.A.; Alatar, A.A.; Cantini, C.; Cai, G.; Hausman, J.-F.; et al. Production of Plant Secondary Metabolites: Examples, Tips and Suggestions for Biotechnologists. Genes 2018, 9, 309. [Google Scholar] [CrossRef] [PubMed]
- Petrović, M.M.; Roschger, C.; Lang, K.; Zierer, A.; Mladenović, M.; Trifunović, S.; Mandić, B.; Joksović, M.D. Synthesis and biological evaluation of new quinoline-4-carboxylic acid-chalcone hybrids as dihydroorotate dehydrogenase inhibitors. Arch. Pharm. 2023, 356, 2200374. [Google Scholar] [CrossRef] [PubMed]
- Trifunović, S.; Isaković, A.M.; Isaković, A.; Vučković, I.; Mandić, B.; Novaković, M.; Vajs, V.; Milosavljević, S.; Trajković, V. Isolation, Characterization, and In Vitro Cytotoxicity of New Sesquiterpenoids from Achillea clavennae. Planta Med. 2014, 80, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Mandić, B.M.; Gođevac, D.M.; Vujisić, L.V.; Trifunović, S.S.; Tesević, V.V.; Vajs, V.V.; Milosavljević, S.M. Semiquinol and phenol compounds from seven Senecio species. Chem. Pap. 2011, 65, 90–92. [Google Scholar] [CrossRef]
- Mandić, B.; Simić, K.; Trifunović, S.; Vujisić, L.V.; Novaković, M.; Tešević, V.; Miljanić, O.Š. Inhibition potency of disulphides and trisulphides on various tumor cell lines growth. Dig. J. Nanomater. Biostructures 2021, 16, 585–592. [Google Scholar] [CrossRef]
- Damjanović, A.; Zdunić, G.; Šavikin, K.; Mandić, B.; Jadranin, M.; Matić, I.Z.; Stanojković, T. Evaluation of the anti-cancer potential of Mahonia aquifolium extracts via apoptosis and anti-angiogenesis. Bangladesh J. Pharmacol. 2016, 11, 741–749. [Google Scholar] [CrossRef]
- Ivanović, S.; Mandrone, M.; Simić, K.; Ristić, M.; Todosijević, M.; Mandić, B.; Gođevac, D. GC–MS-based metabolomics for the detection of adulteration in oregano samples. J. Serb. Chem. Soc. 2021, 86, 1195–1203. [Google Scholar] [CrossRef]
- Mandić, B.M.; Simić, M.R.; Vučković, I.M.; Vujisić, L.V.; Novaković, M.M.; Trifunović, S.S.; Nikolić-Mandić, S.D.; Tešević, V.V.; Vajs, V.V.; Milosavljević, S.M. Pyrrolizidine Alkaloids and Fatty Acids from the Endemic Plant Species Rindera umbellata and the Effect of Lindelofine-N-oxide on Tubulin Polymerization. Molecules 2013, 18, 10694–10706. [Google Scholar] [CrossRef]
- Mandić, B.M.; Vlajić, M.D.; Trifunović, S.S.; Simić, M.R.; Vujisić, L.V.; Vučković, I.M.; Novaković, M.M.; Nikolić-Mandić, S.D.; Tešević, V.V.; Vajs, V.V.; et al. Optimisation of isolation procedure for pyrrolizidine alkaloids from Rindera umbellata Bunge. Nat. Prod. Res. 2015, 29, 887–890. [Google Scholar] [CrossRef]
- Todorović, S.; Perić, M.; Nikolić, B.; Mandić, B.; Cvetković, S.; Bogdanović, M.; Živković, S. Chemical Characterization, Antioxidant Activity, and Cytotoxity of Wild-Growing and In Vitro Cultivated Rindera umbellata (Waldst. and Kit.) Bunge. Horticulturae 2023, 9, 381. [Google Scholar] [CrossRef]
- Mandić, B.M.; Gođevac, D.; Beškoski, V.P.; Simić, M.; Trifunović, S.; Tešević, V.; Vajs, V.; Milosavljević, S. Pyrrolizidine alkaloids from seven wild-growing Senecio species in Serbia and Montenegro. J. Serb. Chem. Soc. 2009, 74, 27–34. [Google Scholar] [CrossRef]
- Hallmann, E.; Rusaczonek, A.; Muszyńska, E.; Ziółkowski, D.; Kuliński, S.; Jasek, J.; Ponder, A. A Long-Term Study on Chemical Compounds and Their Location in Sweet Basil Leaves from Organic and Conventional Producers. Foods 2024, 13, 383. [Google Scholar] [CrossRef] [PubMed]
- Javanmardi, J.; Khalighi, A.; Kashi, A.; Bais, H.P.; Vivanco, J.M. Chemical Characterization of Basil (Ocimum basilicum L.) Found in Local Accessions and Used in Traditional Medicines in Iran. J. Agric. Food Chem. 2002, 50, 5878–5883. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Sun, W.; Cheng, Q. Chemical components and pharmacological benefits of Basil (Ocimum basilicum): A review. Int. J. Food Prop. 2020, 23, 1961–1970. [Google Scholar] [CrossRef]
- Kaurinovic, B.; Popovic, M.; Vlaisavljevic, S.; Trivic, S. Antioxidant Capacity of Ocimum basilicum L. and Origanum vulgare L. Extracts. Molecules 2011, 16, 7401–7414. [Google Scholar] [CrossRef]
- Kadan, S.; Saad, B.; Sasson, Y.; Zaid, H. In vitro evaluation of anti-diabetic activity and cytotoxicity of chemically analysed Ocimum basilicum extracts. Food Chem. 2016, 196, 1066–1074. [Google Scholar] [CrossRef]
- Chatterjee, A.; Sukul, N.C.; Laskar, S.; Ghoshmajumdar, S. Nematicidal Principles from Two Species of Lamiaceae. J. Nematol. 1982, 14, 118–120. [Google Scholar] [PubMed]
- Reuveni, R.; Fleischer, A.; Putievsky, E. Fungistatic Activity of Essential Oils from Ocimum basilicum Chemotypes. J. Phytopathol. 1984, 110, 20–22. [Google Scholar] [CrossRef]
- Wang, M.; Cantrell, C.L.; Mathews, S.T.; Paudel, P.; Lee, J.; Mentreddy, S.R. Agronomy, Chemical Analysis, and Antidiabetic Activity of Basil (Ocimum Species). ACS Food Sci. Technol. 2022, 2, 1243–1256. [Google Scholar] [CrossRef]
- Zahran, E.M.; Abdelmohsen, U.R.; Khalil, H.E.; Desoukey, S.Y.; Fouad, M.A.; Kamel, M.S. Diversity, phytochemical and medicinal potential of the genus Ocimum L. (Lamiaceae). Phytochem. Rev. 2020, 19, 907–953. [Google Scholar] [CrossRef]
- Ghavami, A.; Coward, W.A.; Bluck, L.J.C. The effect of food preparation on the bioavailability of carotenoids from carrots using intrinsic labelling. Br. J. Nutr. 2012, 107, 1350–1366. [Google Scholar] [CrossRef] [PubMed]
- Blois, M.S. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- National Committee for Clinical Laboratory Standards, 5th ed.; Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically: Approved Standards. NCCLS Document M7-A5; NCCLS: Wayne, PA, USA, 2000.
- Vujić, B.; Vidaković, V.; Jadranin, M.; Novaković, I.; Trifunović, S.; Tešević, V.; Mandić, B. Composition, Antioxidant Potential, and Antimicrobial Activity of Helichrysum plicatum DC. Various Extracts. Plants 2020, 9, 337. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 10 September 2024).
- Casanova, L.M.; Espíndola-Netto, J.M.; Tinoco, L.W.; Sola-Penna, M.; Costa, S.S. The Use of NMR Metabolite Profiling and in vivo Hypoglycemic Assay for Comparison of Unfractionated Aqueous Leaf Extracts of Two Ocimum Species. Chem. Biodivers. 2016, 13, 686–694. [Google Scholar] [CrossRef] [PubMed]
- Złotek, U.; Szymanowska, U.; Karaś, M.; Świeca, M. Antioxidative and anti-inflammatory potential of phenolics from purple basil (Ocimum basilicum L.) leaves induced by jasmonic, arachidonic and β-aminobutyric acid elicitation. Int. J. Food Sci. Technol. 2016, 51, 163–170. [Google Scholar] [CrossRef]
- Kumar, S.; Bouic, P.J.; Rosenkranz, B. In Vitro Assessment of the Interaction Potential of Ocimum basilicum (L.) Extracts on CYP2B6, 3A4, and Rifampicin Metabolism. Front. Pharmacol. 2020, 11, 517. [Google Scholar] [CrossRef]
- Dou, H.; Zhou, Y.; Chen, C.; Peng, S.; Liao, X.; Ding, L. Chemical Constituents of the Aerial Parts of Schnabelia tetradonta. J. Nat. Prod. 2002, 65, 1777–1781. [Google Scholar] [CrossRef] [PubMed]
- Zgórka, G.; Głowniak, K. Variation of free phenolic acids in medicinal plants belonging to the Lamiaceae family. J. Pharm. Biomed. Anal. 2001, 26, 79–87. [Google Scholar] [CrossRef]
- Canonica, L.; Gramatica, P.; Manitto, P.; Monti, D. Biosynthesis of caffeic acid in Ocimum basilicum L. J. Chem. Soc., Chem. Commun. 1979, 23, 1073b–1075. [Google Scholar] [CrossRef]
- Pandey, R.; Chandra, P.; Kumar, B.; Dutt, B.; Sharma, K.R. A rapid and highly sensitive method for simultaneous determination of bioactive constituents in leaf extracts of six Ocimum species using ultra high performance liquid chromatography-hybrid linear ion trap triple quadrupole mass spectrometry. Anal. Methods 2015, 8, 333–341. [Google Scholar] [CrossRef]
- Carocho, M.; Barros, L.; Barreira, J.C.M.; Calhelha, R.C.; Soković, M.; Fernández-Ruiz, V.; Buelga, C.S.; Morales, P.; Ferreira, I.C.F.R. Basil as functional and preserving ingredient in “Serra da Estrela” cheese. Food Chem. 2016, 207, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Beltrán-Noboa, A.; Proaño-Ojeda, J.; Guevara, M.; Gallo, B.; Berrueta, L.A.; Giampieri, F.; Perez-Castillo, Y.; Battino, M.; Álvarez-Suarez, J.M.; Tejera, E. Metabolomic profile and computational analysis for the identification of the potential anti-inflammatory mechanisms of action of the traditional medicinal plants Ocimum basilicum and Ocimum tenuiflorum. Food Chem. Toxicol. 2022, 164, 113039. [Google Scholar] [CrossRef]
- Ciriello, M.; Formisano, L.; Graziani, G.; Romano, R.; De Pascale, S.; Rouphael, Y.; Corrado, G. Comparative analysis of aromatic and nutraceutical traits of six basils from Ocimum genus grown in floating raft culture. Sci. Hortic. 2023, 322, 112382. [Google Scholar] [CrossRef]
- Fecka, I.; Turek, S. Determination of Water-Soluble Polyphenolic Compounds in Commercial Herbal Teas from Lamiaceae: Peppermint, Melissa, and Sage. J. Agric. Food Chem. 2007, 55, 10908–10917. [Google Scholar] [CrossRef] [PubMed]
- Skaltsa, H.; Philianos, S. Contribution to the chemical study of Ocimum basilicum L. 2nd communication. Plant. Med. Phytother. 1990, 24, 193–196. [Google Scholar]
- Grayer, R.J.; Bryan, S.E.; Veitch, N.C.; Goldstone, F.J.; Paton, A.; Wollenweber, E. External flavones in sweet basil, Ocimum basilicum, and related taxa. Phytochemistry 1996, 43, 1041–1047. [Google Scholar] [CrossRef]
- Berim, A.; Hyatt, D.C.; Gang, D.R. A Set of Regioselective O-Methyltransferases Gives Rise to the Complex Pattern of Methoxylated Flavones in Sweet Basil. Plant Physiol. 2012, 160, 1052–1069. [Google Scholar] [CrossRef] [PubMed]
- Grayer, R.J.; Vieira, R.F.; Price, A.M.; Kite, G.C.; Simon, J.E.; Paton, A.J. Characterization of cultivars within species of Ocimum by exudate flavonoid profiles. Biochem. Syst. Ecol. 2004, 32, 901–913. [Google Scholar] [CrossRef]
- Fatope, M.O.; Takeda, Y. The Constituents of the Leaves of Ocimum basilicum. Planta Med. 1988, 54, 190. [Google Scholar] [CrossRef]
- Celikcan, F.; Kocak, M.Z.; Kulak, M. Vermicompost applications on growth, nutrition uptake and secondary metabolites of Ocimum basilicum L. under water stress: A comprehensive analysis. Ind. Crops Prod. 2021, 171, 113973. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, X.-H.; Zhou, S.; Gao, H.; Li, G.-L.; Guo, W.-J.; Fang, X.-Y.; Wang, W. Perillanolides A and B, new monoterpene glycosides from the leaves of Perilla frutescens. Rev. Bras. Farmacogn. 2017, 27, 564–568. [Google Scholar] [CrossRef]
- Yin, F.; Hu, L.-H.; Lou, F.-C. Chemical studies on the herb of Ocimum basilicum L. Chin. J. Nat. Med. 2004, 2, 20–24. [Google Scholar]
- Proz, M.d.l.Á.; da Silva, M.A.S.; Rodrigues, E.; Bender, R.J.; Rios, A.d.O. Effects of indoor, greenhouse, and field cultivation on bioactive compounds from parsley and basil. J. Sci. Food Agric. 2021, 101, 6320–6330. [Google Scholar] [CrossRef] [PubMed]
- Skaltsa, H.; Philianos, S. Contribution to the chemical study of Ocimum basilicum L. 1st communication. Plant. Med. Phytother. 1986, 20, 291–299. [Google Scholar]
- Vlase, L.; Benedec, D.; Hanganu, D.; Damian, G.; Csillag, I.; Sevastre, B.; Mot, A.C.; Silaghi-Dumitrescu, R.; Tilea, I. Evaluation of Antioxidant and Antimicrobial Activities and Phenolic Profile for Hyssopus officinalis, Ocimum basilicum and Teucrium chamaedrys. Molecules 2014, 19, 5490–5507. [Google Scholar] [CrossRef] [PubMed]
- Hawrył, A.; Hawrył, M. Chromatographic fingerprinting of some basils and the evaluation of their antioxidant properties with chemometric calculations. J. Liq. Chromatogr. Relat. Technol. 2020, 43, 750–760. [Google Scholar] [CrossRef]
- Grayer, R.J.; Kite, G.C.; Veitch, N.C.; Eckert, M.R.; Marin, P.D.; Senanayake, P.; Paton, A.J. Leaf flavonoid glycosides as chemosystematic characters in Ocimum. Biochem. Syst. Ecol. 2002, 30, 327–342. [Google Scholar] [CrossRef]
- Furukawa, M.; Oikawa, N.; Imohata, T.; Makino, M.; Ogawa, S.; Iida, T.; Fujimoto, Y.; Kitanaka, S. Monoterpene Glucosides from Ziziphora clinopodioides (Labiatae). Chem. Pharm. Bull. 2012, 60, 397–401. [Google Scholar] [CrossRef]
- He, X.-F.; Geng, C.-A.; Huang, X.-Y.; Ma, Y.-B.; Zhang, X.-M.; Chen, J.-J. Chemical Constituents from Mentha haplocalyx Briq. (Mentha canadensis L.) and Their α-Glucosidase Inhibitory Activities. Nat. Prod. Bioprospect. 2019, 9, 223–229. [Google Scholar] [CrossRef]
- Kamel, M.S.; Assaf, M.H.; Hasanean, H.A.; Ohtani, K.; Kasai, R.; Yamasaki, K. Monoterpene glucosides from Origanum syriacum. Phytochemistry 2001, 58, 1149–1152. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Bao, Y.; Wang, M.; Wang, Q. Two new glycosides from Panzerina lanata (L.) Soják. Nat. Prod. Res. 2022, 36, 3567–3571. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Tan, S.-B.; Wang, Y.; Chang, J. Two new monoterpenoid glycosides from the fresh rhizome of Tongling White Ginger (Zingiber officinale). Nat. Prod. Res. 2018, 32, 71–76. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, H.-T.; Tian, T.; Wei, X.-H.; Chou, G.-X. Phenylethanoid Glycosides from Caryopteris aureoglandulosa and Their Biological Activities. Chem. Biodivers. 2023, 20, e202201037. [Google Scholar] [CrossRef]
- Pande, J.; Chanda, S. Determination of phytochemical profile and antioxidant efficacy of Lavendula bipinnata leaves collected during Magha Nakshatra days and Normal days using LC-QTOF-MS technique. J. Pharm. Biomed. Anal. 2020, 186, 113347. [Google Scholar] [CrossRef]
- Li, S.-M.; Yang, X.-W.; Li, Y.-L.; Shen, Y.-H.; Feng, L.; Wang, Y.-H.; Zeng, H.-W.; Liu, X.-H.; Zhang, C.-S.; Long, C.-L.; et al. Chemical Constituents of Dracocephalum forrestii. Planta Med. 2009, 75, 1591–1596. [Google Scholar] [CrossRef] [PubMed]
- Kherkhache, H.; Benabdelaziz, I.; Silva, A.M.S.; Lahrech, M.B.; Benalia, M.; Haba, H. A new indole alkaloid, antioxidant and antibacterial activities of crude extracts from Saccocalyx satureioides. Nat. Prod. Res. 2020, 34, 1528–1534. [Google Scholar] [CrossRef]
- Thi Dung, D.; Thi Trang, D.; Hai Yen, P.; Huy Hoang, N.; Huu Tai, B.; Van Kiem, P. Elsholblanosides A−D, Four New Oleuropeic Acid Derivatives Isolated from Elsholtzia blanda and Their Inhibition of NO Production in LPS-activated RAW264.7 Cells. Chem. Biodivers. 2023, 20, e202300785. [Google Scholar] [CrossRef]
- Seo, Y.H.; Trinh, T.A.; Ryu, S.M.; Kim, H.S.; Choi, G.; Moon, B.C.; Shim, S.H.; Jang, D.S.; Lee, D.; Kang, K.S.; et al. Chemical Constituents from the Aerial Parts of Elsholtzia ciliata and Their Protective Activities on Glutamate-Induced HT22 Cell Death. J. Nat. Prod. 2020, 83, 3149–3155. [Google Scholar] [CrossRef]
- Zhang, C.-G.; Wang, L.; Lu, Y.; Ye, Z.; Han, Z.-Z.; Xu, H.; Chou, G.-X. Diterpenoids from the Whole Plant of Lagochilus platyacanthus. Planta Med. 2015, 81, 1345–1352. [Google Scholar] [CrossRef]
- Deng, Z.-T.; Chen, J.-J.; Geng, C.-A. ent-Labdane and ent-kaurane diterpenoids from Chelonopsis odontochila with α-glucosidase inhibitory activity. Bioorg. Chem. 2020, 95, 103571. [Google Scholar] [CrossRef] [PubMed]
- Gul, I.; Hassan, A.; Haq, E.; Ahmad, S.M.; Shah, R.A.; Ganai, N.A.; Chikan, N.A.; Abdul-Careem, M.F.; Shabir, N. An Investigation of the Antiviral Potential of Phytocompounds against Avian Infectious Bronchitis Virus through Template-Based Molecular Docking and Molecular Dynamics Simulation Analysis. Viruses 2023, 15, 847. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.-B.; Yang, S.-J.; Yan, Z.-R.; Zhang, X.-J.; Pu, D.-B.; Wang, L.-X.; Li, X.-L.; Zhang, R.-H.; Xiao, W.-L. Isolation, Characterization, and Structure–Activity Relationship Analysis of Abietane Diterpenoids from Callicarpa bodinieri as Spleen Tyrosine Kinase Inhibitors. J. Nat. Prod. 2018, 81, 998–1006. [Google Scholar] [CrossRef]
- Tanaka, N.; Tsuji, E.; Sakai, K.; Gonoi, T.; Kobayashi, J. Hikiokoshins A–I, diterpenes from the leaves of Isodon japonicus. Phytochemistry 2014, 102, 205–210. [Google Scholar] [CrossRef]
- Li, H.; Li, M.-M.; Su, X.-Q.; Sun, J.; Gu, Y.-F.; Zeng, K.-W.; Zhang, Q.; Zhao, Y.-F.; Ferreira, D.; Zjawiony, J.K.; et al. Anti-inflammatory Labdane Diterpenoids from Lagopsis supina. J. Nat. Prod. 2014, 77, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- Faiella, L.; Piaz, F.D.; Bader, A.; Braca, A. Diterpenes and phenolic compounds from Sideritis pullulans. Phytochemistry 2014, 106, 164–170. [Google Scholar] [CrossRef]
- Zheng, X.; Kadir, A.; Zheng, G.; Jin, P.; Qin, D.; Maiwulanjiang, M.; Aisa, H.A.; Yao, G. Antiproliferative abietane quinone diterpenoids from the roots of Salvia deserta. Bioorg. Chem. 2020, 104, 104261. [Google Scholar] [CrossRef]
- Monica, A.A. Identification and validation of palustric acid and andrographolide from Ocimum basilicum leaf as a promising breast cancer inhibitor: An in-vitro and in-silico analysis. Int. J. Pharm. Sci. Res. 2023, 14, 2899–2906. [Google Scholar] [CrossRef]
- Luo, S.-H.; Luo, Q.; Niu, X.-M.; Xie, M.-J.; Zhao, X.; Schneider, B.; Gershenzon, J.; Li, S.-H. Glandular Trichomes of Leucosceptrum canum Harbor Defensive Sesterterpenoids. Angew. Chem., Int. Ed. 2010, 49, 4471–4475. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, S.-H.; Schmidt, A.; Wang, G.-D.; Sun, G.-L.; Grant, M.; Kuang, C.; Yang, M.-J.; Jing, S.-X.; Li, C.-H.; et al. A Geranylfarnesyl Diphosphate Synthase Provides the Precursor for Sesterterpenoid (C25) Formation in the Glandular Trichomes of the Mint Species Leucosceptrum canum. Plant Cell 2016, 28, 804–822. [Google Scholar] [CrossRef]
- Hu, P.; Li, D.-H.; Hu, X.; Li, S.-G.; Sai, C.-M.; Sun, X.-C.; Su, T.; Bai, J.; Wang, Z.-H.; Li, Z.-L.; et al. Lignans and triterpenoids from Vitex negundo var. heterophylla and their biological evaluation. Fitoterapia 2016, 111, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-T.; Liaw, C.-C.; Chiou, C.-T.; Kuo, Y.-H.; Lee, K.-T. Triterpene Acids from Mesona procumbens Exert Anti-inflammatory Effects on LPS-Stimulated Murine Macrophages by Regulating the MAPK Signaling Pathway. J. Agric. Food Chem. 2021, 69, 6271–6280. [Google Scholar] [CrossRef] [PubMed]
- Priya, S.; Peddha, M.S. Physicochemical characterization, polyphenols and flavonoids of different extracts from leaves of four varieties of tulsi (Ocimum sp.). S. Afr. J. Bot. 2023, 159, 381–395. [Google Scholar] [CrossRef]
- Marzouk, A.M. Hepatoprotective Triterpenes from Hairy Root Cultures of Ocimum basilicum L. Z. Naturforsch. C 2009, 64, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-W.; Wang, J.-P.; Wu, P.-P.; Kuo, S.-C.; Lee Chao, P.-D. Terpenoids from Ocimum basilicum. Chin. Pharm. J. 1999, 51, 181–189. [Google Scholar]
- Ma, Z.; Qin, Y.; Wang, X.; Zhang, G.; Zhang, X.; Jiang, H.; Tian, Z. Identification of chemical compounds of Schizonepeta tenuifolia Briq. and screening of neuraminidase inhibitors based on AUF-MS and SPR technology. J. Pharm. Biomed. Anal. 2024, 237, 115787. [Google Scholar] [CrossRef]
- Nicholas, H.J. The Sterol and Triterpene Content of the Labiatae Family. J. Am. Pharm. Assoc. Sci. Ed. 1958, 47, 731–733. [Google Scholar] [CrossRef]
- Qamar, K.A.; Farooq, A.D.; Siddiqui, B.S.; Kabir, N.; Begum, S. Antiproliferative Effects of Ocimum basilicum Methanolic Extract and Fractions, Oleanolic Acid and 3-epi-Ursolic Acid. Curr. Tradit. Med. 2020, 6, 134–146. [Google Scholar] [CrossRef]
- Uysal, S.; Zengin, G.; Sinan, K.I.; Ak, G.; Ceylan, R.; Mahomoodally, M.F.; Uysal, A.; Sadeer, N.B.; Jekő, J.; Cziáky, Z.; et al. Chemical characterization, cytotoxic, antioxidant, antimicrobial, and enzyme inhibitory effects of different extracts from one sage (Salvia ceratophylla L.) from Turkey: Open a new window on industrial purposes. RSC Adv. 2021, 11, 5295–5310. [Google Scholar] [CrossRef]
- Zhan, Z.-J.; Yue, J.-M. New glyceroglycolipid and ceramide from Premna microphylla. Lipids 2003, 38, 1299–1303. [Google Scholar] [CrossRef]
- Diyaolu, O.A.; Oluwabusola, E.T.; Attah, A.F.; Olori, E.O.; Fagbemi, A.A.; Preet, G.; Soldatou, S.; Moody, J.O.; Jaspars, M.; Ebel, R. Can Crude Oil Exploration Influence the Phytochemicals and Bioactivity of Medicinal Plants? A Case of Nigerian Vernonia amygdalina and Ocimum gratissimum. Molecules 2022, 27, 8372. [Google Scholar] [CrossRef] [PubMed]
- Dandekar, P.D.; Kotmale, A.S.; Chavan, S.R.; Kadlag, P.P.; Sawant, S.V.; Dhavale, D.D.; RaviKumar, A. Insights into the Inhibition Mechanism of Human Pancreatic α-Amylase, a Type 2 Diabetes Target, by Dehydrodieugenol B Isolated from Ocimum tenuiflorum. ACS Omega 2021, 6, 1780–1786. [Google Scholar] [CrossRef] [PubMed]
- Prinsi, B.; Morgutti, S.; Negrini, N.; Faoro, F.; Espen, L. Insight into Composition of Bioactive Phenolic Compounds in Leaves and Flowers of Green and Purple Basil. Plants 2020, 9, 22. [Google Scholar] [CrossRef]
- Prasongdee, P.; Posridee, K.; Oonsivilai, A.; Oonsivilai, R. A Culinary and Medicinal Gem: Exploring the Phytochemical and Functional Properties of Thai Basil. Foods 2024, 13, 632. [Google Scholar] [CrossRef]
- Amoah, S.K.S.; Sandjo, L.P.; Kratz, J.M.; Biavatti, M.W. Rosmarinic Acid—Pharmaceutical and Clinical Aspects. Planta Med. 2016, 82, 388–406. [Google Scholar] [CrossRef]
- Jayasinghe, C.; Gotoh, N.; Aoki, T.; Wada, S. Phenolics Composition and Antioxidant Activity of Sweet Basil (Ocimum basilicum L.). J. Agric. Food Chem. 2003, 51, 4442–4449. [Google Scholar] [CrossRef] [PubMed]
- Alseekh, S.; Perez de Souza, L.; Benina, M.; Fernie, A.R. The style and substance of plant flavonoid decoration; towards defining both structure and function. Phytochemistry 2020, 174, 112347. [Google Scholar] [CrossRef]
- Bernini, R.; Crisante, F.; Ginnasi, M.C. A Convenient and Safe O-Methylation of Flavonoids with Dimethyl Carbonate (DMC). Molecules 2011, 16, 1418–1425. [Google Scholar] [CrossRef]
- Pandey, P.; Singh, S.; Banerjee, S. Ocimum basilicum suspension culture as resource for bioactive triterpenoids: Yield enrichment by elicitation and bioreactor cultivation. Plant Cell Tissue Organ Cult. 2019, 137, 65–75. [Google Scholar] [CrossRef]
- Pai, S.R.; Joshi, R.K. Variations in Pentacyclic Triterpenoids in Different Parts of Four Ocimum Species Using Reverse Phase-High Performance Liquid Chromatography. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2017, 87, 1153–1158. [Google Scholar] [CrossRef]
- Loussouarn, M.; Krieger-Liszkay, A.; Svilar, L.; Bily, A.; Birtić, S.; Havaux, M. Carnosic Acid and Carnosol, Two Major Antioxidants of Rosemary, Act through Different Mechanisms. Plant Physiol. 2017, 175, 1381–1394. [Google Scholar] [CrossRef] [PubMed]
- Ladeiras, D.; Monteiro, C.M.; Pereira, F.; Reis, C.P.; Afonso, C.A.M.; Rijo, P. Reactivity of Diterpenoid Quinones: Royleanones. Curr. Pharm. Des. 2016, 22, 1682–1714. [Google Scholar] [CrossRef] [PubMed]
- Tarchoune, I.; Baâtour, O.; Harrathi, J.; Hamdaoui, G.; Lachaâl, M.; Ouerghi, Z.; Marzouk, B. Effects of two sodium salts on fatty acid and essential oil composition of basil (Ocimum basilicum L.) leaves. Acta Physiol. Plant. 2013, 35, 2365–2372. [Google Scholar] [CrossRef]
- Lenti, L.; Rigano, D.; Woo, S.L.; Nartea, A.; Pacetti, D.; Maggi, F.; Fiorini, D. A Rapid Procedure for the Simultaneous Determination of Eugenol, Linalool and Fatty Acid Composition in Basil Leaves. Foods 2022, 11, 3315. [Google Scholar] [CrossRef] [PubMed]
- Teofilović, B.; Grujić-Letić, N.; Goločorbin-Kon, S.; Stojanović, S.; Vastag, G.; Gadžurić, S. Experimental and chemometric study of antioxidant capacity of basil (Ocimum basilicum) extracts. Ind. Crops Prod. 2017, 100, 176–182. [Google Scholar] [CrossRef]
- Spiegel, M.; Kapusta, K.; Kołodziejczyk, W.; Saloni, J.; Żbikowska, B.; Hill, G.A.; Sroka, Z. Antioxidant Activity of Selected Phenolic Acids–Ferric Reducing Antioxidant Power Assay and QSAR Analysis of the Structural Features. Molecules 2020, 25, 3088. [Google Scholar] [CrossRef]
- Filipović, M.; Marković, Z.; Đorović, J.; Marković, J.D.; Lučić, B.; Amić, D. QSAR of the free radical scavenging potency of selected hydroxybenzoic acids and simple phenolics. Comptes Rendus Chim. 2015, 18, 492–498. [Google Scholar] [CrossRef]
- Mancuso, G.; Midiri, A.; Gerace, E.; Marra, M.; Zummo, S.; Biondo, C. Urinary Tract Infections: The Current Scenario and Future Prospects. Pathogens 2023, 12, 623. [Google Scholar] [CrossRef]
- Alby, K.; Nachamkin, I. Gastrointestinal Infections. Microbiol. Spectrum 2016, 4, DMIH2-0005-2015. [Google Scholar] [CrossRef]
- Rytter, H.; Jamet, A.; Coureuil, M.; Charbit, A.; Ramond, E. Which Current and Novel Diagnostic Avenues for Bacterial Respiratory Diseases? Front. Microbiol. 2020, 11, 616971. [Google Scholar] [CrossRef]
- WHO Publishes List of Bacteria for Which New Antibiotics Are Urgently Needed. Available online: https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed (accessed on 13 August 2024).
- Flores-Mireles, A.L.; Walker, J.N.; Caparon, M.; Hultgren, S.J. Urinary tract infections: Epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 2015, 13, 269–284. [Google Scholar] [CrossRef] [PubMed]
- Backiam, A.D.S.; Duraisamy, S.; Karuppaiya, P.; Balakrishnan, S.; Sathyan, A.; Kumarasamy, A.; Raju, A. Analysis of the main bioactive compounds from Ocimum basilicum for their antimicrobial and antioxidant activity. Biotechnol. Appl. Biochem. 2023, 70, 2038–2051. [Google Scholar] [CrossRef] [PubMed]
- Ababutain, I.M. Antimicrobial Activity and Gas Chromatography-Mass Spectrometry (GC-MS) Analysis of Saudi Arabian Ocimum basilicum Leaves Extracts. J. Pure Appl. Microbiol. 2019, 13, 823–833. [Google Scholar] [CrossRef]
- Benedict, K.; Chiller, T.M.; Mody, R.K. Invasive Fungal Infections Acquired from Contaminated Food or Nutritional Supplements: A Review of the Literature. Foodborne Pathog. Dis. 2016, 13, 343–349. [Google Scholar] [CrossRef]
- Daglia, M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. [Google Scholar] [CrossRef]
- Bais, H.P.; Walker, T.S.; Schweizer, H.P.; Vivanco, J.M. Root specific elicitation and antimicrobial activity of rosmarinic acid in hairy root cultures of Ocimum basilicum. Plant Physiol. Biochem. 2002, 40, 983–995. [Google Scholar] [CrossRef]
- Shukla, R.; Singh, P.; Prakash, B.; Anuradha; Dubey, N.K. Antifungal, Aflatoxin Inhibitory and Free Radical-Scavenging Activities of Some Medicinal Plants Extracts. J. Food Qual. 2012, 35, 182–189. [Google Scholar] [CrossRef]
- Teodoro, G.R.; Ellepola, K.; Seneviratne, C.J.; Koga-Ito, C.Y. Potential Use of Phenolic Acids as Anti-Candida Agents: A Review. Front. Microbiol. 2015, 6, 1420. [Google Scholar] [CrossRef] [PubMed]
- Grayer, R.J.; Harborne, J.B. A survey of antifungal compounds from higher plants, 1982–1993. Phytochemistry 1994, 37, 19–42. [Google Scholar] [CrossRef]
- Saha, P.; Rahman, F.I.; Hussain, F.; Rahman, S.M.A.; Rahman, M.M. Antimicrobial Diterpenes: Recent Development From Natural Sources. Front. Pharmacol. 2022, 12, 820312. [Google Scholar] [CrossRef]
- Abdissa, N.; Frese, M.; Sewald, N. Antimicrobial Abietane-Type Diterpenoids from Plectranthus punctatus. Molecules 2017, 22, 1919. [Google Scholar] [CrossRef] [PubMed]
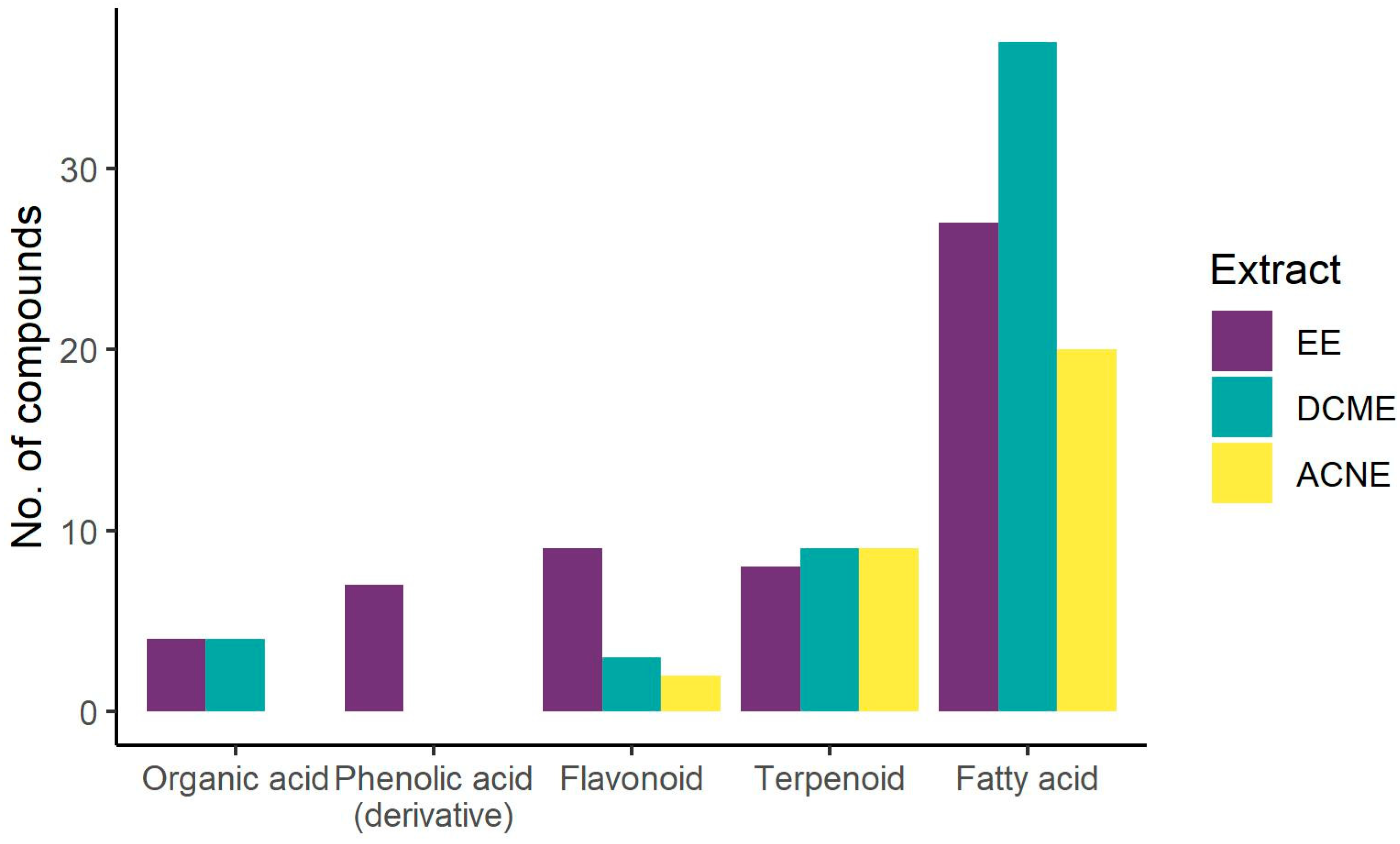
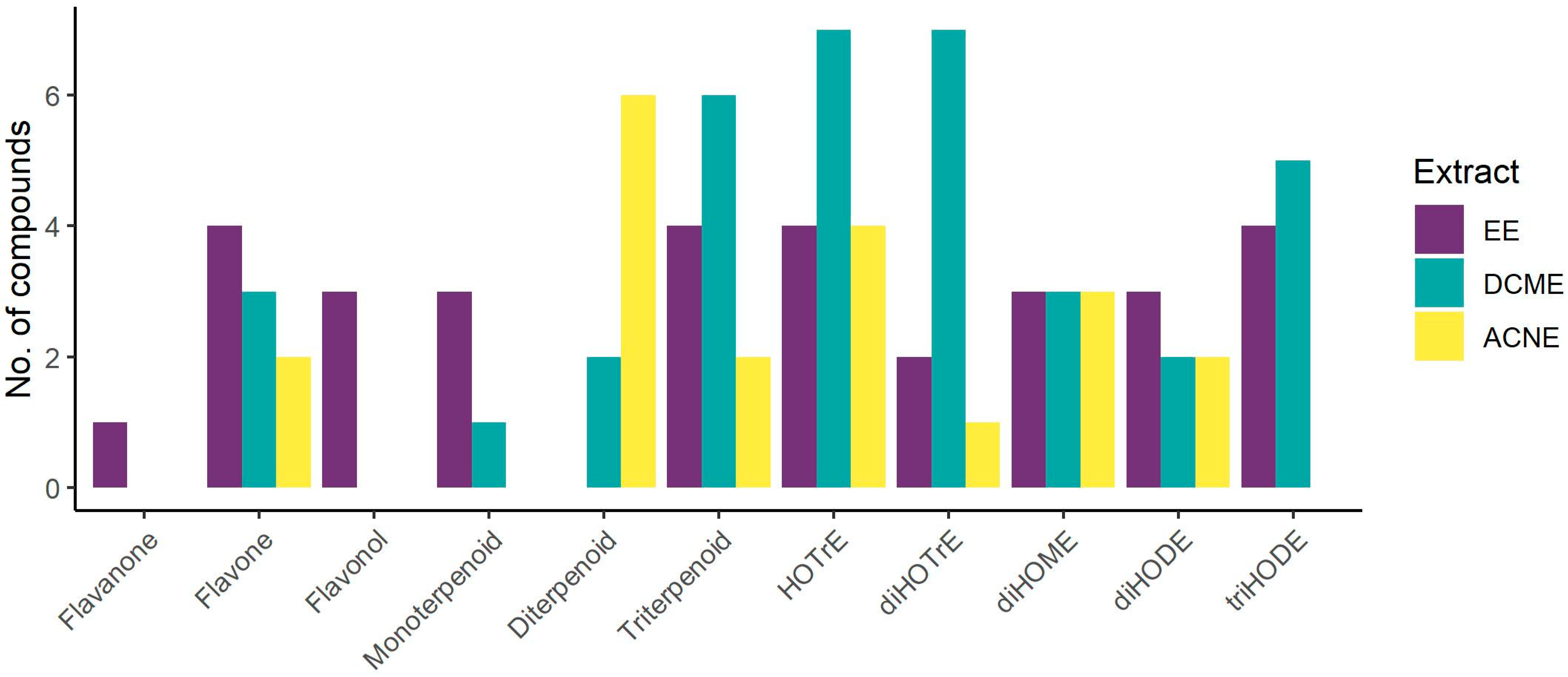
| No | Compound Class/Name | CAS Registry Number | Molecular Formula | Ion Species | Pseudomolecular Ion (m/z) | Rt (min) | UV Maximum (nm) | Extract | Reference | |
|---|---|---|---|---|---|---|---|---|---|---|
| ESI– | LC-DAD | |||||||||
| Organic acid | ||||||||||
| 1 | Succinic acid | 110-15-6 | C4H6O4 | [M−H]− | 117.0205 | 2.70 | 2.62 | 192, 218, 234 | EE | [27] |
| 2 | Benzoic acid | 65-85-0 | C7H6O2 | [M−H]− | 121.0297 | 7.78 | 7.66 | 232, 284 | EE, DCME | [28] |
| 3 | 12-Hydroxyjasmonic acid | 140631-27-2 | C12H18O4 | [M−H]− [M+HCO2]− [2M−H]− | 225.1141 271.1216 451.2346 | 8.00 | n.a. 1 | n.a. | EE, DCME | [29] |
| 4 | Cinnamic acid | 621-82-9 | C9H8O2 | [M−H]− | 147.0454 | 11.58 | n.a. | n.a. | DCME | [17] |
| 5 | Azelaic acid | 123-99-9 | C9H16O4 | [M−H]− | 187.0982 | 13.50 | n.a. | n.a. | EE, DCME | [30] |
| Phenolic acid (derivative) | ||||||||||
| 6 | Protocatechuic acid | 99-50-3 | C7H6O4 | [M−H]− [2M−H]− | 153.0195 307.0498 | 4.57 | 4.49 | 232, 258, 296 | EE | [31] |
| 7 | 4-Hydroxybenzoic acid | 99-96-7 | C7H6O3 | [M−H]− [2M−H]− | 137.0245 275.0607 | 5.80 | 5.70 | 238, 280, 310 | EE | [31] |
| 8 | Caffeic acid | 331-39-5 | C9H8O4 | [M−H]− | 179.0354 | 7.16 | 7.06 | 244, 296sh, 324 | EE | [32] |
| 9 | p-Coumaric acid | 7400-08-0 | C9H8O3 | [M−H]− | 163.0372 | 9.41 | 9.28 | 296sh, 310 | EE | [14] |
| 10 | Rosmarinic acid | 20283-92-5 | C18H16O8 | [M−H]− [M+HCO2]− [2M−H]− | 359.0783 405.0845 719.1621 | 15.53 | 15.35 | 234, 288, 330 | EE | [33] |
| 11 | Salvianolic acid A | 96574-01-5 | C26H22O10 | [M−H]− | 493.1158 | 15.88 | n.a. | n.a. | EE | [34] |
| 12 | Salvianolic acid B Salvianolic acid E Salvianolic acid L | 121521-90-2 142998-46-7 389065-74-1 | C36H30O16 | [M+CF3CO2]− | 717.1473 | 18.91 | n.a. | n.a. | EE | [14] [35] [36] |
| Flavonoid | ||||||||||
| flavanone | ||||||||||
| 13 | Eriocitrin | 13463-28-0 | C27H32O15 | [M−H]− [M+HCO2]− [2M−H]− | 595.1514 641.1612 1191.3053 | 9.20 | n.a. | n.a. | EE | [37] |
| flavone | ||||||||||
| 14 | Vicenin 2 | 23666-13-9 | C27H30O15 | [M−H]− [M+HCO2]− | 593.1525 639.1586 | 13.16 | n.a. | n.a. | EE | [38] |
| 15 | Apigenin | 520-36-5 | C15H10O5 | [M−H]− | 269.0466 | 25.64 | 25.30 | 232, 266, 336 | EE | [33] |
| 16 | Cirsimaritin Pectolinarigenin Ladanein | 6601-62-3 520-12-7 10176-71-3 | C17H14O6 | [M−H]− | 313.0729 | 28.12 | 27.56 | 230, 258, 268, 280 | EE, DCME, ACNE | [39] [40] [39] |
| 17 | Cirsilineol Eupatorin Xanthomicrol Nevadensin 5,8-Dihydroxy-4′,6,7-trimethoxy-flavone (7CI) | 41365-32-6 855-96-9 16545-23-6 10176-66-6 2798-22-3 | C18H16O7 | [M−H]− | 343.0841 | 29.60 | n.a. | n.a. | DCME | [41] [39] [42] [39] [41] |
| 18 | Acacetin Biochanin Genkwanin Negletein | 480-44-4 491-80-5 437-64-9 29550-13-8 | C16H12O5 | [M−H]− | 283.0623 | 29.76 | 29.26 | 230, 282, 330 | EE, DCME | [39] [43] [39] [44] |
| 19 | Cirsilineol Eupatorin Xanthomicrol Nevadensin 5,8-Dihydroxy-4′,6,7-trimethoxy-flavone (7CI) | 41365-32-6 855-96-9 16545-23-6 10176-66-6 2798-22-3 | C18H16O7 | [M−H]− | 343.0824 | 29.91 | n.a. | n.a. | ACNE | [41] [39] [42] [39] [41] |
| flavonol | ||||||||||
| 20 | Rutin | 153-18-4 | C27H30O16 | [M−H]− [M+HCO2]− [2M−H]− | 609.1468 655.1522 1219. 2982 | 10.32 | 10.20 | 354, 266sh, 298sh, 352 | EE | [33] |
| 21 | Kaempferol 3-O-rutinoside | 17650-84-9 | C27H30O15 | [M−H]− [2M−H]− | 593.1525 1187.3085 | 10.36 | n.a. | n.a. | EE | [33] |
| 22 | Quercetin 3-glucoside Hyperoside Quercetin 7-O-glucoside Quercetin 3-O-hexoside Isoquercitrin | 482-35-9 482-36-0 491-50-9 21637-25-2 905846-12-0 | C21H20O12 | [M−H]− [M+HCO2]− [2M−H]− | 463.0895 509.0958 927.1835 | 11.27 | 11.11 | 256, 264sh, 296sh, 352 | EE | [45] [45] [43] [46] [47] |
| flavone/flavonol | ||||||||||
| 23 | Astragalin Quercitrin Luteolin 7-O-glucoside Luteolin 4′-O-glucoside Galuteolin Orientin | 480-10-4 522-12-3 5373-11-5 6920-38-3 20344-46-1 28608-75-5 | C21H20O11 | [M−H]− [2M−H]− | 447.0948 895.1926 | 14.50 | 14.31 | 232, 266, 288, 342 | EE | [43] [48] [20] [49] [50] [20] |
| Terpenoid | ||||||||||
| monoterpenoid | ||||||||||
| 24 | 2-[2-(β-D-Glucopyra- nosyloxy)-1-meth- ylethyl]-5-methyl-cy- clohexanone [2R- [2α(R*),5β]]-(9CI) (3S,6S)-6-Ethenyltetra- hydro-2,2,6-trimethyl- 2H-pyran-3-yl β-D-glu- copyranoside (ACI) p-Menth-1-ene-3,4-diol 4-O-β-glucopyranoside Betulalbuside A (1S,4R,5S)-1,3,3-Trime- thyl-2-oxabicyclo [2.2.2]oct-5-yl β-D-glu- copyranoside (ACI) (1R,2S,4R,5S)-5-Hy- droxy-1,3,3-trime- thylbicyclo [2.2.1]hept- 2-yl β-D-glucopyra- noside (ACI) (1R,4S,5S)-1,3,3-Trime- thyl-2-oxabicyclo [2.2.2]oct-5-yl β-D-glu- copyranoside (ACI) (1S,2S,4R)-2-Hydroxy- 1,8-cineole β-D-gluco- pyranoside | 78916-66-2 174760-79-3 403613-11-6 64776-96-1 2104786-86-7 217960-83-3 155836-27-4 113270-15-8 | C16H28O7 | [M+HCO2]− | 377.1829 | 8.32 | n.a. | n.a. | EE | [51] [52] [53] [54] [55] [56] [57] [58] |
| 25 | 2-[2-(β-D-Glucopyra- nosyloxy)-1-meth- ylethyl]-5-methyl-cy- clohexanone [2R- [2α(R*),5β]]-(9CI) (3S,6S)-6-Ethenyltetra- hydro-2,2,6-trimethyl- 2H-pyran-3-yl β-D-glu- copyranoside (ACI) p-Menth-1-ene-3,4-diol 4-O-β-glucopyranoside Betulalbuside A (1S,4R,5S)-1,3,3-Trime- thyl-2-oxabicyclo [2.2.2]oct-5-yl β-D-glu- copyranoside (ACI) (1R,2S,4R,5S)-5-Hy- droxy-1,3,3-trime- thylbicyclo [2.2.1]hept- 2-yl β-D-glucopyra- noside (ACI) (1R,4S,5S)-1,3,3-Trime- thyl-2-oxabicyclo [2.2.2]oct-5-yl β-D-glu- copyranoside (ACI) (1S,2S,4R)-2-Hydroxy- 1,8-cineole β-D-gluco- pyranoside | 78916-66-2 174760-79-3 403613-11-6 64776-96-1 2104786-86-7 217960-83-3 155836-27-4 113270-15-8 | C16H28O7 | [M+HCO2]− | 377.1826 | 9.17 | n.a. | n.a. | EE | [51] [52] [53] [54] [55] [56] [57] [58] |
| 26 | (-)-α-Terpineol 8-O-β-D-glucopyranoside Geranyl glucoside Neryl glucoside Linalool glucoside | 89616-07-9 22850-13-1 22850-14-2 82928-12-9 | C16H28O6 | [M+HCO2]− | 361.1878 | 22.70 | n.a. | n.a. | EE, DCME | [59] [60] [61] [58] |
| sesquiterpenoid | ||||||||||
| 27 | Roseoside | 54835-70-0 | C19H30O8 | [M+HCO2]− | 431.1936 | 6.69 | n.a. | n.a. | EE | [45] |
| diterpenoid | ||||||||||
| 28 | Carnosic acid | 3650-09-7 | C20H28O4 | [M−H]− | 331.1919 | 29.65 | n.a. | n.a. | ACNE | [20] |
| 29 | 15-Nor-14-oxolabda-8(17),12E-diene-18-oic acid ent-15-Nor-14-oxolabda-8(17),12E-dien-18-oic acid | 1039673-32-9 81920-05-0 | C19H28O3 | [M−H]− | 303.1957 | 30.55 | n.a. | n.a. | ACNE | [62] [63] |
| 30 | Royleanone | 6812-87-9 | C20H28O3 | [M−H]− | 315.1975 | 32.25 | n.a. | n.a. | ACNE | [64] |
| 31 | Bodinieric acid A | 2227130-30-3 | C19H24O4 | [M−H]− | 315.1616 | 33.43 | n.a. | n.a. | DCME | [65] |
| 32 | Odonicin | 51419-51-3 | C24H30O7 | [M−H]− | 429.1914 | 33.76 | n.a. | n.a. | ACNE | [66] |
| 33 | Lagopsin C 15-epi-Lagopsin C Lagopsin D 15-epi-Lagopsin D Sideripullol C | 1590387-64-6 1590387-65-7 1590387-66-8 1590387-67-9 1621480-82-7 | C22H36O6 | [M−H]− | 395.2454 | 34.29 | 33.60 | 258sh, 276 | DCME | [67] [67] [67] [67] [68] |
| 34 | 1,4-Phenanthrenedione, 10-butoxy-4b,5,6,7,8,8a,9,10-octahydro-3-hydroxy-4b,8,8-trimethyl-2-(1-methylethyl)-, (4bS,8aS,10R)-(ACI) | 3024059-23-9 | C24H36O4 | [M−H]− | 387.2531 | 36.11 | n.a. | n.a. | ACNE | [69] |
| 35 | Palustric acid | 1945-53-5 | C20H30O2 | [M−H]− | 301.2168 | 36.47 | n.a. | n.a. | ACNE | [70] |
| sesterpenoid | ||||||||||
| 36 | Leucosceptroid B (1R,3S,3aR,4aS,5S,7aS,8S,8aR)-Decahydro-8a-hydroxy-3,5,8-trimethyl-3-[2-(3-methyl-2-furanyl)ethyl]-1-(2-methyl-1-propen-1-yl)-4H-cyclopent[f]isobenzofuran-4-one (ACI) | 1239975-37-1 1443528-32-2 | C25H36O4 | [M+HCO2]− | 399.2555 | 36.81 | n.a. | n.a. | ACNE | [71] [72] |
| triterpenoid | ||||||||||
| 37 | Vitexnegheteroin H Sanguic acid | 2173172-57-9 821797-62-0 | C30H46O7 | [M−H]− [2M−H]− | 517.3185 1035; 6401 | 28.68 | n.a. | n.a. | EE | [73] [74] |
| 38 | Madecassic acid | 18449-41-7 | C30H48O6 | [M−H]− [2M−H]− | 503.3388 1007; 6836 | 28.81 | n.a. | n.a. | EE, DCME | [75] |
| 39 | Euscaphic acid Tormentic acid | 53155-25-2 13850-16-3 | C30H48O5 | [M−H]− | 487.3441 | 30.90 | 30.62 | 196, 210, 218, 228 | EE, DCME | [76] [77] |
| 40 | Euscaphic acid Tormentic acid | 53155-25-2 13850-16-3 | C30H48O5 | [M−H]− [M+HCO2]− | 487.3426 533.3427 | 30.98 | n.a. | n.a. | ACNE | [76] [77] |
| 41 | Alphitolic acid Pomolic acid 3-epi-Maslinic acid | 19533-92-7 13849-91-7 26563-68-8 | C30H48O4 | [M−H]− | 471.3491 | 31.02 | n.a. | n.a. | DCME | [76,77] [77] [76] |
| 42 | Glycyrrhetinic acid | 471-53-4 | C30H46O4 | [M−H]− | 469.3339 | 33.98 | n.a. | n.a. | DCME | [78] |
| 43 | Alphitolic acid Pomolic acid 3-epi-Maslinic acid | 19533-92-7 13849-91-7 26563-68-8 | C30H48O4 | [M−H]− | 471.3493 | 34.48 | 33.74 | 206, 212, 216sh, 280 | EE, DCME | [76,77] [77] [76] |
| 44 | Alphitolic acid Pomolic acid 3-epi-Maslinic acid | 19533-92-7 13849-91-7 26563-68-8 | C30H48O4 | [M−H]− | 471.3465 | 34.70 | n.a. | n.a. | ACNE | [76,77] [77] [76] |
| 45 | Oleanolic acid (+)-Ursolic acid 3-epi-Ursolic acid Betulinic acid | 508-02-1 77-52-1 989-30-0 472-15-1 | C30H48O3 | [M−H]− | 455.3546 | 37.45 | n.a. | n.a. | DCME | [79] [20,33] [80] [20] |
| Fatty acid | ||||||||||
| 46 | FA 2 18:2;O3 | C18H32O5 | [M−H]− [M+HCO2]− | 327.2188 373.2204 | 25.33 | 24.98 | 234, 292 | EE, DCME | ||
| 47 | FA 18:2;O3 | C18H32O5 | [M−H]− | 327.2187 | 25.64 | n.a. | n.a. | EE, DCME | ||
| 48 | FA 18:2;O3 | C18H32O5 | [M−H]− | 327.2181 | 25.84 | n.a. | n.a. | EE, DCME | ||
| 49 | FA 18:2;O3 | C18H32O5 | [M−H]− | 327.2191 | 26.20 | 25.60 | 250 | EE, DCME | ||
| 50 | FA 18:1;O3 | C18H34O5 | [M−H]− | 329.2345 | 26.52 | 26.31 | 234, 282 | EE, DCME | ||
| 51 | FA 18:2;O3 | C18H32O5 | [M−H]− | 327.2192 | 26.90 | n.a. | n.a. | DCME | ||
| 52 | FA 16:0;O2 | C16H32O4 | [M−H]− | 287.2241 | 27.40 | 27.26 | 232, 258, 268, 278 | DCME | ||
| 53 | FA 18:3;O2 | C18H30O4 | [M−H]− | 309.2080 355.2159 | 27.88 | 27.57 | 260, 268, 278 | DCME | ||
| 54 | FA 18:3;O2 | C18H30O4 | [M−H]− | 309.2080 355.2159 | 28.06 | 27.74 | 258, 268, 278 | DCME | ||
| 55 | FA 18:3;O3 | C18H30O5 | [M−H]− | 325.2031 | 28.28 | n.a. | n.a. | EE, DCME | ||
| 56 | FA 18:4;O2 | C18H28O4 | [M−H]− [M+HCO2]− [2M−H]− | 307.1925 353.1996 615.3859 | 28.47 | 28.15 | 314 | EE, DCME | ||
| 57 | FA 18:4;O2 | C18H28O4 | [M−H]− | 307.1925 | 28.64 | 28.28 | 254sh, 320 | EE, DCME | ||
| 58 | FA 18:3;O4 | C18H30O6 | [M−H]− | 341.1982 | 28.87 | n.a. | n.a. | EE | ||
| 59 | FA 17:3;O3 FA 16:3;O | C17H28O5 C16H26O3 | [M−H]− [M+HCO2]− | 311.1878 | 29.04 | 28.69 | 264, 280, 334 | DCME | ||
| 60 | FA 18:3;O3 | C18H30O5 | [M−H]− [M+HCO2]− | 325.2035 371.2107 | 29.22 | n.a. | n.a. | DCME | ||
| 61 | FA 18:3;O2 | C18H30O4 | [M−H]− | 309.2081 | 29.42 | n.a. | n.a. | DCME | ||
| 62 | FA 18:3;O2 | C18H30O4 | [M−H]− [M+HCO2]− | 309.2080 355.2110 | 29.58 | n.a. | n.a. | DCME | ||
| 63 | FA 18:3;O2 | C18H30O4 | [M−H]− [M+HCO2]− | 309.2080 355.2122 | 29.80 | n.a. | n.a. | EE, DCME | ||
| 64 | FA 18:3;O2 | C18H30O4 | [M−H]− | 309.2069 | 29.80 | n.a. | n.a. | ACNE | ||
| 65 | FA 18:2;O2 | C18H32O4 | [M−H]− [M+HCO2]− | 311.2238 357.2308 | 29.94 | 29.44 | 262sh, 270, 282sh, 338 | EE, DCME | ||
| 66 | FA 18:2;O2 | C18H32O4 | [M−H]− | 311.2225 | 30.13 | n.a. | n.a. | ACNE | ||
| 67 | FA 18:2;O4 | C18H32O6 | [M−H]− | 343.2143 | 30.31 | n.a. | n.a. | DCME | ||
| 68 | FA 18:3;O2 | C18H30O4 | [M−H]− | 309.1802 | 30.62 | n.a. | n.a. | DCME | ||
| 69 | FA 18:3;O3 | C18H30O5 | [M−H]− | 325.2017 | 30.66 | n.a. | n.a. | ACNE | ||
| 70 | FA 18:1;O2 | C18H34O4 | [M−H]− | 313.2379 | 30.90 | n.a. | n.a. | ACNE | ||
| 71 | FA 18:1;O2 | C18H34O4 | [M−H]− | 313.2396 | 31.15 | n.a. | n.a. | EE, DCME | ||
| 72 | FA 18:1;O2 | C18H34O4 | [M−H]- [M+HCO2]− | 313.2373 359.2435 | 31.37 | 30.91 | 282 | ACNE | ||
| 73 | FA 18:1;O2 | C18H34O4 | [M−H]− | 313.2381 | 32.15 | n.a. | n.a. | ACNE | ||
| 74 | FA 18:3;O | C18H30O3 | [M−H]− [M+HCO2]− | 293.2124 339.2205 | 32.18 | 31.56 | 276, 332, 410 | EE, DCME | ||
| 75 | FA 18:3;O | C18H30O3 | [M−H]− | 293.2112 | 32.31 | 31.71 | 230, 278, 286sh | DCME | ||
| 76 | FA 18:3;O | C18H30O3 | [M−H]− | 293.2113 | 32.46 | n.a. | n.a. | ACNE | ||
| 77 | FA 18:3;O2 | C18H30O4 | [M−H]− [M+HCO2]− [2M−H]− | 309.2082 355.2160 619.4192 | 32.55 | 31.90 | 200, 218sh, 232 | EE, DCME | ||
| 78 | FA 18:0;O2 | C18H36O4 | [M−H]− [M+HCO2]− | 315.2528 361.2556 | 32.64 | n.a. | n.a. | DCME, ACNE | ||
| 79 | FA 18:1;O3 | C18H34O5 | [M−H]− | 329.1906 | 33.05 | 32.45 | 202, 212, 220 | EE | ||
| 80 | FA 18:3;O | C18H30O3 | [M−H]− | 293.2112 | 33.06 | n.a. | n.a. | DCME | ||
| 81 | FA 18:2;O | C18H32O3 | [M−H]− [M+HCO2]− | 295.2294 341.2336 | 33.36 | 32.63 | 280, 414 | EE, DCME | ||
| 82 | FA 18:1;O3 | C18H34O5 | [M−H]− | 329.1889 | 33.36 | n.a. | n.a. | ACNE | ||
| 83 | FA 18:2;O2 | C18H32O4 | [M−H]− | 311.2241 | 33.52 | n.a. | n.a. | EE | ||
| 84 | FA 18:3;O | C18H30O3 | [M−H]− | 293.2133 | 33.55 | n.a. | n.a. | EE, DCME | ||
| 85 | FA 18:2;O2 | C18H32O4 | [M−H]− | 311.2241 | 33.61 | n.a. | n.a. | DCME | ||
| 86 | FA 18:2;O | C18H32O3 | [M−H]− | 295.2271 | 33.65 | n.a. | n.a. | ACNE | ||
| 87 | FA 18:2;O | C18H32O4 | [M−H]− | 311.2242 | 33.66 | 33.04 | 208, 214, 220sh | EE | ||
| 88 | FA 18:3;O | C18H30O3 | [M−H]− | 293.2135 | 33.83 | 33.05 | 234, 268, 326 | DCME | ||
| 89 | FA 18:2;O2 | C18H32O4 | [M−H]− | 311.2227 | 33.90 | n.a. | n.a. | ACNE | ||
| 90 | FA 18:3;O | C18H30O3 | [M−H]− | 293.2133 | 34.10 | 33.43 | 204, 216, 280 | EE, DCME, ACNE | ||
| 91 | FA 18:3;O | C18H30O3 | [M−H]- [M+HCO2]− | 293.2116 339.2167 | 34.34 | n.a. | n.a. | ACNE | ||
| 92 | FA 18:1;O | C18H34O3 | [M−H]− | 297.2429 | 34.37 | n.a. | n.a. | ACNE | ||
| 93 | FA 18:1;O | C18H34O3 | [M−H]− | 297.2449 | 34.55 | n.a. | n.a. | DCME | ||
| 94 | FA 18:1;O2 | C18H34O4 | [M−H]− [M+HCO2]− | 313.2402 359.2452 | 34.65 | n.a. | n.a. | EE, DCME | ||
| 95 | FA 18:3;O | C18H30O3 | [M−H]− [M+HCO2]− | 293.2115 339.2178 | 34.75 | 34.36 | 276 | EE, DCME, ACNE | ||
| 96 | FA 18:1;O2 | C18H34O4 | [M−H]− | 313.2401 | 34.82 | 34.04 | 276 | EE, DCME | ||
| 97 | FA 18:2;O | C18H32O3 | [M−H]− | 295.2271 | 34.91 | n.a. | n.a. | ACNE | ||
| 98 | FA 18:2;O | C18H32O3 | [M−H]− | 295.2268 | 35.15 | n.a. | n.a. | ACNE | ||
| 99 | FA 18:1;O | C18H34O3 | [M−H]− | 297.2429 | 36.32 | n.a. | n.a. | ACNE | ||
| 100 | FA 18:3 | C18H30O2 | [M−H]− [M+HCO2]− | 277.2194 323.2194 | 36.47 | n.a. | n.a. | EE, DCME | ||
| 101 | FA 18:3 | C18H30O2 | [M−H]− [M+HCO2]− | 277.2166 323.2261 | 36.80 | n.a. | n.a. | ACNE | ||
| 102 | FA 18:2 | C18H32O2 | [M−H]− [M+HCO2]− | 279.2323 325.2383 | 37.83 | n.a. | n.a. | ACNE | ||
| 103 | Undecanedioic acid | 1852-04-6 | C11H20O4 | [M−H]− | 215.1295 | 24.94 | 24.62 | 202, 208, 214, 232 | EE, DCME | [81] |
| Glycosylmonoacylglycerol | ||||||||||
| 104 | Gingerglycolipid A | 145937-22-0 | C33H56O14 | [M−H]− [M+HCO2]− [2M−H]− | 675.3613 721.3657 1351.7256 | 31.32 | n.a. | n.a. | EE | [82] |
| 105 | Panaxcerol B | 171520-42-6 | C27H46O9 | [M−H]− | 513.3079 559.3137 | 33.07 | n.a. | n.a. | EE, DCME | [82] |
| Monoacylglycerol | ||||||||||
| 106 | 2-Monolinolein | 3443-82-1 | C21H38O4 | [M−H]− | 353.2718 | 37.16 | n.a. | n.a. | ACNE | [83] |
| Coumarin | ||||||||||
| 107 | Esculetin | 305-01-1 | C9H6O4 | [M−H]− | 177.0197 | 6.92 | n.a. | n.a. | EE | [38] |
| Xanthone | ||||||||||
| 108 | Moreollic acid | 173792-68-2 | C34H40O9 | [M−H]− [2M−H]− | 591.2630 1183.5283 | 35.62 | n.a. | n.a. | DCME | [83] |
| Neolignan | ||||||||||
| 109 | Dehydrodieugenol B | 75225-33-1 | C20H22O4 | [M−H]− | 325.1442 | 32.89 | n.a. | n.a. | ACNE | [84] |
| EE | DCME | ACNE | OE | BHT (Methanol) | BHT (Toluene) | |
|---|---|---|---|---|---|---|
| EC50 1 (mg/mL) | 1.73 ± 0.06 | 9.55 ± 0.15 | 15.92 ± 0.31 | 52.69 ± 1.39 | 0.33 ± 0.01 | 1.42 ± 0.01 |
| EE | DCME | ACNE | OE | |
|---|---|---|---|---|
| BHTE 1 | 189.27 ± 6.61 a | 149.19 ± 1.71 b | 89.49 ± 1.73 c | 27.05 ± 0.72 d |
| Escherichia coli | Pseudomonas aeruginosa | Proteus hauseri | Klebsiella pneumoniae | Salmonella enterica subsp. enterica | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| mg/mL | mg/mL | mg/mL | mg/mL | mg/mL | ||||||
| EE | 2.5 | >10 | 0.313 | 1.25 | 2.5 | >10 | 0.625 | 2.5 | 0.625 | 2.5 |
| DCME | / | / | / | / | / | / | / | / | / | / |
| ACNE | 2.5 | >10 | 1.25 | 5 | 0.313 | 1.25 | 0.625 | 1.25 | 1.25 | 5 |
| OE | 1.25 | 10 | 1.25 | 5 | 2.5 | 10 | 2.5 | 10 | 0.625 | 2.5 |
| Chloramphenicol | 0.062 | 0.25 | 0.125 | 0.062 | 0.125 | |||||
| Staphylococcus aureus | Bacillus subtilis | Clostridium sporogenes | ||||
|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | |
| mg/mL | mg/mL | mg/mL | ||||
| EE | 0.625 | 2.5 | 1.25 | 10 | 0.625 | 2.5 |
| DCME | / | / | / | / | / | / |
| ACNE | 0.313 | 1.25 | 1.25 | 5 | 2.5 | 10 |
| OE | 2.5 | 10 | 2.5 | 10 | 2.5 | 10 |
| Chloramphenicol | 0.015 | 0.015 | 0.25 | |||
| Aspergillus brasiliensis | Saccharomyces cerevisiae | Candida albicans | ||||
|---|---|---|---|---|---|---|
| MIC | MFC | MIC | MFC | MIC | MFC | |
| mg/mL | mg/mL | mg/mL | ||||
| EE | 1.25 | >10 | 1.25 | 10 | 2.5 | 5 |
| DCME | 1.25 | 5 | 1.25 | 5 | 2.5 | 5 |
| ACNE | 1.25 | 5 | 1.25 | 5 | 2.5 | 5 |
| OE | 1.25 | 10 | 1.25 | 5 | 2.5 | 5 |
| Nystatin | 2.5 | 1.25 | 2.5 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vidaković, V.; Vujić, B.; Jadranin, M.; Novaković, I.; Trifunović, S.; Tešević, V.; Mandić, B. Qualitative Profiling, Antioxidant and Antimicrobial Activities of Polar and Nonpolar Basil Extracts. Foods 2024, 13, 2993. https://doi.org/10.3390/foods13182993
Vidaković V, Vujić B, Jadranin M, Novaković I, Trifunović S, Tešević V, Mandić B. Qualitative Profiling, Antioxidant and Antimicrobial Activities of Polar and Nonpolar Basil Extracts. Foods. 2024; 13(18):2993. https://doi.org/10.3390/foods13182993
Chicago/Turabian StyleVidaković, Vera, Bojan Vujić, Milka Jadranin, Irena Novaković, Snežana Trifunović, Vele Tešević, and Boris Mandić. 2024. "Qualitative Profiling, Antioxidant and Antimicrobial Activities of Polar and Nonpolar Basil Extracts" Foods 13, no. 18: 2993. https://doi.org/10.3390/foods13182993






