Hardness Analysis of Foods in a Diet Based on the Mediterranean Diet and Adapted to Chilean Gastronomy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Groups of Foods Based on Mediterranean Diet
2.2. TPA Analysis
2.2.1. TPA on Dairy Products
2.2.2. TPA on Animal Protein
2.2.3. TPA on Fruits
2.2.4. TPA on Vegetables
2.2.5. TPA on Cereals and Grains
2.2.6. TPA on High Lipid Food and Others
2.2.7. TPA on Vegetal Protein
2.3. Statistical Analysis
3. Results
3.1. Dairy Group
3.2. Animal Protein
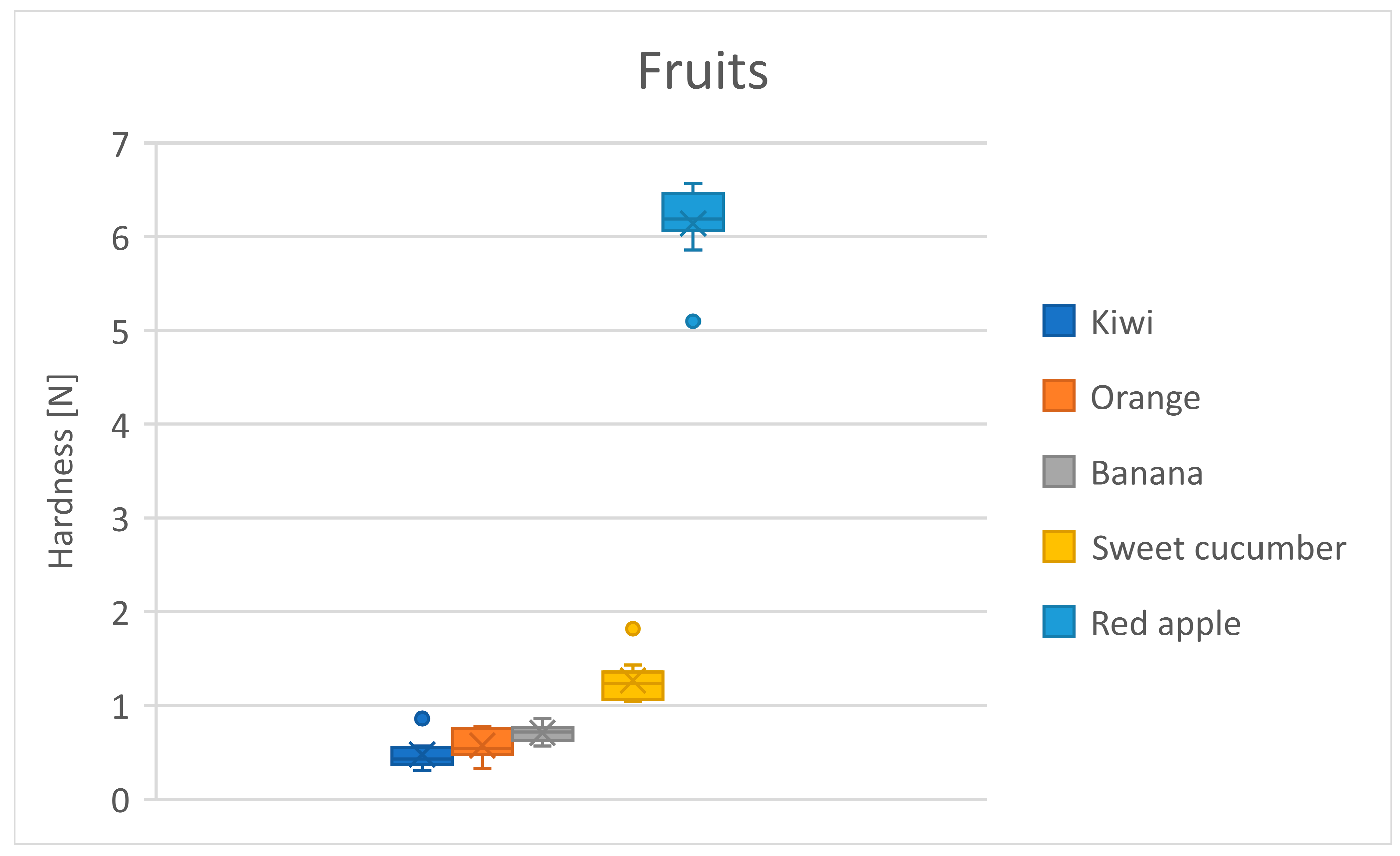
3.3. Fruits
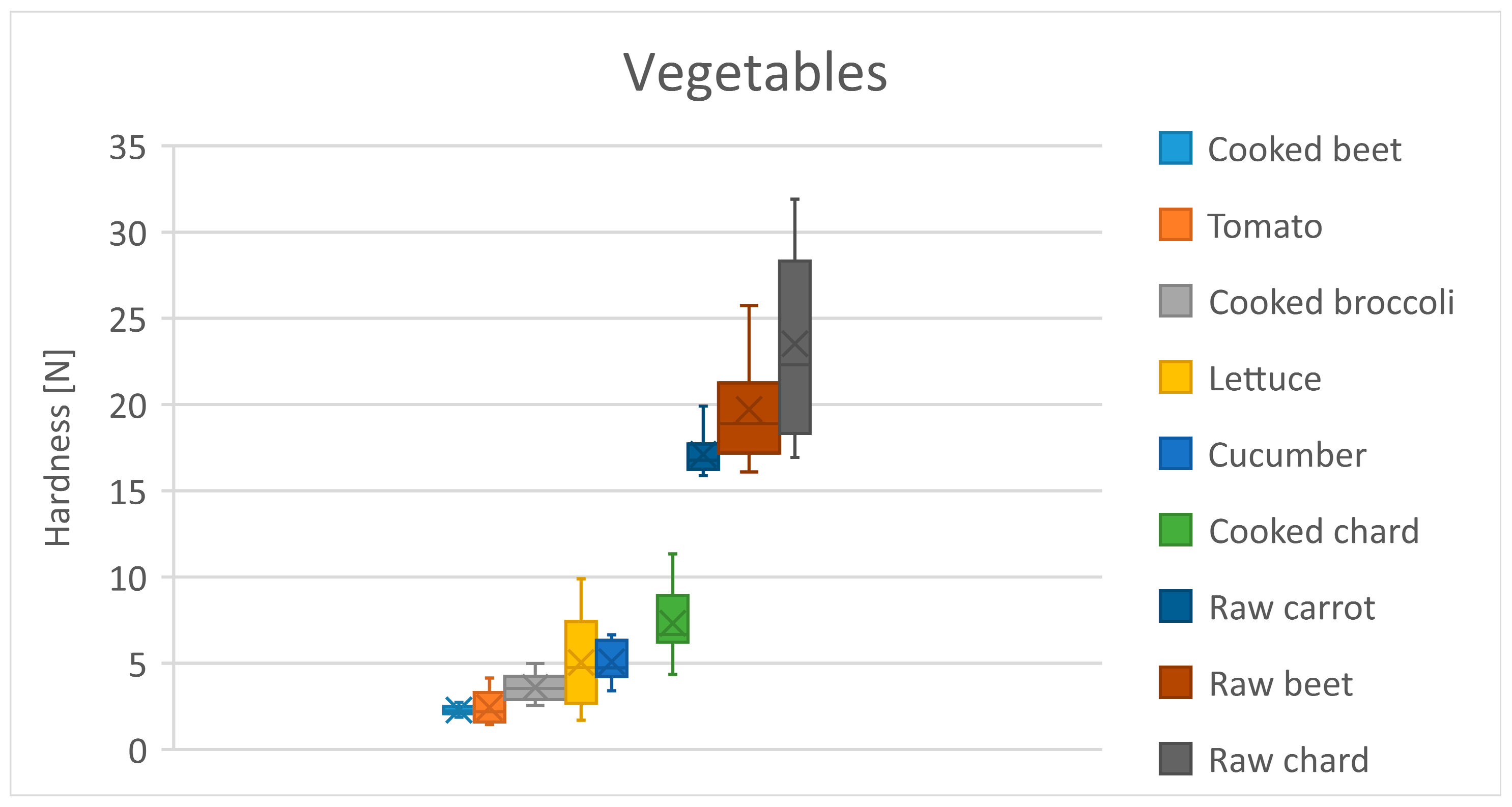
3.4. Vegetables
3.5. Cereals and Grains
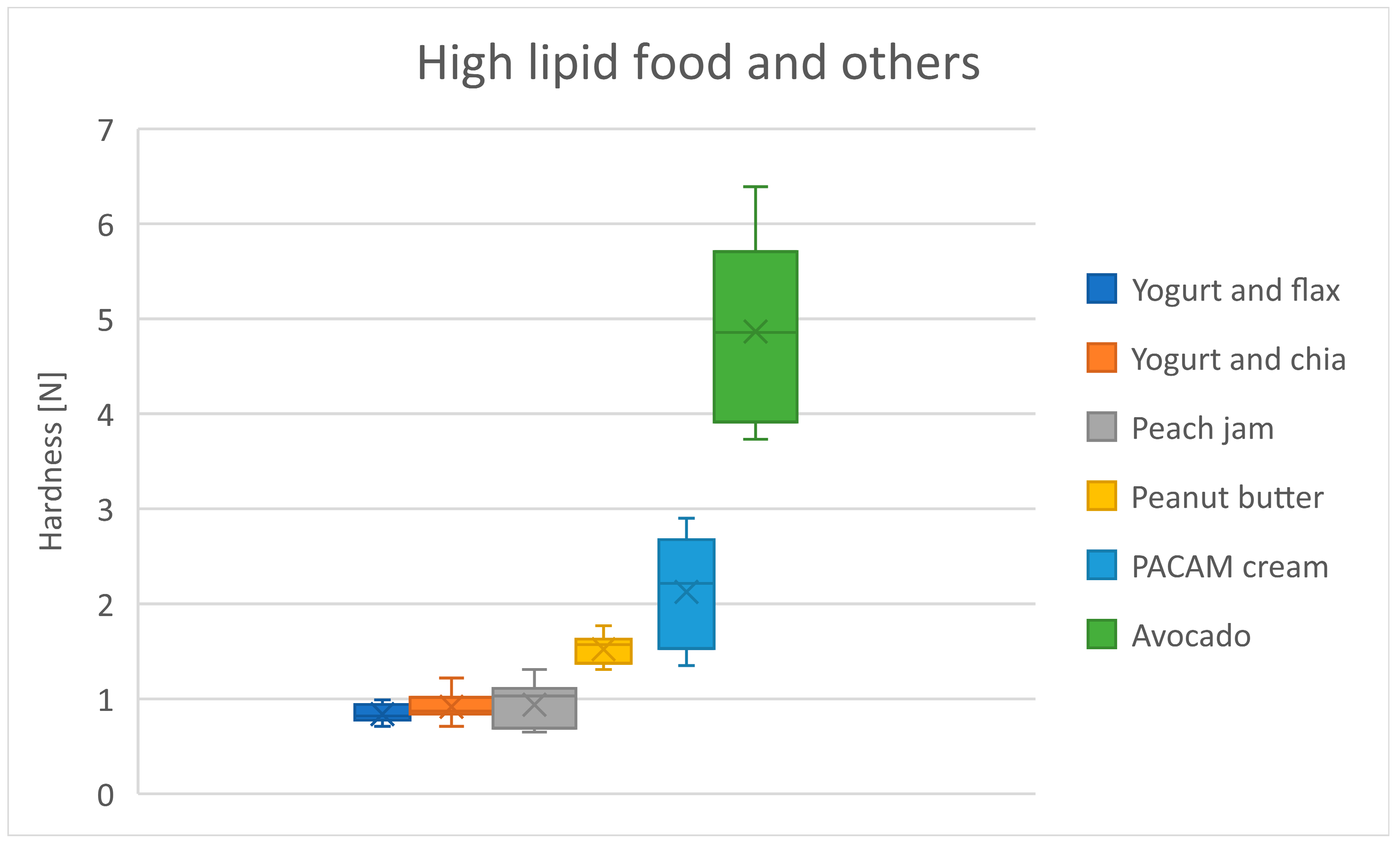
3.6. High Lipid Foods and Others
3.7. Vegetal Protein
4. Discussion
4.1. Food Hardness Ranking
4.2. TPA Analysis and Comparison of Results
4.3. Dental Status and Food Selection
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Diet, Nutrition, and the Prevention of Chronic Diseases: Report of a WHO-FAO Expert Consultation; Joint WHO-FAO Expert Consultation on Diet, Nutrition, and the Prevention of Chronic Diseases, 2002, Geneva, Switzerland; Weltgesundheitsorganisation, FAO, Ed.; WHO Technical Report Series; World Health Organization: Geneva, Switzerland, 2003; ISBN 978-92-4-120916-8.
- Iwasaki, M.; Yoshihara, A.; Ogawa, H.; Sato, M.; Muramatsu, K.; Watanabe, R.; Ansai, T.; Miyazaki, H. Longitudinal Association of Dentition Status with Dietary Intake in Japanese Adults Aged 75 to 80 Years. J. Oral Rehabil. 2016, 43, 737–744. [Google Scholar] [CrossRef]
- Tsakos, G.; Herrick, K.; Sheiham, A.; Watt, R.G. Edentulism and Fruit and Vegetable Intake in Low-Income Adults. J. Dent. Res. 2010, 89, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Gil-Montoya, J.A.; Subirá, C.; Ramón, J.M.; González-Moles, M.A. Oral Health-Related Quality of Life and Nutritional Status. J. Public Health Dent. 2008, 68, 88–93. [Google Scholar] [CrossRef]
- Saarela, R.K.T.; Lindroos, E.; Soini, H.; Hiltunen, K.; Muurinen, S.; Suominen, M.H.; Pitkälä, K.H. Dentition, Nutritional Status and Adequacy of Dietary Intake among Older Residents in Assisted Living Facilities. Gerodontology 2016, 33, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Nowjack-Raymer, R.E.; Sheiham, A. Association of Edentulism and Diet and Nutrition in US Adults. J. Dent. Res. 2003, 82, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Passia, N.; Chaar, M.S.; Krummel, A.; Nagy, A.; Freitag-Wolf, S.; Ali, S.; Kern, M. Influence of the Number of Implants in the Edentulous Mandible on Chewing Efficacy and Oral Health-related Quality of Life—A Within-subject Design Study. Clin. Oral Implant. Res. 2022, 33, 1030–1037. [Google Scholar] [CrossRef]
- Mojon, P. Relationship between Oral Health and Nutrition in Very Old People. Age Ageing 1999, 28, 463–468. [Google Scholar] [CrossRef]
- Oliveira, T.R.C.D.; Frigerio, M.L.M.A. Association between Nutrition and the Prosthetic Condition in Edentulous Elderly. Gerodontology 2004, 21, 205–208. [Google Scholar] [CrossRef]
- Chari, M. The Relationships among Consumption of Fruits, Tooth Loss and Obesity. Community Dent. Health 2018, 35, 148–152. [Google Scholar] [CrossRef]
- Nascimento, G.G.; Leite, F.R.M.; Conceição, D.A.; Ferrúa, C.P.; Singh, A.; Demarco, F.F. Is There a Relationship between Obesity and Tooth Loss and Edentulism? A Systematic Review and Meta-analysis. Obes. Rev. 2016, 17, 587–598. [Google Scholar] [CrossRef]
- Nanri, H.; Yamada, Y.; Itoi, A.; Yamagata, E.; Watanabe, Y.; Yoshida, T.; Miyake, M.; Date, H.; Ishikawa-Takata, K.; Yoshida, M.; et al. Frequency of Fruit and Vegetable Consumption and the Oral Health-Related Quality of Life among Japanese Elderly: A Cross-Sectional Study from the Kyoto-Kameoka Study. Nutrients 2017, 9, 1362. [Google Scholar] [CrossRef] [PubMed]
- Brennan, D.; Singh, K.; Liu, P.; Spencer, A. Fruit and Vegetable Consumption among Older Adults by Tooth Loss and Socio-economic Status. Aust. Dent. J. 2010, 55, 143–149. [Google Scholar] [CrossRef]
- Kumari, S.; Khushboo, B.; Arvind, A.; Singh, S.; Ahsan, A.; Singh, C. Assessment of Role of Complete Dentures in Improving the Chewing Efficiency of Edentulous Patients. J. Pharm. Bioall. Sci. 2022, 14, 792. [Google Scholar] [CrossRef] [PubMed]
- Bessadet, M.; Nicolas, E.; Sochat, M.; Hennequin, M.; Veyrune, J.-L. Impact of Removable Partial Denture Prosthesis on Chewing Efficiency. J. Appl. Oral Sci. 2013, 21, 392–396. [Google Scholar] [CrossRef] [PubMed]
- Taira, K.; Watanabe, Y.; Okada, K.; Kondo, M.; Takeda, M.; Ito, K.; Nakajima, J.; Iwasaki, M.; Itoda, M.; Inohara, K.; et al. Association between Denture Use and Food Form in Older Adults Requiring Long-Term Care: A Multicenter Cross-Sectional Study. J. Prosthodont. Res. 2022, 67, 366–375. [Google Scholar] [CrossRef]
- Su, Y.; Yuki, M.; Hirayama, K.; Sato, M.; Han, T. Denture Wearing and Malnutrition Risk Among Community-Dwelling Older Adults. Nutrients 2020, 12, 151. [Google Scholar] [CrossRef]
- Budiman, L.; Masulili, C.; Ariani, N. Can Denture Wearing Increase the Nutritional Status in Pre-Elderly and Elderly Patients? Pesqui. Bras. Odontopediatria Clín. Integr. 2020, 20, e5679. [Google Scholar] [CrossRef]
- Prakash, N.; Kalavathy, N.; Sridevi, J.; Premnath, K. Nutritional Status Assessment in Complete Denture Wearers. Gerodontology 2012, 29, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Natsui, S.; Sato, M.; Yokoyama, T.; Inaba, H.; Kuboyama, N.; Shibuya, K. Effects of Chewing Frequency on Cerebral Blood Flow and Cognitive Function. J. Behav. Brain Sci. 2020, 10, 287–295. [Google Scholar] [CrossRef]
- Allen, A.P.; Smith, A.P. Effects of Chewing Gum and Time-on-Task on Alertness and Attention. Nutr. Neurosci. 2012, 15, 176–185. [Google Scholar] [CrossRef]
- Smith, A. Effects of Chewing Gum on Cognitive Function, Mood and Physiology in Stressed and Non-Stressed Volunteers. Nutr. Neurosci. 2010, 13, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Hirano, Y.; Obata, T.; Kashikura, K.; Nonaka, H.; Tachibana, A.; Ikehira, H.; Onozuka, M. Effects of Chewing in Working Memory Processing. Neurosci. Lett. 2008, 436, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Tada, A.; Miura, H. Association between Mastication and Cognitive Status: A Systematic Review. Arch. Gerontol. Geriatr. 2017, 70, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Lahoud, T.; Yu, A.Y.; King, S. Masticatory Dysfunction in Older Adults: A Scoping Review. J. Oral Rehabil. 2023, 50, 724–737. [Google Scholar] [CrossRef] [PubMed]
- Rakyoo, K.; Vichayanrat, T.; Anunmana, C.; Kriengsinyos, W.; Gaewkhiew, P. Effect of Dentures and Dietary Advice on Protein Intake in Older Thai Adults with Missing Posterior Occluding Teeth. Gerodontology 2023, 40, 382–389. [Google Scholar] [CrossRef]
- Komagamine, Y.; Suzuki, H.; Iwaki, M.; Minakuchi, S.; Kanazawa, M. Effect of New Complete Dentures and Simple Dietary Advice on Cognitive Screening Test among Edentulous Older Adults: A Randomized Controlled Trial. J. Clin. Med. 2023, 12, 4709. [Google Scholar] [CrossRef]
- Echeverría, G.; Dussaillant, C.; McGee, E.; Urquiaga, I.; Velasco, N.; Rigotti, A. Applying the Mediterranean Diet for Chronic Disease Prevention and Treatment beyond the Mediterranean Basin. In Mediterranean Identities—Environment, Society, Culture; Fuert-Bjelis, B., Ed.; IntechOpenLimited: London, UK, 2017; pp. 401–414. [Google Scholar]
- Sikalidis, A.K.; Kelleher, A.H.; Kristo, A.S. Mediterranean Diet. Encyclopedia 2021, 1, 371–387. [Google Scholar] [CrossRef]
- Romaguera, D.; Norat, T.; Vergnaud, A.-C.; Mouw, T.; May, A.M.; Agudo, A.; Buckland, G.; Slimani, N.; Rinaldi, S.; Couto, E.; et al. Mediterranean Dietary Patterns and Prospective Weight Change in Participants of the EPIC-PANACEA Project. Am. J. Clin. Nutr. 2010, 92, 912–921. [Google Scholar] [CrossRef]
- Steffen, L.M.; Van Horn, L.; Daviglus, M.L.; Zhou, X.; Reis, J.P.; Loria, C.M.; Jacobs, D.R.; Duffey, K.J. A Modified Mediterranean Diet Score Is Associated with a Lower Risk of Incident Metabolic Syndrome over 25 Years among Young Adults: The CARDIA (Coronary Artery Risk Development in Young Adults) Study. Br. J. Nutr. 2014, 112, 1654–1661. [Google Scholar] [CrossRef]
- Martínez-González, M.A.; Gea, A.; Ruiz-Canela, M. The Mediterranean Diet and Cardiovascular Health: A Critical Review. Circ. Res. 2019, 124, 779–798. [Google Scholar] [CrossRef]
- Sofi, F.; Cesari, F.; Abbate, R.; Gensini, G.F.; Casini, A. Adherence to Mediterranean Diet and Health Status: Meta-Analysis. BMJ 2008, 337, a1344. [Google Scholar] [CrossRef]
- Maraki, M.I.; Yannakoulia, M.; Stamelou, M.; Stefanis, L.; Xiromerisiou, G.; Kosmidis, M.H.; Dardiotis, E.; Hadjigeorgiou, G.M.; Sakka, P.; Anastasiou, C.A.; et al. Mediterranean Diet Adherence Is Related to Reduced Probability of Prodromal Parkinson’s Disease. Mov. Disord. 2019, 34, 48–57. [Google Scholar] [CrossRef]
- García-Casares, N.; Gallego Fuentes, P.; Barbancho, M.Á.; López-Gigosos, R.; García-Rodríguez, A.; Gutiérrez-Bedmar, M. Alzheimer’s Disease, Mild Cognitive Impairment and Mediterranean Diet. A Systematic Review and Dose-Response Meta-Analysis. J. Clin. Med. 2021, 10, 4642. [Google Scholar] [CrossRef]
- Wu, Y.; He, B.; Chen, Q.; Yu, R.; Wu, Y.; Yang, H.; Yu, Y.; Yan, F.; Li, Y.; Chen, F. Association between Mediterranean Diet and Periodontitis among US Adults: The Mediating Roles of Obesity Indicators. J. Periodontal. Res. 2023, 59, 32–41. [Google Scholar] [CrossRef]
- Urquiaga, I.; Echeverría, G.; Dussaillant, C.; Rigotti, A. Origen, Componentes y Posibles Mecanismos de Acción de La Dieta Mediterránea. Rev. Méd. Chile 2017, 145, 85–95. [Google Scholar] [CrossRef]
- Tonni, I.; Ricciardi, G.; Piancino, M.G.; Stretti, C.; Costantinides, F.; Paganelli, C. The Influence of Food Hardness on the Physiological Parameters of Mastication: A Systematic Review. Arch. Oral Biol. 2020, 120, 104903. [Google Scholar] [CrossRef]
- Park, H.S.; Kim, D.; Lee, S.Y.; Park, K. The Effect of Aging on Mastication and Swallowing Parameters According to the Hardness Change of Solid Food. J. Texture Stud. 2017, 48, 362–369. [Google Scholar] [CrossRef]
- Laird, M.F. Variation in Human Gape Cycle Kinematics and Occlusal Topography. Am. J Phys Anthr. 2017, 164, 574–585. [Google Scholar] [CrossRef] [PubMed]
- Pematilleke, N.; Kaur, M.; Adhikari, B.; Torley, P. Influence of Meat Texture on Oral Processing and Bolus Formation. J. Food Eng. 2020, 283, 110038. [Google Scholar] [CrossRef]
- Peleg, M. The Instrumental Texture Profile Analysis Revisited. J. Texture Stud. 2019, 50, 362–368. [Google Scholar] [CrossRef]
- Trịnh, K.T.; Glasgow, S. On the Texture Profile Analysis Test. In Proceedings of the Chemeca 2012, Wellington, New Zealand, 23–26 September 2012. [Google Scholar]
- Davis, C.; Bryan, J.; Hodgson, J.; Murphy, K. Definition of the Mediterranean Diet; A Literature Review. Nutrients 2015, 7, 9139–9153. [Google Scholar] [CrossRef] [PubMed]
- Riquelme, N.; Robert, P.; Arancibia, C. Understanding Older People Perceptions about Desserts Using Word Association and Sorting Task Methodologies. Food Qual. Prefer. 2022, 96, 104423. [Google Scholar] [CrossRef]
- Ando, S.; Sakuma, M.; Morimoto, Y.; Arai, H. The Effect of Various Boiling Conditions on Reduction of Phosphorus and Protein in Meat. J. Ren. Nutr. 2015, 25, 504–509. [Google Scholar] [CrossRef]
- Arias-Rico, J.; Macías-León, F.J.; Alanís-García, E.; Cruz-Cansino, N.D.S.; Jaramillo-Morales, O.A.; Barrera-Gálvez, R.; Ramírez-Moreno, E. Study of Edible Plants: Effects of Boiling on Nutritional, Antioxidant, and Physicochemical Properties. Foods 2020, 9, 599. [Google Scholar] [CrossRef] [PubMed]
- Sasmazer, R.C.; Korukluoglu, M.; Ginoyan, R.V.; Platova, G.I. White Cheese Texture Profile at Different Storage Temperatures. IOP Conf. Ser. Earth Environ. Sci. 2022, 1052, 012061. [Google Scholar] [CrossRef]
- Ismail, H.A.; El-Sawah, T.H.; Ayyash, M.; Adhikari, B.; Elkot, W.F. Functionalization of Ricotta Cheese with Powder of Spirulina platensis: Physicochemical, Sensory, and Microbiological Properties. Int. J. Food Prop. 2023, 26, 1968–1983. [Google Scholar] [CrossRef]
- Aguirre, M.E.; Owens, C.M.; Miller, R.K.; Alvarado, C.Z. Descriptive Sensory and Instrumental Texture Profile Analysis of Woody Breast in Marinated Chicken. Poult. Sci. 2018, 97, 1456–1461. [Google Scholar] [CrossRef]
- Segundo, C.; Román, L.; Gómez, M.; Martínez, M.M. Mechanically Fractionated Flour Isolated from Green Bananas (M. cavendishii Var. Nanica) as a Tool to Increase the Dietary Fiber and Phytochemical Bioactivity of Layer and Sponge Cakes. Food Chem. 2017, 219, 240–248. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, H.; Ye, L.; Fan, D.; Wang, Z. Effects of the Combination of Phytic Acid and Vacuum Packaging on Storage Quality of Fresh-Cut Lettuce. Food Sci. Technol. Res. 2022, 28, 45–52. [Google Scholar] [CrossRef]
- Nyorere, O. Instrumental Texture Profile Analysis (TPA) of Cucumber Fruit as Influenced by Its Part and Maturity Stage. Am. J. Eng. Technol. Manag. 2018, 3, 54. [Google Scholar] [CrossRef]
- Gebregziabher, A.A.; Supriyadi, S.; Indarti, S.; Setyowati, L. Texture Profile and Pectinase Activity in Tomato Fruit (Solanum lycopersicum, Servo F1) at Different Maturity Stages and Storage Temperatures. Plant Trop. 2021, 9, 20–34. [Google Scholar] [CrossRef]
- Sert, D.; Mercan, E.; Tongur, A.; Kara, Ü. Production of Bread from Doughs Composed of High-Pressure Homogenisation Treated Flour Slurries: Effects on Physicochemical, Crumb Grain and Textural Characteristics. Food Meas. 2021, 15, 3052–3059. [Google Scholar] [CrossRef]
- Bragança, G.; Ávila, B.; Rockenbach, R.; Santos, M.; Alves, G.; Santos, M.D.L.; Bortolini, V.; Monks, J.; Peres, W.; Elias, M. Effects of Different Hydration Treatments on Technological, Physical, Nutritional, and Bioactive Parameters of Lentils. Rev. Chil. Nutr. 2020, 47, 658–668. [Google Scholar] [CrossRef]
- Department of Cattle, Sheep Breeding and Milk Evaluation, Siedlce University of Natural Sciences and Humanities, Siedlce, Poland; Department of Cattle Breeding, UTP University of Science and Technology, Bydgoszcz, Poland; Młynek, K.; Oler, A.; Zielińska, K.; Tkaczuk, J.; Zawadzka, W. The Effect of Selected Components of Milk and Ripening Time on the Development of the Hardness and Melting Properties of Cheese. Acta Sci. Pol. Technol. Aliment. 2018, 17, 133–140. [Google Scholar] [CrossRef]
- Joshi, N.S.; Jhala, R.P.; Muthukumarappan, K.; Acharya, M.R.; Mistry, V.V. Textural and Rheological Properties of Processed Cheese. Int. J. Food Prop. 2004, 7, 519–530. [Google Scholar] [CrossRef]
- Bruce, H.L.; Roy, B.C. Production Factors Affecting the Contribution of Collagen to Beef Toughness. J. Anim. Sci. 2019, 97, 2270–2278. [Google Scholar] [CrossRef]
- Pematilleke, N.; Kaur, M.; Adhikari, B.; Torley, P.J. Relationship between Masticatory Variables and Bolus Characteristics of Meat with Different Textures. J. Texture Stud. 2021, 52, 552–560. [Google Scholar] [CrossRef]
- Singh, V.; Guizani, N.; Al-Alawi, A.; Claereboudt, M.; Rahman, M.S. Instrumental Texture Profile Analysis (TPA) of Date Fruits as a Function of Its Physico-Chemical Properties. Ind. Crops Prod. 2013, 50, 866–873. [Google Scholar] [CrossRef]
- Rahman, M.S.; Al-Farsi, S.A. Instrumental Texture Profile Analysis (TPA) of Date Flesh as a Function of Moisture Content. J. Food Eng. 2005, 66, 505–511. [Google Scholar] [CrossRef]
- Abu El-Maaty, E.A. Some Factors Affecting the Mechanical Coating of Quinoa Seeds. J. Soil Sci. Agric. Eng. 2021, 12, 591–596. [Google Scholar] [CrossRef]
- Singh, R.; Guerrero, M.; Nickerson, M.T.; Koksel, F. Effects of Extrusion Screw Speed, Feed Moisture Content, and Barrel Temperature on the Physical, Techno-functional, and Microstructural Quality of Texturized Lentil Protein. J. Food Sci. 2024, 89, 2040–2053. [Google Scholar] [CrossRef]
- Bargale, P.C.; Irudayaraj, J.; Marquis, B. Some Mechanical Properties and Stress Relaxation Characteristics of Lentils. Agric. Food Sci. 1994, 36, 247–253. [Google Scholar]
- Sahu, U.; Prasad, K.; Sahoo, P.; Sahu, B.B. Studies on Engineering Properties of Raw and Roasted Pulses. Int. J. Process. Post Harvest. Technol. 2014, 5, 184–188. [Google Scholar] [CrossRef]
- Rosenthal, A.J. Texture profile analysis—How important are the parameters? Parameters in texture profile analysis. J. Texture Stud. 2010, 41, 672–684. [Google Scholar] [CrossRef]
- Alvarez, M.; Canet, W.; López, M. Influence of Deformation Rate and Degree of Compression on Textural Parameters of Potato and Apple Tissues in Texture Profile Analysis. Eur. Food Res. Technol. 2002, 215, 13–20. [Google Scholar] [CrossRef]
- Nishinari, K.; Fang, Y.; Rosenthal, A. Human Oral Processing and Texture Profile Analysis Parameters: Bridging the Gap between the Sensory Evaluation and the Instrumental Measurements. J. Texture Stud. 2019, 50, 369–380. [Google Scholar] [CrossRef]
- Kohyama, K.; Mioche, L.; Martin, J. Chewing patterns of various texture foods studied by electromyography in young and elderly populations. J. Texture Stud. 2002, 33, 269–283. [Google Scholar] [CrossRef]
- Wee, M.S.M.; Goh, A.T.; Stieger, M.; Forde, C.G. Correlation of Instrumental Texture Properties from Textural Profile Analysis (TPA) with Eating Behaviours and Macronutrient Composition for a Wide Range of Solid Foods. Food Funct. 2018, 9, 5301–5312. [Google Scholar] [CrossRef]
- Di Monaco, R.; Cavella, S.; Masi, P. Predicting sensory cohesiveness, hardness and springiness of solid foods from instrumental measurements. J. Texture Stud. 2008, 39, 129–149. [Google Scholar] [CrossRef]
- Marcenes, W.; Steele, J.G.; Sheiham, A.; Walls, A.W.G. The Relationship between Dental Status, Food Selection, Nutrient Intake, Nutritional Status, and Body Mass Index in Older People. Cad. Saúde Pública 2003, 19, 809–815. [Google Scholar] [CrossRef]
- Watson, S.; McGowan, L.; McCrum, L.-A.; Cardwell, C.R.; McGuinness, B.; Moore, C.; Woodside, J.V.; McKenna, G. The Impact of Dental Status on Perceived Ability to Eat Certain Foods and Nutrient Intakes in Older Adults: Cross-Sectional Analysis of the UK National Diet and Nutrition Survey 2008–2014. Int. J. Behav. Nutr. Phys. Act. 2019, 16, 43. [Google Scholar] [CrossRef] [PubMed]
- Al-Sultani, H.F.F.; Breckons, M.; Field, J.; Thomason, J.M.; Moynihan, P. Development of Patient-centric Eating Advice for Complete Denture Wearers. Gerodontology 2023, 40, 56–73. [Google Scholar] [CrossRef] [PubMed]





| Dairy | Animal Protein | Fruits | Vegetables | Cereals and Grains | High Lipid Food and Others | Vegetal Protein |
|---|---|---|---|---|---|---|
| Ricotta | Eggs | Kiwi | Cooked beet | Oatmeal and milk | Yogurt and flax | Lentil 4 mm |
| Yogurt | Jack Mackerel | Orange | Tomato | Fusilli noodles | Yogurt and chia | Lentil 5 mm |
| Soft unsalted cheese | Chicken thigh | Banana | Cooked broccoli | Whole-wheat sliced bread | Peach jam | Beans |
| Buttery cheese | Turkey breast | Sweet cucumber | Lettuce | Whole-wheat bread with seeds | Peanut butter | Chickpeas |
| Cucumber | Cooked quinoa | PACAM cream | ||||
| Chanco cheese | Beef | Red apple | Cooked chard | Whole-grain rice | Avocado | Split peas |
| Raw carrot | Couscous | |||||
| Raw beets | Husked wheatberries | |||||
| Raw chard |
| Foods | Cooking Process | Cooking Time (min) | Size Sample | Probe |
|---|---|---|---|---|
| Buttery cheese | Raw | - | 4 × 4 × 2 cm | TA-10 (12.7 mm Ø) |
| Laminate cheese | ||||
| Soft unsalted cheese | ||||
| Chanco cheese | ||||
| Ricotta | Raw | - | Beaker filled with 40 mL, reaching a height of 4 cm | TA4-1000 probe (38.1 mm Ø) |
| Yogurt |
| Foods | Cooking Process | Cooking Time (min) | Sample Size | Probe |
|---|---|---|---|---|
| Canned jack mackerel | Raw | - | 4 × 4 × 2 cm height | TA-10 (12.7 mm Ø) |
| White eggs | Boiled | 10 | ||
| Chicken thighs | Boiled | 30 | ||
| Beef | Boiled | 50 | ||
| Turkey breast | Baked 180 °C | 80 |
| Foods | Cooking Process | Cooking Time (min) | Size Sample | Probe |
|---|---|---|---|---|
| Kiwi | - | - | 4 × 4 × 2 cm | Magnes Tylor (3 mm Ø) |
| Orange | 2 cm | |||
| Banana | 2 cm | |||
| Sweet cucumber | 2 cm | |||
| Red apple | 4 × 4 × 2 cm |
| Foods | Cooking Process | Cooking Time (min) | Sample Size | Probe |
|---|---|---|---|---|
| Raw chard | Raw | - | 5 × 5 × 1 cm | TA44 (4 mm Ø) |
| Cooked chard | Boiled | 3 | ||
| Lettuce | Raw | - | ||
| Cooked broccoli | Boiled | 5 | 2 cm height | Magnes Tylor (3 mm Ø) |
| Tomato | Raw | - | ||
| Cucumber | Raw | - | ||
| Raw carrot | Raw | - | ||
| Raw beets | Raw | - | ||
| Cooked beet | Boiled | 25 |
| Foods | Cooking Process | Cooking Time (min) | Sample Size | Probe |
|---|---|---|---|---|
| Oatmeal and milk | 4 teaspoons with 200 mL of milk | 60 resting | Beaker filled with 40 mL, reaching a height of 4 cm | TA4-1000 probe (38.1 mm Ø) |
| Fusilli noodles | Boiled | 10 | Ottawa cell (447 mL) filled until reaching a height of 2 cm | 36 mm Ø |
| Whole-wheat sliced bread | Raw | - | ||
| Whole-wheat sliced bread with seeds | Raw | - | ||
| Cooked quinoa | Boiled | 5 | ||
| Whole-grain rice | Boiled | 10 | ||
| Couscous | Boiled | 5 | ||
| Husked wheatberries | Boiled | 30 |
| Foods | Cooking Process | Cooking Time (min) | Sample Size | Probe |
|---|---|---|---|---|
| Yogurt and flax | 12 g of flax mixed with 600 mL of yogurt | - | Beaker filled with 40 mL, reaching a height of 4 cm | TA4-1000 probe (38.1 mm Ø) |
| Yogurt and chia | 15 g of chi mixed with 600 mL of yogurt | |||
| Peach jam | Raw | |||
| Peanut butter | Raw | |||
| PACAM cream | Package instructions | |||
| Avocado | Raw | 2 cm height | TA-10 (12.7 mm Ø) |
| Foods | Cooking Process | Cooking Time (min) | Sample Size | Probe |
|---|---|---|---|---|
| 4 mm Lentil | Boiled | 20 | Ottawa cell (447 mL) filled until reaching a height of 2 cm | TA4-1000 probe (38.1 mm Ø) |
| 5 mm Lentil | ||||
| White beans | 30 | |||
| Chickpeas | ||||
| Split Peas |
| Group | Food | Mean [N] | ±SD [N] |
|---|---|---|---|
| Dairy | Ricotta | 0.6 | 0.2 |
| Yogurt | 0.7 | 0.1 | |
| Soft unsalted cheese | 2.7 | 0.4 | |
| Buttery cheese | 6.3 | 1.2 | |
| Chanco cheese | 27.3 | 4.0 | |
| Animal Protein | Eggs | 4.4 | 1.7 |
| Jack mackerel | 8.2 | 3.7 | |
| Chicken thigh | 17.5 | 5.1 | |
| Turkey breast | 19.4 | 5.7 | |
| Beef | 47.0 | 9.3 | |
| Fruits | Kiwi * | 0.5 | 0.2 |
| Orange | 0.6 | 0.2 | |
| Banana | 0.7 | 0.1 | |
| Sweet cucumber | 1.3 | 0.2 | |
| Red apple * | 6.1 | 0.4 | |
| Vegetables | Cooked beet | 2.3 | 0.3 |
| Tomato | 2.4 | 1.0 | |
| Cooked broccoli | 3.6 | 0.8 | |
| Lettuce | 5.0 | 2.8 | |
| Cucumber | 5.1 | 1.1 | |
| Cooked chard | 7.3 | 2.1 | |
| Raw carrot | 17.1 | 1.2 | |
| Raw beets * | 19.7 | 3.3 | |
| Raw chard | 23.5 | 5.6 | |
| Cereals and Grains | Oatmeal and milk * | 0.3 | 0.1 |
| Fusilli noodles | 2.2 | 0.6 | |
| Integral sliced bread | 5.9 | 1.1 | |
| Integral sliced bread with seeds | 7.7 | 2.2 | |
| Cooked quinoa | 10.0 | 2.2 | |
| Integral rice | 10.4 | 2.8 | |
| Couscous | 11.2 | 2.7 | |
| Husked wheat berries | 22.2 | 6.7 | |
| High lipid food and others | Yogurt and flax | 0.8 | 0.1 |
| Yogurt and chia | 0.9 | 0.1 | |
| Peach jam | 0.9 | 0.2 | |
| Peanut butter | 1.5 | 0.2 | |
| PACAM cream | 2.1 | 0.6 | |
| Avocado | 4.9 | 0.9 | |
| Vegetal protein | Lentil 4 mm | 14.9 | 3.3 |
| Lentil 5 mm | 23.0 | 6.2 | |
| Beans | 48.1 | 14.3 | |
| Chickpea | 76.3 | 14.4 | |
| Split peas | 88.1 | 24.5 |
| Dairy | Yogurt | Soft Unsalted Cheese | Buttery Cheese | Chanco Cheese |
|---|---|---|---|---|
| Ricotta | 0.1 | 2.1 | 5.7 *** | 26.7 *** |
| Yogurt | - | 2.0 | 5.6 *** | 26.6 *** |
| Soft unsalted cheese | - | - | 3.6 *** | 24.6 *** |
| Buttery cheese | - | - | - | 21.0 *** |
| Animal Protein | Jack Mackerel | Chicken Thigh | Turkey Breast | Beef |
|---|---|---|---|---|
| Eggs | 3.8 | 13.1 *** | 15.1 *** | 42.6 *** |
| Jack mackerel | - | 9.3 ** | 11.2 *** | 38.8 *** |
| Chicken thigh | - | - | 1.9 | 29.5 *** |
| Turkey breast | - | - | - | 27.5 *** |
| Fruits | Orange | Banana | Sweet Cucumber | Red Apple |
|---|---|---|---|---|
| Kiwi | 0.1 | 0.2 * | 0.8 *** | 5.7 *** |
| Orange | - | 0.1 | 0.7 *** | 5.6 *** |
| Banana | - | - | 0.6 *** | 5.4 *** |
| Sweet cucumber | - | - | - | 4.9 *** |
| Vegetables | Tomato | Cooked Broccoli | Lettuce | Cucumber | Cooked Chard | Raw Carrot | Raw Beet | Raw Chard |
|---|---|---|---|---|---|---|---|---|
| Cooked beet | 0.2 | 1.3 | 2.8 | 2.8 | 5.0 ** | 14.8 *** | 17.4 * | 21.3 *** |
| Tomato | - | 1.2 | 2.6 | 2.7 | 4.9 ** | 14.7 *** | 17.3 * | 21.1 *** |
| Cooked broccoli | - | - | 1.5 | 1.5 | 3.72 * | 13.5 *** | 16.1 * | 20.0 *** |
| Lettuce | - | - | - | 0.05 | 2.3 | 12.1 *** | 14.7 * | 18.5 *** |
| Cucumber | - | - | - | - | 2.2 | 12.0 *** | 14.6 * | 18.5 *** |
| Cooked chard | - | - | - | - | - | 9.8 *** | 12.4 * | 16.2 *** |
| Raw carrot | - | - | - | - | - | - | 2.6 | 6.4 *** |
| Raw beet | - | - | - | - | - | - | - | 3.8 |
| Cereals and Grains | Fusilli Noodles | Integral Sliced Bread | Integral Sliced Bread with Seeds | Cooked Quinoa | Integral Rice | Couscous | Husked Wheat Berries |
|---|---|---|---|---|---|---|---|
| Oatmeal and milk | 1.9 ** | 5.6 ** | 7.4 ** | 9.7 ** | 10.1 ** | 11.0 ** | 21.9 ** |
| Fusilli noodles | - | 3.7 | 5.5 ** | 7.8 *** | 8.2 *** | 9.0 *** | 20.0 *** |
| Integral sliced bread | - | - | 1.8 | 4.1 | 4.5 * | 5.3 ** | 16.3 *** |
| Integral sliced bread with seeds | - | - | - | 2.3 | 2.7 | 3.5 | 14.4 *** |
| Cooked quinoa | - | - | - | - | 0.4 | 1.2 | 12.2 *** |
| Integral rice | - | - | - | - | - | 0.8 | 11.8 *** |
| Couscous | - | - | - | - | - | - | 11.0 *** |
| High Lipid Foods and Others | Yogurt and Chia | Peach Jam | Peanut Butter | PACAM Cream | Avocado |
|---|---|---|---|---|---|
| Yogurt and flax | 0.1 | 0.1 | 0.7 * | 1.3 *** | 4.0 *** |
| Yogurt and chia | - | 0.0 | 0.6 | 1.2 *** | 4.0 *** |
| Peach jam | - | - | 0.6 | 1.2 *** | 4.0 *** |
| Peanut butter | - | - | - | 0.6 | 3.3 *** |
| PACAM cream | - | - | - | - | 2.7 *** |
| Vegetal Protein | Lentil 5 mm | Beans | Chickpea | Split Peas |
|---|---|---|---|---|
| Lentil 4 mm | 8.1 | 33.1 *** | 61.3 *** | 73.2 *** |
| Lentil 5 mm | - | 25.0 ** | 53.2 *** | 65.1 *** |
| Beans | - | - | 28.2 *** | 40.0 *** |
| Chickpea | - | - | - | 12.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marinelli, F.; Venegas, C.; Pirce, F.; del Carmen Silva Celedón, J.; Navarro, P.; Jarpa-Parra, M.; Fuentes, R. Hardness Analysis of Foods in a Diet Based on the Mediterranean Diet and Adapted to Chilean Gastronomy. Foods 2024, 13, 3061. https://doi.org/10.3390/foods13193061
Marinelli F, Venegas C, Pirce F, del Carmen Silva Celedón J, Navarro P, Jarpa-Parra M, Fuentes R. Hardness Analysis of Foods in a Diet Based on the Mediterranean Diet and Adapted to Chilean Gastronomy. Foods. 2024; 13(19):3061. https://doi.org/10.3390/foods13193061
Chicago/Turabian StyleMarinelli, Franco, Camila Venegas, Fanny Pirce, Jennifer del Carmen Silva Celedón, Pablo Navarro, Marcela Jarpa-Parra, and Ramón Fuentes. 2024. "Hardness Analysis of Foods in a Diet Based on the Mediterranean Diet and Adapted to Chilean Gastronomy" Foods 13, no. 19: 3061. https://doi.org/10.3390/foods13193061





