Genetic Profile and Toxigenic Potential of Bacillus cereus Isolates from a Norwegian Ice Cream Production Plant
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains and Growth Conditions
2.2. Genome Sequencing and Phylogenetic Analysis
2.3. Typing, Virulome Determination, and Clustering
2.4. PCR
2.5. Growth Curves
2.6. Cereulide Production in Ice Cream Matrix
2.7. Quantification of Cereulide by LC-MS/MS
2.8. Statistics
3. Results
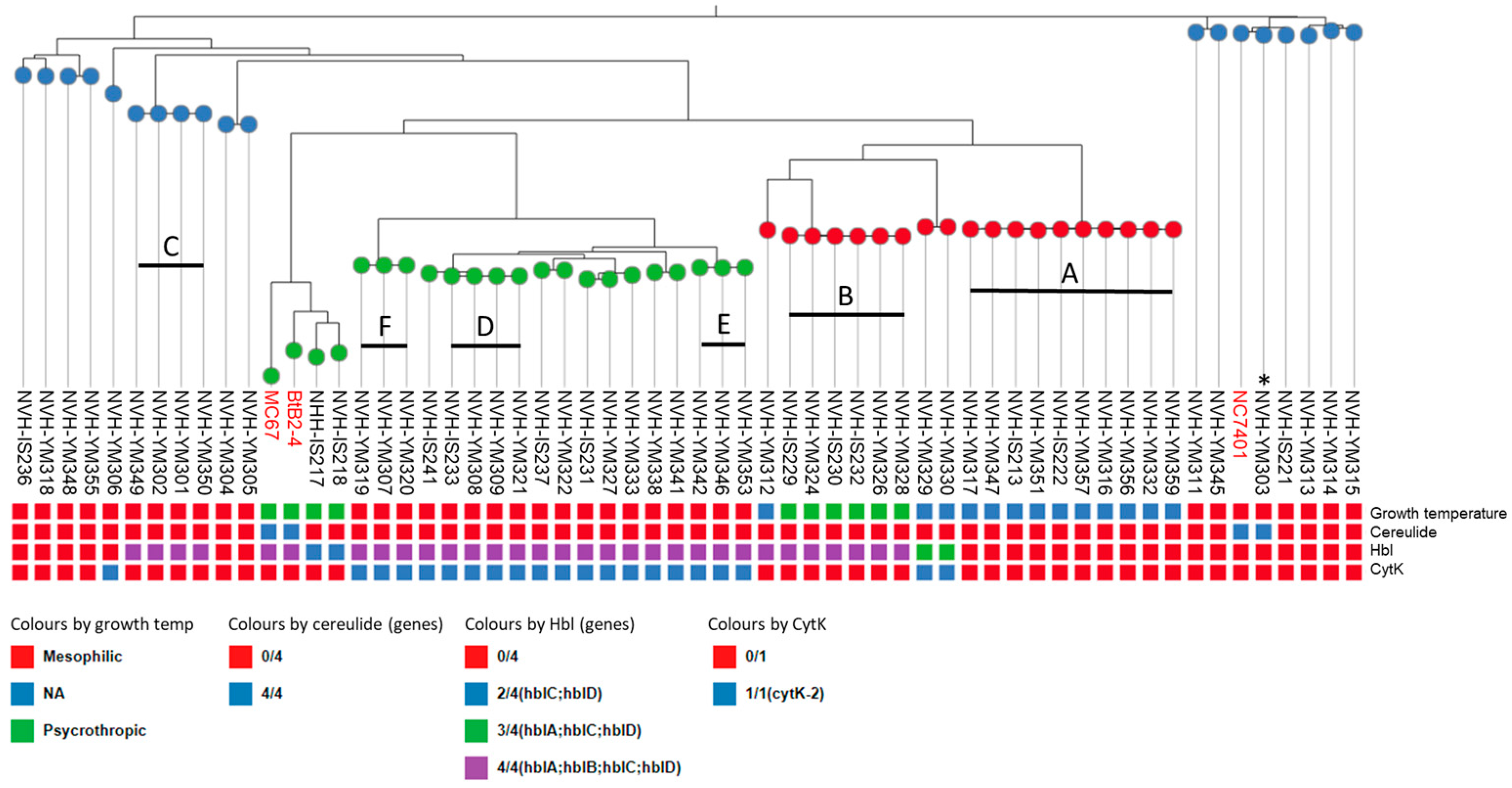
3.1. Genetic Characterization of B. cereus Ice Cream Isolates
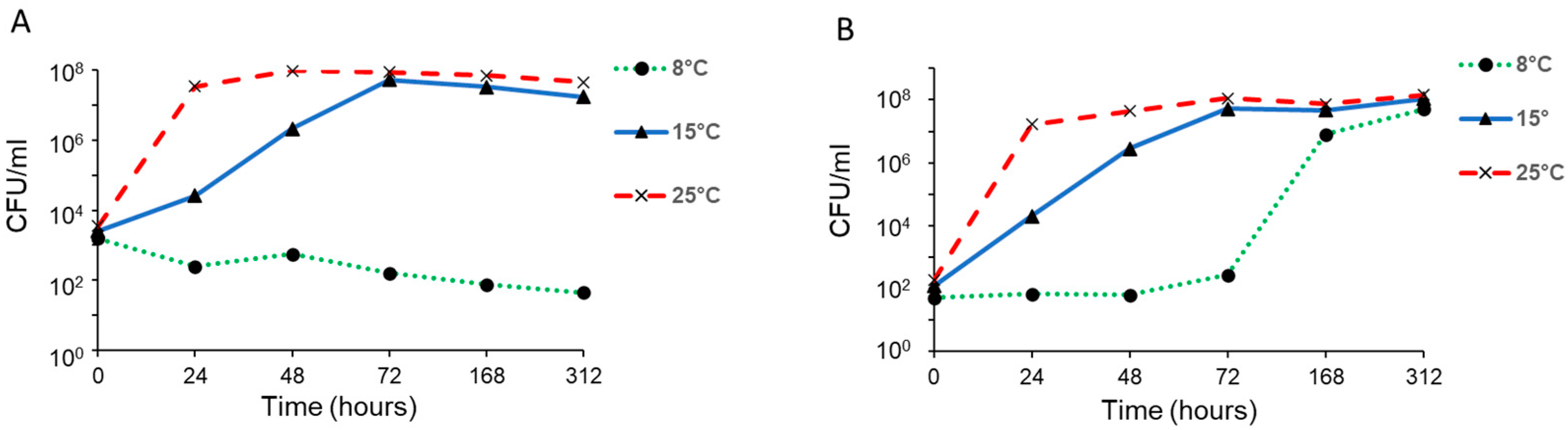
3.2. Toxigenic Potential and Temperature Requirements of Isolates
3.3. Growth in Milk
3.4. Cereulide Production in Milk and Ice Cream Mixture
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kable, M.E.; Srisengfa, Y.; Laird, M.; Zaragoza, J.; McLeod, J.; Heidenreich, J.; Marco, M.L. The Core and Seasonal Microbiota of Raw Bovine Milk in Tanker Trucks and the Impact of Transfer to a Milk Processing Facility. mBio 2016, 7, e00836-16. [Google Scholar] [CrossRef] [PubMed]
- Porcellato, D.; Aspholm, M.; Skeie, S.B.; Monshaugen, M.; Brendehaug, J.; Mellegård, H. Microbial diversity of consumption milk during processing and storage. Int. J. Food Microbiol. 2018, 266, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Quigley, L.; McCarthy, R.; O’Sullivan, O.; Beresford, T.P.; Fitzgerald, G.F.; Ross, R.P.; Stanton, C.; Cotter, P.D. The microbial content of raw and pasteurized cow milk as determined by molecular approaches. J. Dairy Sci. 2013, 96, 4928–4937. [Google Scholar] [CrossRef] [PubMed]
- Beecher, D.J.; Schoeni, J.L.; Wong, A.C. Enterotoxic activity of hemolysin BL from Bacillus cereus. Infect. Immun. 1995, 63, 4423–4428. [Google Scholar] [CrossRef]
- Lund, T.; Granum, P.E. Characterisation of a non-haemolytic enterotoxin complex from Bacillus cereus isolated after a foodborne outbreak. FEMS Microbiol. Lett. 1996, 141, 151–156. [Google Scholar] [CrossRef]
- Lund, T.; De Buyser, M.L.; Granum, P.E. A new cytotoxin from Bacillus cereus that may cause necrotic enteritis. Mol. Microbiol. 2000, 38, 254–261. [Google Scholar] [CrossRef]
- Agata, N.; Ohta, M.; Mori, M.; Isobe, M. A novel dodecadepsipeptide, cereulide, is an emetic toxin of Bacillus cereus. FEMS Microbiol. Lett. 1995, 129, 17–20. [Google Scholar] [CrossRef]
- Dierick, K.; Van Coillie, E.; Swiecicka, I.; Meyfroidt, G.; Devlieger, H.; Meulemans, A.; Hoedemaekers, G.; Fourie, L.; Heyndrickx, M.; Mahillon, J. Fatal family outbreak of Bacillus cereus-associated food poisoning. J. Clin. Microbiol. 2005, 43, 4277–4279. [Google Scholar] [CrossRef]
- Mahler, H.; Pasi, A.; Kramer, J.M.; Schulte, P.; Scoging, A.C.; Bär, W.; Krähenbühl, S. Fulminant liver failure in association with the emetic toxin of Bacillus cereus. N. Engl. J. Med. 1997, 336, 1142–1148. [Google Scholar] [CrossRef]
- Naranjo, M.; Denayer, S.; Botteldoorn, N.; Delbrassinne, L.; Veys, J.; Waegenaere, J.; Sirtaine, N.; Driesen, R.B.; Sipido, K.R.; Mahiilon, J.; et al. Sudden death of a young adult associated with Bacillus cereus food poisoning. J. Clin. Microbiol. 2011, 49, 4379–4381. [Google Scholar] [CrossRef]
- Shiota, M.; Saitou, K.; Mizumoto, H.; Matsusaka, M.; Agata, N.; Nakayama, M.; Kage, M.; Tatsumi, S.; Okamoto, A.; Yameguchi, S.; et al. Rapid detoxification of cereulide in Bacillus cereus food poisoning. Pediatrics 2010, 125, e951–e955. [Google Scholar] [CrossRef] [PubMed]
- Thery, M.; Cousin, V.L.; Tissieres, P.; Enault, M.; Morin, L. Multi-organ failure caused by lasagnas: A case report of Bacillus cereus food poisoning. Front. Pediatr. 2022, 10, 978250. [Google Scholar] [CrossRef] [PubMed]
- Ehling-Schulz, M.; Vukov, N.; Schulz, A.; Shaheen, R.; Andersson, M.; Märtlbauer, E.; Scherer, S. Identification and partial characterization of the nonribosomal peptide synthetase gene responsible for cereulide production in emetic Bacillus cereus. Appl. Environ. Microbiol. 2005, 71, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Ehling-Schulz, M.; Fricker, M.; Grallert, H.; Rieck, P.; Wagner, M.; Scherer, S. Cereulide synthetase gene cluster from emetic Bacillus cereus: Structure and location on a mega virulence plasmid related to Bacillus anthracis toxin plasmid pXO1. BMC Microbiol. 2006, 6, 20. [Google Scholar] [CrossRef] [PubMed]
- Lechner, S.; Mayr, R.; Francis, K.P.; Prüss, B.M.; Kaplan, T.; Wiessner-Gunkel, E.; Stewart, G.S.; Scherer, S. Bacillus weihenstephanensis sp. nov. is a new psychrotolerant species of the Bacillus cereus group. Int. J. Syst. Bacteriol. 1998, 48, 1373–1382. [Google Scholar] [CrossRef]
- Francis, K.P.; Mayr, R.; von Stetten, F.; Stewart, G.S.; Scherer, S. Discrimination of psychrotrophic and mesophilic strains of the Bacillus cereus group by PCR targeting of major cold shock protein genes. Appl. Environ. Microbiol. 1998, 64, e3525-9. [Google Scholar] [CrossRef]
- Liu, Y.; Lai, Q.; Shao, Z. Genome analysis-based reclassification of Bacillus weihenstephanensis as a later heterotypic synonym of Bacillus mycoides. Int. J. Syst. Evol. Microbiol. 2018, 68, 106–112. [Google Scholar] [CrossRef]
- Altayar, M.; Sutherland, A.D. Bacillus cereus is common in the environment but emetic toxin producing isolates are rare. J. Appl. Microbiol. 2006, 100, 7–14. [Google Scholar] [CrossRef]
- Hoton, F.M.; Fornelos, N.; N’Guessan, E.; Hu, X.; Swiecicka, I.; Dierick, K.; Jääskeläinen, E.; Salkinoja-Salonen, M.; Mahillon, J. Family portrait of Bacillus cereus and Bacillus weihenstephanensis cereulide-producing strains. Environ. Microbiol. Rep. 2009, 1, 177–183. [Google Scholar] [CrossRef]
- Thorsen, L.; Hansen, B.M.; Nielsen, K.F.; Hendriksen, N.B.; Phipps, R.K.; Budde, B.B. Characterization of emetic Bacillus weihenstephanensis, a new cereulide-producing bacterium. Appl. Environ. Microbiol. 2006, 72, 5118–5121. [Google Scholar] [CrossRef]
- Jovanovic, J.; Tretiak, S.; Begyn, K.; Rajkovic, A. Detection of Enterotoxigenic Psychrotrophic Presumptive Bacillus cereus and Cereulide Producers in Food Products and Ingredients. Toxins 2022, 14, 289. [Google Scholar] [CrossRef] [PubMed]
- Guérin, A.; Rønning, H.T.; Dargaignaratz, C.; Clavel, T.; Broussolle, V.; Mahillon, J.; Granum, P.E.; Nguyen-The, C. Cereulide production by Bacillus weihenstephanensis strains during growth at different pH values and temperatures. Food Microbiol. 2017, 65, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Webb, M.D.; Barker, G.C.; Goodburn, K.E.; Peck, M.W. Risk presented to minimally processed chilled foods by psychrotrophic Bacillus cereus. Trends Food Sci. Technol. 2019, 93, 94–105. [Google Scholar] [CrossRef] [PubMed]
- NMKL: Nordic Committe on Food Analysis. NMKL 67 Presumptive Bacillus cereus Determination in Foods; NMKL-Nordic Committe on Food Analysis: Bergen, Norway, 2010. [Google Scholar]
- Agata, N.; Ohta, M.; Yokoyama, K. Production of Bacillus cereus emetic toxin (cereulide) in various foods. Int. J. Food Microbiol. 2002, 73, 23–27. [Google Scholar] [CrossRef]
- Pospiech, A.; Neumann, B. A versatile quick-prep of genomic DNA from gram-positive bacteria. Trends Genet. 1995, 11, 217–218. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Mikheenko, A.; Prjibelski, A.; Saveliev, V.; Antipov, D.; Gurevich, A. Versatile genome assembly evaluation with QUAST-LG. Bioinformatics 2018, 34, i142–i150. [Google Scholar] [CrossRef]
- Manni, M.; Berkeley, M.R.; Seppey, M.; Simão, F.A.; Zdobnov, E.M. BUSCO Update: Novel and Streamlined Workflows along with Broader and Deeper Phylogenetic Coverage for Scoring of Eukaryotic, Prokaryotic, and Viral Genomes. Mol. Biol. Evol. 2021, 38, 4647–4654. [Google Scholar] [CrossRef]
- Haft, D.H.; DiCuccio, M.; Badretdin, A.; Brover, V.; Chetvernin, V.; O’Neill, K.; Li, W.; Chitsaz, F.; Derbyshire, M.K.; Gonzales, N.R.; et al. RefSeq: An update on prokaryotic genome annotation and curation. Nucleic Acids Res. 2018, 46, D851–D860. [Google Scholar] [CrossRef]
- Li, W.; O’Neill, K.R.; Haft, D.H.; DiCuccio, M.; Chetvernin, V.; Badretdin, A.; Coulouris, G.; Chirsaz, F.; Derbyshire, M.K.; Durkin, A.S.; et al. RefSeq: Expanding the Prokaryotic Genome Annotation Pipeline reach with protein family model curation. Nucleic Acids Res. 2021, 49, D1020–D1028. [Google Scholar] [CrossRef]
- Tatusova, T.; DiCuccio, M.; Badretdin, A.; Chetvernin, V.; Nawrocki, E.P.; Zaslavsky, L.; Lomsadze, A.; Pruitt, K.D.; Borodoysky, M.; Ostell, J. NCBI prokaryotic genome annotation pipeline. Nucleic Acids Res. 2016, 44, 6614–6624. [Google Scholar] [CrossRef] [PubMed]
- Katz, L.S.; Griswold, T.; Morrison, S.S.; Caravas, J.A.; Zhang, S.; den Bakker, H.C.; Deng, X.; Carleton, H.A. Mashtree: A rapid comparison of whole genome sequence files. J. Open Source Softw. 2019, 4, 1762. [Google Scholar] [CrossRef] [PubMed]
- Takeno, A.; Okamoto, A.; Tori, K.; Oshima, K.; Hirakawa, H.; Toh, H.; Agata, N.; Yamada, K.; Ogasawara, N.; Hayashi, T.; et al. Complete genome sequence of Bacillus cereus NC7401, which produces high levels of the emetic toxin cereulide. J. Bacteriol. 2012, 194, 4767–4768. [Google Scholar] [CrossRef] [PubMed]
- Seemann, T. Snippy: Rapid Haploid Variant Calling and Core Genome Alignment. Snippy. 2020. Available online: https://github.com/tseemann/snippy (accessed on 15 August 2024).
- Carroll, L.M.; Cheng, R.A.; Kovac, J. No Assembly Required: Using BTyper3 to Assess the Congruency of a Proposed Taxonomic Framework for the Bacillus cereus Group with Historical Typing Methods. Front. Microbiol. 2020, 11, 580691. [Google Scholar] [CrossRef]
- Carroll, L.M.; Pierneef, R.; Mathole, A.; Atanda, A.; Matle, I. Genomic Sequencing of Bacillus cereus Sensu Lato Strains Isolated from Meat and Poultry Products in South Africa Enables Inter- and Intranational Surveillance and Source Tracking. Microbiol. Spectr. 2022, 10, e0070022. [Google Scholar] [CrossRef]
- Guinebretière, M.H.; Thompson, F.L.; Sorokin, A.; Normand, P.; Dawyndt, P.; Ehling-Schulz, M.; Svensson, B.; Sanchis, V.; Nguyen-The, C.; Heyndriclx, M.; et al. Ecological diversification in the Bacillus cereus Group. Environ. Microbiol. 2008, 10, 851–865. [Google Scholar] [CrossRef]
- Carroll, L.M.; Kovac, J.; Miller, R.A.; Wiedmann, M. Rapid, High-Throughput Identification of Anthrax-Causing and Emetic Bacillus cereus Group Genome Assemblies via BTyper, a Computational Tool for Virulence-Based Classification of Bacillus cereus Group Isolates by Using Nucleotide Sequencing Data. Appl. Environ. Microbiol. 2017, 83, e01096-17. [Google Scholar] [CrossRef]
- Horwood, P.F.; Burgess, G.W.; Oakey, H.J. Evidence for non-ribosomal peptide synthetase production of cereulide (the emetic toxin) in Bacillus cereus. FEMS Microbiol. Lett. 2004, 236, 319–324. [Google Scholar] [CrossRef]
- Rønning, H.T.; Asp, T.N.; Granum, P.E. Determination and quantification of the emetic toxin cereulide from Bacillus cereus in pasta, rice and cream with liquid chromatography-tandem mass spectrometry. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2015, 32, 911–921. [Google Scholar] [CrossRef]
- ISO 18465:2017; Microbiology of the Food Chain—Quantitative Determination of Emetic Toxin (Cereulide) Using LC-MS/MS, Edition 1. International Standard: Geneva, Switzerland, 2017. Available online: https://www.iso.org/standard/62512.html (accessed on 15 August 2024).
- Rasko, D.A.; Rosovitz, M.J.; Økstad, O.A.; Fouts, D.E.; Jiang, L.; Cer, R.Z.; Kolstø, A.B.; Gill, S.R.; Ravel, J. Complete sequence analysis of novel plasmids from emetic and periodontal Bacillus cereus isolates reveals a common evolutionary history among the B. cereus-group plasmids, including Bacillus anthracis pXO1. J. Bacteriol. 2007, 189, 52–64. [Google Scholar] [CrossRef]
- Carroll, L.M.; Wiedmann, M.; Mukherjee, M.; Nicholas, D.C.; Mingle, L.A.; Dumas, N.B.; Cole, J.A.; Kovac, J. Characterization of Emetic and Diarrheal Bacillus cereus Strains From a 2016 Foodborne Outbreak Using Whole-Genome Sequencing: Addressing the Microbiological, Epidemiological, and Bioinformatic Challenges. Front. Microbiol. 2019, 10, 144. [Google Scholar] [CrossRef] [PubMed]
- Fiedler, G.; Schneider, C.; Igbinosa, E.O.; Kabisch, J.; Brinks, E.; Becker, B.; Stoll, D.A.; Cho, G.-S.; Huch, M.; Franz, C.M.A.P. Antibiotics resistance and toxin profiles of Bacillus cereus-group isolates from fresh vegetables from German retail markets. BMC Microbiol. 2019, 19, 250. [Google Scholar] [CrossRef]
- Gdoura-Ben Amor, M.; Jan, S.; Baron, F.; Grosset, N.; Culot, A.; Gdoura, R.; Gauitier, M.; Techer, C. Toxigenic potential and antimicrobial susceptibility of Bacillus cereus group bacteria isolated from Tunisian foodstuffs. BMC Microbiol. 2019, 19, 196. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.; Carvalho, M.; Teixeira, P. Characterization of the Toxigenic Potential of Bacillus cereus sensu lato Isolated from Raw Berries and Their Products. Foods 2023, 12, 4021. [Google Scholar] [CrossRef] [PubMed]
- Messelhäusser, U.; Kämpf, P.; Fricker, M.; Ehling-Schulz, M.; Zucker, R.; Wagner, B.; Busch, U.; Höller, C. Prevalence of emetic Bacillus cereus in different ice creams in Bavaria. J. Food. Prot. 2010, 73, 395–399. [Google Scholar] [CrossRef]
- Dietrich, R.; Jessberger, N.; Ehling-Schulz, M.; Märtlbauer, E.; Granum, P.E. The Food Poisoning Toxins of Bacillus cereus. Toxins 2021, 13, 98. [Google Scholar] [CrossRef]
- Ehling-Schulz, M.; Svensson, B.; Guinebretiere, M.H.; Lindbäck, T.; Andersson, M.; Schulz, A.; Fricker, M.; Christiansson, A.; Granum, P.E.; Märtlbauer, E.; et al. Emetic toxin formation of Bacillus cereus is restricted to a single evolutionary lineage of closely related strains. Microbiology 2005, 151, 183–197. [Google Scholar] [CrossRef]
- Carroll, L.M.; Wiedmann, M. Cereulide Synthetase Acquisition and Loss Events within the Evolutionary History of Group III Bacillus cereus Sensu Lato Facilitate the Transition between Emetic and Diarrheal Foodborne Pathogens. mBio 2020, 11, e01263-20. [Google Scholar] [CrossRef]
- Dommel, M.K.; Frenzel, E.; Strasser, B.; Blöchinger, C.; Scherer, S.; Ehling-Schulz, M. Identification of the main promoter directing cereulide biosynthesis in emetic Bacillus cereus and its application for real-time monitoring of ces gene expression in foods. Appl. Environ. Microbiol. 2010, 76, 1232–1240. [Google Scholar] [CrossRef]
- Ehling-Schulz, M.; Fricker, M.; Scherer, S. Bacillus cereus, the causative agent of an emetic type of food-borne illness. Mol. Nutr. Food Res. 2004, 48, 479–487. [Google Scholar] [CrossRef]
- Carlin, F.; Fricker, M.; Pielaat, A.; Heisterkamp, S.; Shaheen, R.; Salonen, M.S.; Svensson, B.; Nguyen-The, C.; Ehling-Schulz, M. Emetic toxin-producing strains of Bacillus cereus show distinct characteristics within the Bacillus cereus group. Int. J. Food Microbiol. 2006, 109, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Finlay, W.J.; Logan, N.A.; Sutherland, A.D. Bacillus cereus produces most emetic toxin at lower temperatures. Lett. Appl. Microbiol. 2000, 31, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Apetroaie-Constantin, C.; Shaheen, R.; Andrup, L.; Smidt, L.; Rita, H.; Salkinoja-Salonen, M. Environment driven cereulide production by emetic strains of Bacillus cereus. Int. J. Food Microbiol. 2008, 127, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Frentzel, H.; Kraemer, M.; Kelner-Burgos, Y.; Uelze, L.; Bodi, D. Cereulide production capacities and genetic properties of 31 emetic Bacillus cereus group strains. Int. J. Food Microbiol. 2024, 417, 110694. [Google Scholar] [CrossRef]
- Stenfors, L.P.; Granum, P.E. Psychrotolerant species from the Bacillus cereus group are not necessarily Bacillus weihenstephanensis. FEMS Microbiol. Lett. 2001, 197, 223–228. [Google Scholar] [CrossRef]

| Primer | Gene | Sequence | Reference |
|---|---|---|---|
| BcAPF1/BcAPR1 | cspA | 5′-GAGGAAATAATTATGACAGTT-3′/ 5′-CTTYTTGGCCTTCTTCTAA-3′ | [16] |
| Cluster | Isolate | % Aligned * | SNPs | Date of Isolation | Isolated from |
|---|---|---|---|---|---|
| A | NVH-IS213 | 99.6 | 20 | August 2021 | Product a, line 1 |
| NVH-IS222 | 98.8 | 1 | June 2021 | Product b, line 2 | |
| NVH-YM316 | 98.8 | 3 | January 2022 | Product c, line 2 | |
| NVH-YM332 | 98.9 | 2 | September 2022 | Licm ** | |
| NVH-YM347 | 99.8 | 3 | September 2022 | Licm | |
| NVH-YM351 | 99.6 | 24 | September 2022 | Licm | |
| NVH-YM356 | 99.2 | 2 | September 2022 | Freezer | |
| NVH-YM357 | 99.1 | 0 | September 2022 | Product m | |
| NVH-YM359 | 98.9 | 2 | September 2022 | Cream | |
| Reference NVH-YM317 (5,763,304 bp) | 100 | 0 | January 2022 | Product c, line 2 | |
| B | NVH-IS229 | 99.2 | 1 | October 2021 | Product c, line 2 |
| NVH-IS230 | 99.9 | 1 | October 2021 | Product c, line 2 | |
| NVH-IS232 | 99.9 | 8 | December 2021 | Cream | |
| NVH-YM324 | 99.9 | 0 | December 2021 | Product c, line 2 | |
| NVH-YM328 | 99.9 | 0 | December 2021 | Product d, line 2 | |
| Reference NVH-YM326 (5,795,337 bp) | 100 | 0 | December 2021 | Product c, line 2 | |
| C | NVH-YM301 | 100 | 0 | December 2022 | Product g, line 1 |
| NVH-YM349 | 100 | 2 | September 2022 | Pipeline | |
| NVH-YM350 | 100 | 2 | September 2022 | Licm | |
| Reference NVH-YM302 (5,324,995 bp) | 100 | 0 | December 2022 | Product g, line 1 | |
| D | NVH-IS241 | 99.9 | 11 | November 2021 | Product f |
| NVH-YM308 | 99.9 | 0 | December 2021 | Product c, line 2 | |
| NVH-YM309 | 99.9 | 1 | March 2022 | Product c, line 2 | |
| NVH-YM321 | 99.9 | 4 | January 2022 | Product c, line 2 | |
| Reference NVH-IS233 (5,344,339 bp) | 100 | 0 | December 2021 | Product d, line 3 | |
| E | NVH-YM342 | 99.7 | 0 | October 2022 | Freezer |
| NVH-YM353 | 99.7 | 1 | September 2022 | Freezer | |
| Reference NVH-YM346 (5,691,691 bp) | 100 | 0 | September 2022 | Freezer | |
| F | NVH-YM307 | 100 | 2 | December 2021 | Product c, line 2 |
| NVH-YM319 | 100 | 1 | December 2021 | Product h, line 2 | |
| Reference NVH-YM320 (5,706,417 bp) | 100 | 0 | January 2022 | Product c, line 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lindbäck, T.; Llarena, A.-K.; Aanrud, S.G.; Monshaugen, M.; Mekonnen, Y.B.; Holmemo, C.W.; Aspholm, M. Genetic Profile and Toxigenic Potential of Bacillus cereus Isolates from a Norwegian Ice Cream Production Plant. Foods 2024, 13, 3029. https://doi.org/10.3390/foods13193029
Lindbäck T, Llarena A-K, Aanrud SG, Monshaugen M, Mekonnen YB, Holmemo CW, Aspholm M. Genetic Profile and Toxigenic Potential of Bacillus cereus Isolates from a Norwegian Ice Cream Production Plant. Foods. 2024; 13(19):3029. https://doi.org/10.3390/foods13193029
Chicago/Turabian StyleLindbäck, Toril, Ann-Katrin Llarena, Stine Göransson Aanrud, Marte Monshaugen, Yohannes B. Mekonnen, Carina Wiker Holmemo, and Marina Aspholm. 2024. "Genetic Profile and Toxigenic Potential of Bacillus cereus Isolates from a Norwegian Ice Cream Production Plant" Foods 13, no. 19: 3029. https://doi.org/10.3390/foods13193029







