Co-Encapsulation of Coffee and Coffee By-Product Extracts with Probiotic Kluyveromyces lactis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Extraction of Bioactive Compounds from Green Coffee Beans, Coffee Silverskin, and PVA Beans
2.2. High-Performance Liquid Chromatography Analysis of the Bioactive Compounds
2.3. Inoculum Preparation and Encapsulation of Yeast and Extracts
2.3.1. Encapsulation Efficiency
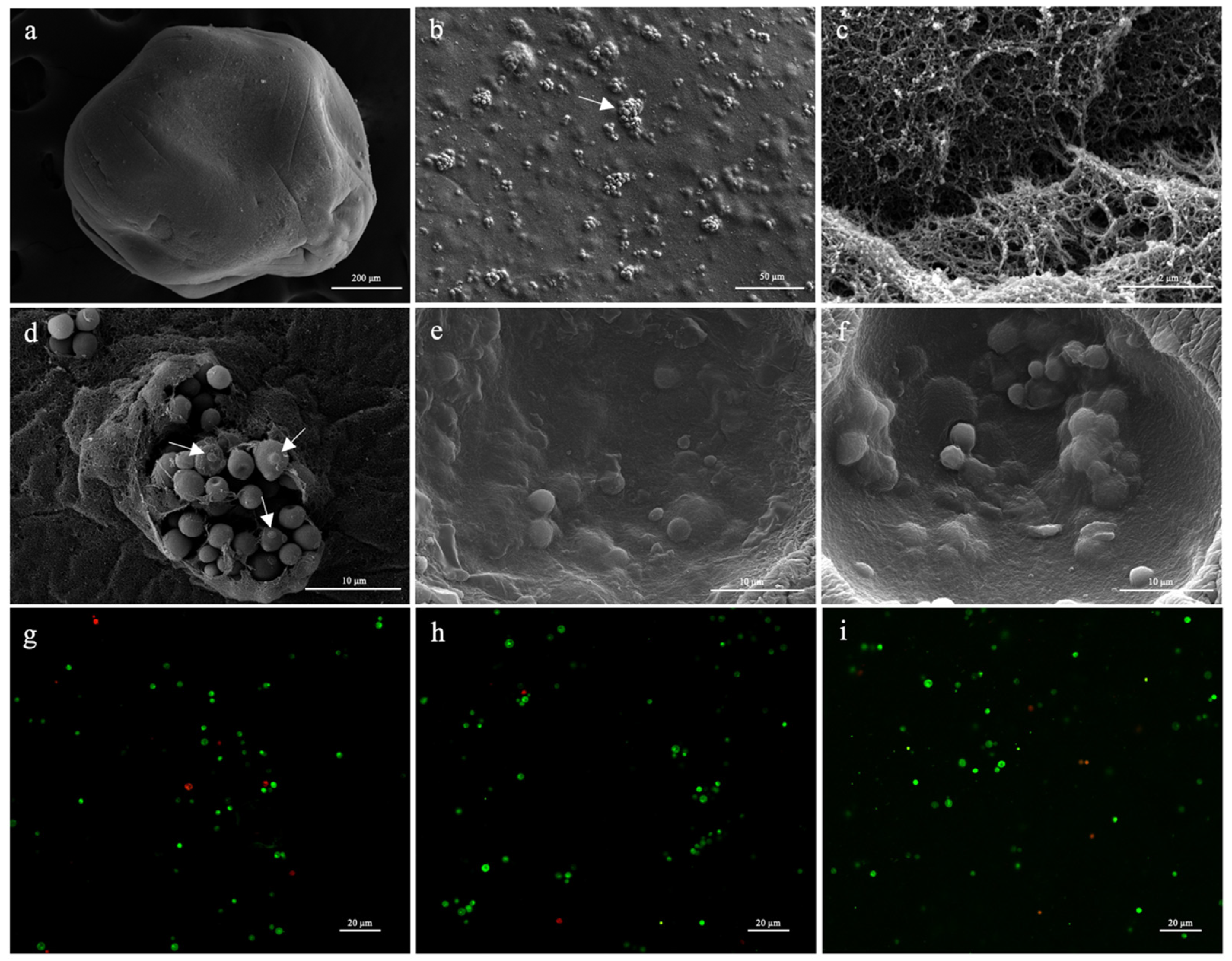
2.3.2. Scanning Electron Microscopy (SEM)
2.3.3. Confocal Laser Microscopy
2.4. Probiotic Yeast Survival under Simulated Gastrointestinal Conditions
2.5. Determination of Antibacterial Activity
2.6. Determination of Antioxidant Activity
2.7. Determination of Total Phenolic Compounds
2.8. Statistical Analysis
3. Results and Discussion
3.1. Analysis of the Bioactive Compounds from Green Coffee Beans, Coffee Silverskin, and PVA Beans
3.2. Determination of Antibacterial Activity
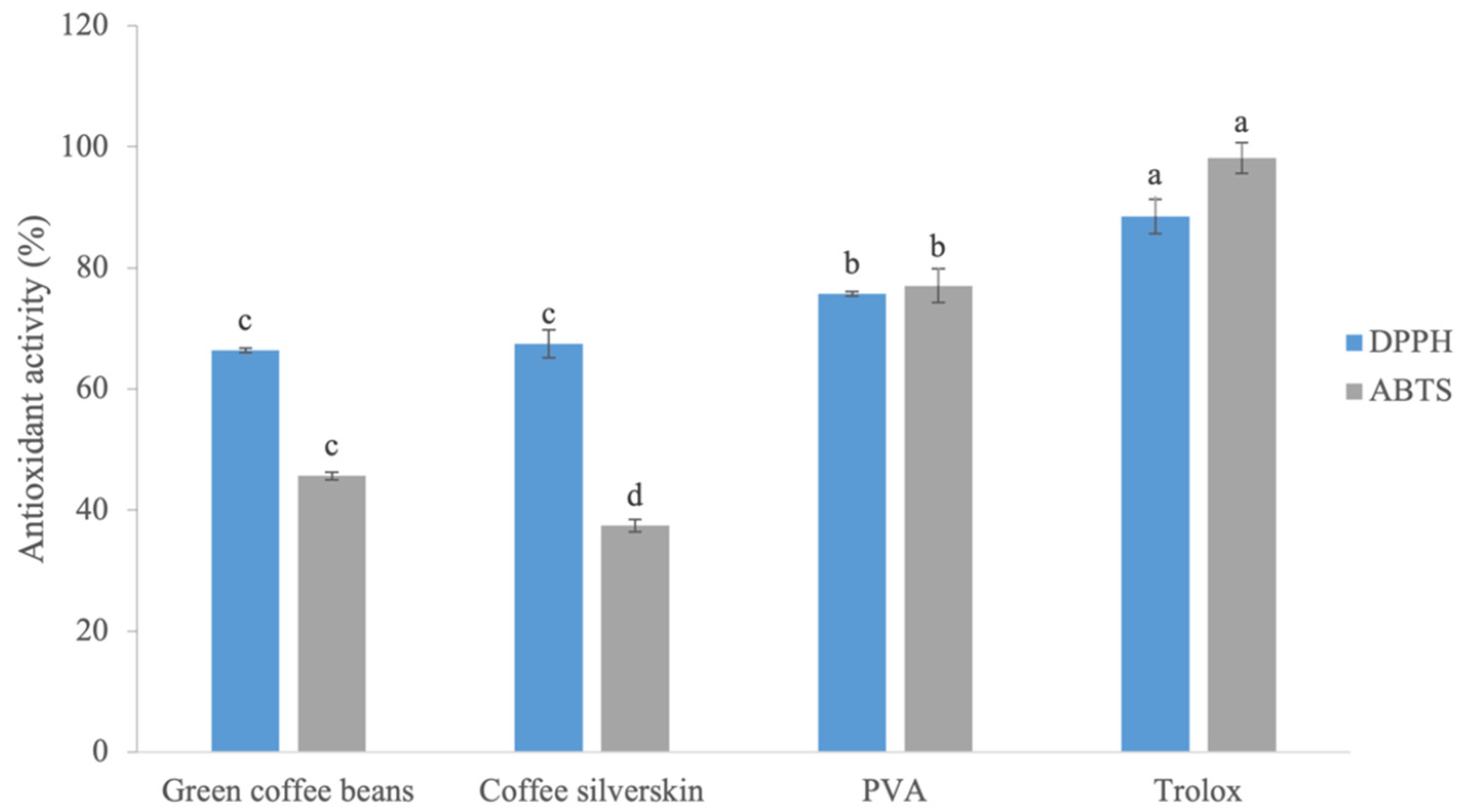
3.3. Antioxidant Activity and Total Phenolic Compounds of Extracts
3.4. Microcapsule Characterization of Encapsulation Efficiency
3.5. Antioxidant Activity and Total Phenolic Compounds of Microcapsules
3.6. Survival in Gastrointestinal Conditions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- USDA. Coffee Semi-Annual. BR2023-0031. 2023. Available online: https://apps.fas.usda.gov/newgainapi/api/Report/DownloadReportByFileName?fileName=Coffee%20Semi-annual_Brasilia_Brazil_BR2023-0031.pdf (accessed on 12 September 2023).
- Janissen, B.; Huynh, T. Chemical Composition and Value-Adding Applications of Coffee Industry by-Products: A Review. Resour. Conserv. Recycl. 2018, 128, 110–117. [Google Scholar] [CrossRef]
- Gomes, W.P.C.; Pires, J.A.; Teixeira, N.N.; Bortoleto, G.G.; Gutierrez, E.M.R.; Melchert, W.R. Effects of Green Coffee Bean Flour Fortification on the Chemical and Nutritional Properties of Gluten-Free Cake. Food Meas. 2022, 16, 3451–3458. [Google Scholar] [CrossRef]
- International Coffee Agreement 2007. Ica2007e.Pdf. Available online: https://www.ico.org/documents/ica2007e.pdf (accessed on 28 August 2023).
- Pazmiño-Arteaga, J.; Gallardo, C.; González-Rodríguez, T.; Winkler, R. Loss of Sensory Cup Quality: Physiological and Chemical Changes during Green Coffee Storage. Plant Foods Hum. Nutr. 2022, 77, 1–11. [Google Scholar] [CrossRef]
- De Luca, S.; Ciotoli, E.; Biancolillo, A.; Bucci, R.; Magrì, A.D.; Marini, F. Simultaneous Quantification of Caffeine and Chlorogenic Acid in Coffee Green Beans and Varietal Classification of the Samples by HPLC-DAD Coupled with Chemometrics. Env. Sci. Pollut. Res. 2018, 25, 28748–28759. [Google Scholar] [CrossRef]
- Toledo, P.R.A.B.; Pezza, L.; Pezza, H.R.; Toci, A.T. Relationship Between the Different Aspects Related to Coffee Quality and Their Volatile Compounds. Compr. Rev. Food Sci. Food Saf. 2016, 15, 705–719. [Google Scholar] [CrossRef] [PubMed]
- Kalschne, D.L.; Biasuz, T.; De Conti, A.J.; Viegas, M.C.; Corso, M.P.; Benassi, M.D.T. Sensory Characterization and Acceptance of Coffee Brews of C. arabica and C. canephora Blended with Steamed Defective Coffee. Food Res. Int. 2019, 124, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Hejna, A. Potential Applications of By-Products from the Coffee Industry in Polymer Technology—Current State and Perspectives. Waste Manag. 2021, 121, 296–330. [Google Scholar] [CrossRef]
- Lorbeer, L.; Schwarz, S.; Franke, H.; Lachenmeier, D.W. Toxicological Assessment of Roasted Coffee Silver Skin (Testa of Coffea sp.) as Novel Food Ingredient. Molecules 2022, 27, 6839. [Google Scholar] [CrossRef]
- Borrelli, R.C.; Esposito, F.; Napolitano, A.; Ritieni, A.; Fogliano, V. Characterization of a New Potential Functional Ingredient: Coffee Silverskin. J. Agric. Food Chem. 2004, 52, 1338–1343. [Google Scholar] [CrossRef]
- Nzekoue, F.K.; Angeloni, S.; Navarini, L.; Angeloni, C.; Freschi, M.; Hrelia, S.; Vitali, L.A.; Sagratini, G.; Vittori, S.; Caprioli, G. Coffee Silverskin Extracts: Quantification of 30 Bioactive Compounds by a New HPLC-MS/MS Method and Evaluation of Their Antioxidant and Antibacterial Activities. Food Res. Int. 2020, 133, 109128. [Google Scholar] [CrossRef]
- Lemos, M.F.; de Andrade Salustriano, N.; de Souza Costa, M.M.; Lirio, K.; da Fonseca, A.F.A.; Pacheco, H.P.; Endringer, D.C.; Fronza, M.; Scherer, R. Chlorogenic Acid and Caffeine Contents and Anti-Inflammatory and Antioxidant Activities of Green Beans of Conilon and Arabica Coffees Harvested with Different Degrees of Maturation. J. Saudi Chem. Soc. 2022, 26, 101467. [Google Scholar] [CrossRef]
- Mansour, A.; Mohajeri-Tehrani, M.R.; Samadi, M.; Qorbani, M.; Merat, S.; Adibi, H.; Poustchi, H.; Hekmatdoost, A. Effects of Supplementation with Main Coffee Components Including Caffeine and/or Chlorogenic Acid on Hepatic, Metabolic, and Inflammatory Indices in Patients with Non-Alcoholic Fatty Liver Disease and Type 2 Diabetes: A Randomized, Double-Blind, Placebo-Controlled, Clinical Trial. Nutr. J. 2021, 20, 35. [Google Scholar] [CrossRef] [PubMed]
- Pimpley, V.; Patil, S.; Srinivasan, K.; Desai, N.; Murthy, P.S. The Chemistry of Chlorogenic Acid from Green Coffee and Its Role in Attenuation of Obesity and Diabetes. Prep. Biochem. Biotechnol. 2020, 50, 969–978. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Liang, S.; Zhang, M.; Wang, Z.; Wang, Z.; Ren, X. The Effect of Chlorogenic Acid on Bacillus Subtilis Based on Metabolomics. Molecules 2020, 25, 4038. [Google Scholar] [CrossRef]
- Passos, C.P.; Costa, R.M.; Ferreira, S.S.; Lopes, G.R.; Cruz, M.T.; Coimbra, M.A. Role of Coffee Caffeine and Chlorogenic Acids Adsorption to Polysaccharides with Impact on Brew Immunomodulation Effects. Foods 2021, 10, 378. [Google Scholar] [CrossRef]
- Guidelines for the Evaluation of Probiotics in Food. Available online: https://www.mhlw.go.jp/content/11121000/001129364.pdf (accessed on 16 March 2023).
- Andrade, R.P.; Melo, C.N.; Genisheva, Z.; Schwan, R.F.; Duarte, W.F. Yeasts from Canastra Cheese Production Process: Isolation and Evaluation of Their Potential for Cheese Whey Fermentation. Food Res. Int. 2017, 91, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Andrade, R.P.; Oliveira, D.R.; Lopes, A.C.A.; De Abreu, L.R.; Duarte, W.F. Survival of Kluyveromyces Lactis and Torulaspora Delbrueckii to Simulated Gastrointestinal Conditions and Their Use as Single and Mixed Inoculum for Cheese Production. Food Res. Int. 2019, 125, 108620. [Google Scholar] [CrossRef]
- Andrade, G.C.; Andrade, R.P.; Oliveira, D.R.; Quintanilha, M.F.; Martins, F.S.; Duarte, W.F. Kluyveromyces Lactis and Torulaspora Delbrueckii: Probiotic Characterization, Anti-Salmonella Effect, and Impact on Cheese Quality. LWT 2021, 151, 112240. [Google Scholar] [CrossRef]
- Rosolen, M.D.; Bordini, F.W.; de Oliveira, P.D.; Conceição, F.R.; Pohndorf, R.S.; Fiorentini, Â.M.; da Silva, W.P.; Pieniz, S. Symbiotic Microencapsulation of Lactococcus lactis subsp. Lactis R7 Using Whey and Inulin by Spray Drying. LWT 2019, 115, 108411. [Google Scholar] [CrossRef]
- Samedi, L.; Charles, A.L. Viability of 4 Probiotic Bacteria Microencapsulated with Arrowroot Starch in the Simulated Gastrointestinal Tract (GIT) and Yoghurt. Foods 2019, 8, 175. [Google Scholar] [CrossRef]
- Cosgun, G.; Gungor, K.K.; Balci-Torun, F.; Sahin, S.; Torun, M. Design of Encapsulation Method for Chlorogenic Acid and Caffeine in Coffee Waste By-Product. Phytochem. Anal. 2023. [Google Scholar] [CrossRef]
- Suárez-Quiroz, M.L.; Alonso Campos, A.; Valerio Alfaro, G.; González-Ríos, O.; Villeneuve, P.; Figueroa-Espinoza, M.C. Isolation of Green Coffee Chlorogenic Acids Using Activated Carbon. J. Food Compos. Anal. 2014, 33, 55–58. [Google Scholar] [CrossRef]
- Malta, M.R.; Chagas, S.J. de R. Avaliação de compostos não-voláteis em diferentes cultivares de cafeeiro produzidas na região sul de Minas Gerais. Acta Sci. Agron. 2009, 31, 57–61. [Google Scholar] [CrossRef]
- Santiago, W.D.; Teixeira, A.R.; Santiago, J.D.A.; Lopes, A.C.A.; Brandão, R.M.; Caetano, A.R.; Cardoso, M.D.G.; Resende, M.L.V. Development and Validation of Chromatographic Methods to Quantify Organic Compounds in Green Coffee (Coffea arabica) Beans. Aust. J. Crop Sci. 2020, 1275–1282. [Google Scholar] [CrossRef]
- Duarte, W.F.; Dias, D.R.; Oliveira, J.M.; Vilanova, M.; Teixeira, J.A.; e Silva, J.B.A.; Schwan, R.F. Raspberry (Rubus idaeus L.) Wine: Yeast Selection, Sensory Evaluation and Instrumental Analysis of Volatile and Other Compounds. Food Res. Int. 2010, 43, 2303–2314. [Google Scholar] [CrossRef]
- Sathyabama, S.; Ranjith Kumar, M.; Bruntha Devi, P.; Vijayabharathi, R.; Brindha Priyadharisini, V. Co-Encapsulation of Probiotics with Prebiotics on Alginate Matrix and Its Effect on Viability in Simulated Gastric Environment. LWT 2014, 57, 419–425. [Google Scholar] [CrossRef]
- Ferreira Leite Ladislau, H.; Silva De Farias, T.G.; Mendonça Soares, B.L.; Medeiros, J.A.D.C.; Ferrão Castelo Branco Melo, N.; Stamford–Arnaud, T.M.; Stamford, T.C.M.; Stamford, T.L.M. The Effect of Co-encapsulation of Lactobacillus rhamnosus GG ATCC 53103 with Inulin on Alginate/Chitosan Matrix: The Viability in Fermented Soy Blend and Simulated Digestive System. Int. J. Food Sci. Tech. 2021, 56, 5395–5401. [Google Scholar] [CrossRef]
- Moreira, S.I.; Pereira, L.F.; De Souza, E.A.; Alves, E. Fluorochrome-Based Methods for Fungal Sample Examination. In Laboratory Protocols in Fungal Biology; Gupta, V.K., Tuohy, M., Eds.; Fungal Biology; Springer International Publishing: Cham, Switzerland, 2022; pp. 209–234. ISBN 978-3-030-83748-8. [Google Scholar]
- Gudiña, E.J.; Rocha, V.; Teixeira, J.A.; Rodrigues, L.R. Antimicrobial and Antiadhesive Properties of a Biosurfactant Isolated from Lactobacillus paracasei ssp. Paracasei A20. Lett. Appl. Microbiol. 2010, 50, 419–424. [Google Scholar] [CrossRef]
- Krunić, T.Ž.; Rakin, M.B. Enriching Alginate Matrix Used for Probiotic Encapsulation with Whey Protein Concentrate or Its Trypsin-Derived Hydrolysate: Impact on Antioxidant Capacity and Stability of Fermented Whey-Based Beverages. Food Chem. 2022, 370, 130931. [Google Scholar] [CrossRef]
- Gonçalves Tavares, D.; Viana Lessa Barbosa, B.; Lopes Ferreira, R.; Ferreira Duarte, W.; Gomes Cardoso, P. Antioxidant Activity and Phenolic Compounds of the Extract from Pigment-Producing Fungi Isolated from Brazilian Caves. Biocatal. Agric. Biotechnol. 2018, 16, 148–154. [Google Scholar] [CrossRef]
- Scott, A.J.; Knott, M. A Cluster Analysis Method for Grouping Means in the Analysis of Variance. Biometrics 1974, 30, 507–512. [Google Scholar] [CrossRef]
- Habtamu, D.; Belay, A. First Order Derivative Spectra to Determine Caffeine and Chlorogenic Acids in Defective and Nondefective Coffee Beans. Food Sci. Nutr. 2020, 8, 4757–4762. [Google Scholar] [CrossRef] [PubMed]
- Brzezicha, J.; Błażejewicz, D.; Brzezińska, J.; Grembecka, M. Green Coffee VS Dietary Supplements: A Comparative Analysis of Bioactive Compounds and Antioxidant Activity. Food Chem. Toxicol. 2021, 155, 112377. [Google Scholar] [CrossRef] [PubMed]
- Macheiner, L.; Schmidt, A.; Schreiner, M.; Mayer, H.K. Green Coffee Infusion as a Source of Caffeine and Chlorogenic Acid. J. Food Compos. Anal. 2019, 84, 103307. [Google Scholar] [CrossRef]
- Jeszka-Skowron, M.; Sentkowska, A.; Pyrzyńska, K.; De Peña, M.P. Chlorogenic Acids, Caffeine Content and Antioxidant Properties of Green Coffee Extracts: Influence of Green Coffee Bean Preparation. Eur. Food Res. Technol. 2016, 242, 1403–1409. [Google Scholar] [CrossRef]
- Koshiro, Y.; Jackson, M.C.; Katahira, R.; Wang, M.-L.; Nagai, C.; Ashihara, H. Biosynthesis of Chlorogenic Acids in Growing and Ripening Fruits of Coffea arabica and Coffea canephora Plants. Z. Für Naturforschung C 2007, 62, 731–742. [Google Scholar] [CrossRef]
- Bessada, S.M.F.; Alves, R.C.; Costa, A.S.G.; Nunes, M.A.; Oliveira, M.B.P.P. Coffea canephora Silverskin from Different Geographical Origins: A Comparative Study. Sci. Total Environ. 2018, 645, 1021–1028. [Google Scholar] [CrossRef] [PubMed]
- Jeszka-Skowron, M.; Frankowski, R.; Zgoła-Grześkowiak, A. Comparison of Methylxantines, Trigonelline, Nicotinic Acid and Nicotinamide Contents in Brews of Green and Processed Arabica and Robusta Coffee Beans—Influence of Steaming, Decaffeination and Roasting Processes on Coffee Beans. LWT 2020, 125, 109344. [Google Scholar] [CrossRef]
- Wang, X.; Hong, D.-F.; Hu, G.-L.; Li, Z.-R.; Peng, X.-R.; Shi, Q.-Q.; Qiu, M.-H. Morphological Changes and Component Characterization of Coffee Silverskin. Molecules 2021, 26, 4914. [Google Scholar] [CrossRef]
- Arauz, J.; Ramos-Tovar, E.; Muriel, P. Coffee and the Liver. In Liver Pathophysiology; Elsevier: Amsterdam, The Netherlands, 2017; pp. 675–685. ISBN 978-0-12-804274-8. [Google Scholar]
- Zeng, W.-Y.; Tan, L.; Han, C.; Zheng, Z.-Y.; Wu, G.-S.; Luo, H.-R.; Li, S.-L. Trigonelline Extends the Lifespan of C. Elegans and Delays the Progression of Age-Related Diseases by Activating AMPK, DAF-16, and HSF-1. Oxidative Med. Cell. Longev. 2021, 2021, 7656834. [Google Scholar] [CrossRef]
- Farid, M.M.; Yang, X.; Kuboyama, T.; Tohda, C. Trigonelline Recovers Memory Function in Alzheimer’s Disease Model Mice: Evidence of Brain Penetration and Target Molecule. Sci. Rep. 2020, 10, 16424. [Google Scholar] [CrossRef]
- Pimpley, V.A.; Murthy, P.S. Influence of Green Extraction Techniques on Green Coffee: Nutraceutical Compositions, Antioxidant Potential and in Vitro Bio-Accessibility of Phenolics. Food Biosci. 2021, 43, 101284. [Google Scholar] [CrossRef]
- Prandi, B.; Ferri, M.; Monari, S.; Zurlini, C.; Cigognini, I.; Verstringe, S.; Schaller, D.; Walter, M.; Navarini, L.; Tassoni, A.; et al. Extraction and Chemical Characterization of Functional Phenols and Proteins from Coffee (Coffea arabica) By-Products. Biomolecules 2021, 11, 1571. [Google Scholar] [CrossRef]
- Tsai, C.-F.; Jioe, I.P.J. The Analysis of Chlorogenic Acid and Caffeine Content and Its Correlation with Coffee Bean Color under Different Roasting Degree and Sources of Coffee (Coffea arabica Typica). Processes 2021, 9, 2040. [Google Scholar] [CrossRef]
- Clifford, M.N.; Kazi, T. The Influence of Coffee Bean Maturity on the Content of Chlorogenic Acids, Caffeine and Trigonelline. Food Chem. 1987, 26, 59–69. [Google Scholar] [CrossRef]
- Hu, G.; Peng, X.; Wang, X.; Li, X.; Li, X.; Qiu, M. Excavation of Coffee Maturity Markers and Further Research on Their Changes in Coffee Cherries of Different Maturity. Food Res. Int. 2020, 132, 109121. [Google Scholar] [CrossRef] [PubMed]
- Acidri, R.; Sawai, Y.; Sugimoto, Y.; Handa, T.; Sasagawa, D.; Masunaga, T.; Yamamoto, S.; Nishihara, E. Phytochemical Profile and Antioxidant Capacity of Coffee Plant Organs Compared to Green and Roasted Coffee Beans. Antioxidants 2020, 9, 93. [Google Scholar] [CrossRef] [PubMed]
- Khochapong, W.; Ketnawa, S.; Ogawa, Y.; Punbusayakul, N. Effect of in Vitro Digestion on Bioactive Compounds, Antioxidant and Antimicrobial Activities of Coffee (Coffea arabica L.) Pulp Aqueous Extract. Food Chem. 2021, 348, 129094. [Google Scholar] [CrossRef]
- Lou, Z.; Wang, H.; Zhu, S.; Ma, C.; Wang, Z. Antibacterial Activity and Mechanism of Action of Chlorogenic Acid. J. Food Sci. 2011, 76, M398–M403. [Google Scholar] [CrossRef]
- Chaves-Ulate, C.; Rodríguez-Sánchez, C.; Arias-Echandi, M.L.; Esquivel, P. Antimicrobial Activities of Phenolic Extracts of Coffee Mucilage. NFS J. 2023, 31, 50–56. [Google Scholar] [CrossRef]
- Duangjai, A.; Suphrom, N.; Wungrath, J.; Ontawong, A.; Nuengchamnong, N.; Yosboonruang, A. Comparison of Antioxidant, Antimicrobial Activities and Chemical Profiles of Three Coffee (Coffea arabica L.) Pulp Aqueous Extracts. Integr. Med. Res. 2016, 5, 324–331. [Google Scholar] [CrossRef]
- Tasew, T.; Mekonnen, Y.; Gelana, T.; Redi-Abshiro, M.; Chandravanshi, B.S.; Ele, E.; Mohammed, A.M.; Mamo, H. In Vitro Antibacterial and Antioxidant Activities of Roasted and Green Coffee Beans Originating from Different Regions of Ethiopia. Int. J. Food Sci. 2020, 2020, e8490492. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Huang, H.-W.; Wang, C.-Y. Effects of High Pressure-Assisted Extraction on Yield, Antioxidant, Antimicrobial, and Anti-Diabetic Properties of Chlorogenic Acid and Caffeine Extracted from Green Coffee Beans. Food Bioprocess Technol. 2022, 15, 1529–1538. [Google Scholar] [CrossRef]
- Silva, M.D.O.; Honfoga, J.N.B.; Medeiros, L.L.D.; Madruga, M.S.; Bezerra, T.K.A. Obtaining Bioactive Compounds from the Coffee Husk (Coffea arabica L.) Using Different Extraction Methods. Molecules 2020, 26, 46. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Xu, W.; Balan, P.; Wong, M.; Chen, W.; Popovich, D.G. In Vitro Antioxidant Properties of New Zealand Hass Avocado Byproduct (Peel and Seed) Fractions. ACS Food Sci. Technol. 2021, 1, 579–587. [Google Scholar] [CrossRef]
- Dunford, N.T.; Gumus, Z.P.; Gur, C.S. Chemical Composition and Antioxidant Properties of Pecan Shell Water Extracts. Antioxidants 2022, 11, 1127. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas-Hernández, E.; Torres-León, C.; Ascacio-Valdés, J.; Contreras-Esquivel, J.C.; Aguilar, C.N. Influence of Drying and Extraction Technology on the Chemical Profile and Antioxidant Property of Mexican Mango Byproduct. In Food Loss and Waste Reduction; Apple Academic Press: Palm Bay, FL, USA, 2021; ISBN 978-1-00-308390-0. [Google Scholar]
- Oberoi, K.; Tolun, A.; Altintas, Z.; Sharma, S. Effect of Alginate-Microencapsulated Hydrogels on the Survival of Lactobacillus rhamnosus under Simulated Gastrointestinal Conditions. Foods 2021, 10, 1999. [Google Scholar] [CrossRef]
- Nami, Y.; Lornezhad, G.; Kiani, A.; Abdullah, N.; Haghshenas, B. Alginate-Persian Gum-Prebiotics Microencapsulation Impacts on the Survival Rate of Lactococcus Lactis ABRIINW-N19 in Orange Juice. LWT 2020, 124, 109190. [Google Scholar] [CrossRef]
- Afzaal, M.; Khan, A.U.; Saeed, F.; Ahmed, A.; Ahmad, M.H.; Maan, A.A.; Tufail, T.; Anjum, F.M.; Hussain, S. Functional Exploration of Free and Encapsulated Probiotic Bacteria in Yogurt and Simulated Gastrointestinal Conditions. Food Sci. Nutr. 2019, 7, 3931–3940. [Google Scholar] [CrossRef]
- Anani, J.; Noby, H.; Zkria, A.; Yoshitake, T.; ElKady, M. Monothetic Analysis and Response Surface Methodology Optimization of Calcium Alginate Microcapsules Characteristics. Polymers 2022, 14, 709. [Google Scholar] [CrossRef]
- Sakoui, S.; Derdak, R.; Pop, O.L.; Vodnar, D.C.; Addoum, B.; Teleky, B.-E.; Elemer, S.; Elmakssoudi, A.; Suharoschi, R.; Soukri, A.; et al. Effect of Encapsulated Probiotic in Inulin-Maltodextrin-Sodium Alginate Matrix on the Viability of Enterococcus Mundtii SRBG1 and the Rheological Parameters of Fermented Milk. Curr. Res. Food Sci. 2022, 5, 1713–1719. [Google Scholar] [CrossRef] [PubMed]
- Roquero, D.M.; Othman, A.; Melman, A.; Katz, E. Iron(Iii)-Cross-Linked Alginate Hydrogels: A Critical Review. Mater. Adv. 2022, 3, 1849–1873. [Google Scholar] [CrossRef]
- Adilah, R.N.; Chiu, S.-T.; Hu, S.-Y.; Ballantyne, R.; Happy, N.; Cheng, A.-C.; Liu, C.-H. Improvement in the Probiotic Efficacy of Bacillus Subtilis E20-Stimulates Growth and Health Status of White Shrimp, Litopenaeus vannamei via Encapsulation in Alginate and Coated with Chitosan. Fish. Shellfish Immunol. 2022, 125, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Raddatz, G.C.; Fonseca, V.R.; Cichoski, A.J.; Zepka, L.Q.; Jacob-Lopes, E.; Campagnol, P.C.B.; Wagner, R.; Muller, E.I.; de Moraes Flores, E.M.; de Bona da Silva, C.; et al. Viability and Stability Evaluation of Lactobacillus casei LC03 Co-Encapsulated with Red Onion (Allium Cepa L.) Peel Extract. LWT 2022, 153, 112434. [Google Scholar] [CrossRef]
- Bakhtiyari, M.; Hamidi-Esfahani, Z.; Barzegar, M. Optimization of Co-Encapsulation of L. Plantarum Cells and Silybum Marianum Seed Extract and Evaluation of Protective Effect of Extract on Cells Survival in Simulated Gastrointestinal Fluids. LWT 2022, 165, 113733. [Google Scholar] [CrossRef]
- Petraitytė, S.; Šipailienė, A. Enhancing Encapsulation Efficiency of Alginate Capsules Containing Lactic Acid Bacteria by Using Different Divalent Cross-Linkers Sources. LWT 2019, 110, 307–315. [Google Scholar] [CrossRef]
- Aguiar, J.; Estevinho, B.N.; Santos, L. Microencapsulation of Natural Antioxidants for Food Application—The Specific Case of Coffee Antioxidants—A Review. Trends Food Sci. Technol. 2016, 58, 21–39. [Google Scholar] [CrossRef]
- Christodoulou, M.C.; Orellana Palacios, J.C.; Hesami, G.; Jafarzadeh, S.; Lorenzo, J.M.; Domínguez, R.; Moreno, A.; Hadidi, M. Spectrophotometric Methods for Measurement of Antioxidant Activity in Food and Pharmaceuticals. Antioxidants 2022, 11, 2213. [Google Scholar] [CrossRef]
- Danet, A.F. Recent Advances in Antioxidant Capacity Assays. In Antioxidants—Benefits, Sources, Mechanisms of Action; IntechOpen: London, UK, 2021; ISBN 978-1-83968-865-2. [Google Scholar]
- Radünz, M.; da Trindade, M.L.M.; Camargo, T.M.; Radünz, A.L.; Borges, C.D.; Gandra, E.A.; Helbig, E. Antimicrobial and Antioxidant Activity of Unencapsulated and Encapsulated Clove (Syzygium aromaticum L.) Essential Oil. Food Chem. 2019, 276, 180–186. [Google Scholar] [CrossRef]
- Yousefi, M.; Khanniri, E.; Shadnoush, M.; Khorshidian, N.; Mortazavian, A.M. Development, Characterization and in Vitro Antioxidant Activity of Chitosan-Coated Alginate Microcapsules Entrapping Viola odorata Linn. Extract. Int. J. Biol. Macromol. 2020, 163, 44–54. [Google Scholar] [CrossRef]
- Moschona, A.; Liakopoulou-Kyriakides, M. Encapsulation of Biological Active Phenolic Compounds Extracted from Wine Wastes in Alginate-Chitosan Microbeads. J. Microencapsul. 2018, 35, 229–240. [Google Scholar] [CrossRef]
- Sarabandi, K.; Jafari, S.M.; Mahoonak, A.S.; Mohammadi, A. Application of Gum Arabic and Maltodextrin for Encapsulation of Eggplant Peel Extract as a Natural Antioxidant and Color Source. Int. J. Biol. Macromol. 2019, 140, 59–68. [Google Scholar] [CrossRef]
- Bruno Romanini, E.; Misturini Rodrigues, L.; Finger, A.; Perez Cantuaria Chierrito, T.; Regina da Silva Scapim, M.; Scaramal Madrona, G. Ultrasound Assisted Extraction of Bioactive Compounds from BRS Violet Grape Pomace Followed by Alginate-Ca2+ Encapsulation. Food Chem. 2021, 338, 128101. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, D.R.A.; Sedaghat Doost, A.; Gupta, V.; bin Sintang, M.D.; Van de Walle, D.; Van der Meeren, P.; Dewettinck, K. Stability and Functionality of Xanthan Gum–Shellac Nanoparticles for the Encapsulation of Cinnamon Bark Extract. Food Hydrocoll. 2020, 100, 105377. [Google Scholar] [CrossRef]
- Aguirre-Calvo, T.R.; Sosa, N.; López, T.A.; Quintanilla-Carvajal, M.X.; Perullini, M.; Santagapita, P.R. Bioaccessibility Assay, Antioxidant Activity and Consumer-Oriented Sensory Analysis of Beta vulgaris by-Product Encapsulated in Ca(II)-Alginate Beads for Different Foods. Food Chem. Mol. Sci. 2022, 5, 100140. [Google Scholar] [CrossRef]
- Gaudreau, H.; Champagne, C.P.; Remondetto, G.E.; Gomaa, A.; Subirade, M. Co-Encapsulation of Lactobacillus Helveticus Cells and Green Tea Extract: Influence on Cell Survival in Simulated Gastrointestinal Conditions. J. Funct. Foods 2016, 26, 451–459. [Google Scholar] [CrossRef]
- Li, R.; Zhang, Y.; Polk, D.B.; Tomasula, P.M.; Yan, F.; Liu, L. Preserving Viability of Lactobacillus rhamnosus GG in Vitro and in Vivo by a New Encapsulation System. J. Control Release 2016, 230, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Suvarna, S.; Dsouza, J.; Ragavan, M.L.; Das, N. Potential Probiotic Characterization and Effect of Encapsulation of Probiotic Yeast Strains on Survival in Simulated Gastrointestinal Tract Condition. Food Sci. Biotechnol. 2018, 27, 745–753. [Google Scholar] [CrossRef]
- Gheorghita Puscaselu, R.; Lobiuc, A.; Dimian, M.; Covasa, M. Alginate: From Food Industry to Biomedical Applications and Management of Metabolic Disorders. Polymers 2020, 12, 2417. [Google Scholar] [CrossRef]
- Ta, L.P.; Bujna, E.; Antal, O.; Ladányi, M.; Juhász, R.; Szécsi, A.; Kun, S.; Sudheer, S.; Gupta, V.K.; Nguyen, Q.D. Effects of Various Polysaccharides (Alginate, Carrageenan, Gums, Chitosan) and Their Combination with Prebiotic Saccharides (Resistant Starch, Lactosucrose, Lactulose) on the Encapsulation of Probiotic Bacteria Lactobacillus casei 01 Strain. Int. J. Biol. Macromol. 2021, 183, 1136–1144. [Google Scholar] [CrossRef] [PubMed]
| Extracts | Chlorogenic Acid (mg/g) | Caffeine (mg/g) | Trigonelline (mg/g) |
|---|---|---|---|
| PVA | 223.02 ± 0.51 a2 | 145.51 ± 3.47 a2 | 0.79 ± 0.09 a1 |
| Green coffee bean | 204.72 ± 3.64 a1 | 116.59 ± 0.20 a3 | 1.04 ± 0.08 a1 |
| Coffee silverskin | 10.83 ± 0.06 a3 | 62.04 ± 0.42 a1 | 4.38 ± 0.01 a2 |
| Extract | Green Coffee Bean | Coffee Silverskin | PVA | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Salmonella | S. aureus | E. coli | Salmonella | S. aureus | E. coli | Salmonella | S. aureus | E. coli | |
| 50% | 100 ± 0 aA* | 100 ± 0 aA | 100 ± 1 aA* | 100 ± 0 aA | 100 ± 0 aA* | 100 ± 0 aA | 100 ± 0 aA | 100 ± 0 aA | 100 ± 1 aA |
| 25% | 49 ± 3 bC | 100 ± 0 aA* | 93 ± 3.5 bB | 99 ± 0 aA* | 100 ± 0 aA | 100 ± 2 aA* | 100 ± 0 aA* | 99 ± 0.6 aA | 98 ± 2 aA* |
| 12.5% | 38 ± 0.4 cC | 41 ± 1 bB | 59 ± 0.2 cC | 87 ± 2.4 bA | 100 ± 0 aA | 99 ± 0.3 aA | 74 ± 0.7 bB | 98 ± 0.9 aA | 87 ± 0 bB |
| 6.25% | 35 ± 1 cA | 28 ± 2 cC | 46 ± 1.1 dB | 20 ± 1.5 cC | 100 ± 0 aA | 24 ± 1 bC | 30 ± 0.4 cB | 97 ± 1 bA* | 66 ± 0 cA |
| Extracts | EC50 (mg/mL) | Total Phenolics (mg of GAE/g of Extract) | |
|---|---|---|---|
| DPPH | ABTS•+ | ||
| Green coffee bean | * | 5.9 ± 0.1 b | 22.3 ± 1.1 c |
| Coffee silverskin | 3.6 ± 0.2 b | 6.8 ± 0.1 c | 26.9 ± 0.2 b |
| PVA | 0.6 ± 0.03 a | 3.2 ± 0.1 a | 89.9 ± 2.8 a |
| Extracts | Microcapsule Diameter (μm) | Encapsulation Efficiency (%) | Viability of Encapsulated K. lactis B10 (log CFU/mL) | |
|---|---|---|---|---|
| T0 | T1 | |||
| Green coffee bean | 1529.49 | 98.05 | 8.93 | 8.76 |
| Coffee silverskin | 1581.12 | 96.51 | 8.87 | 8.75 |
| PVA | 1451.46 | 96.32 | 8.85 | 8.59 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tavares, D.G.; Souza, M.A.M.d.; Santos, T.L.d.; Silva, A.d.A.D.; Abreu, D.J.M.d.; Duarte, W.F. Co-Encapsulation of Coffee and Coffee By-Product Extracts with Probiotic Kluyveromyces lactis. Foods 2024, 13, 3056. https://doi.org/10.3390/foods13193056
Tavares DG, Souza MAMd, Santos TLd, Silva AdAD, Abreu DJMd, Duarte WF. Co-Encapsulation of Coffee and Coffee By-Product Extracts with Probiotic Kluyveromyces lactis. Foods. 2024; 13(19):3056. https://doi.org/10.3390/foods13193056
Chicago/Turabian StyleTavares, Dérica Gonçalves, Mayara Andrade Martins de Souza, Tamara Leite dos Santos, Adriele do Amor Divino Silva, Danilo José Machado de Abreu, and Whasley Ferreira Duarte. 2024. "Co-Encapsulation of Coffee and Coffee By-Product Extracts with Probiotic Kluyveromyces lactis" Foods 13, no. 19: 3056. https://doi.org/10.3390/foods13193056





