Isorhamnetin in Quinoa Whole-Grain Flavonoids Intervenes in Non-Alcoholic Fatty Liver Disease by Modulating Bile Acid Metabolism through Regulation of FXR Expression
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of CQWF30
2.3. UPLC-ESI-MS/MS Experiment
2.4. Network Pharmacology Analysis
2.5. Cell Culture
2.6. CCK-8 Proliferation Assay
2.7. TG Content and Lipid-Related Indexes Detection Assay
2.8. Oil Red O Staining Experiment
2.9. Nile Red Staining Experiment
2.10. Animal Experiments
2.10.1. Animal Experiment Design
2.10.2. Hematoxylin and Eosin (H&E) Staining Experiment
2.10.3. TBA Content Detection Assay
2.10.4. Bile Acid Metabolic Profiling
2.10.5. Principal Component Analysis (PCA)
2.11. Western Blot Assay
2.12. Statistical Analysis
3. Results
3.1. The Prediction of Active Components and Mechanism (s) in CQWF30 Intervenes with NAFLD
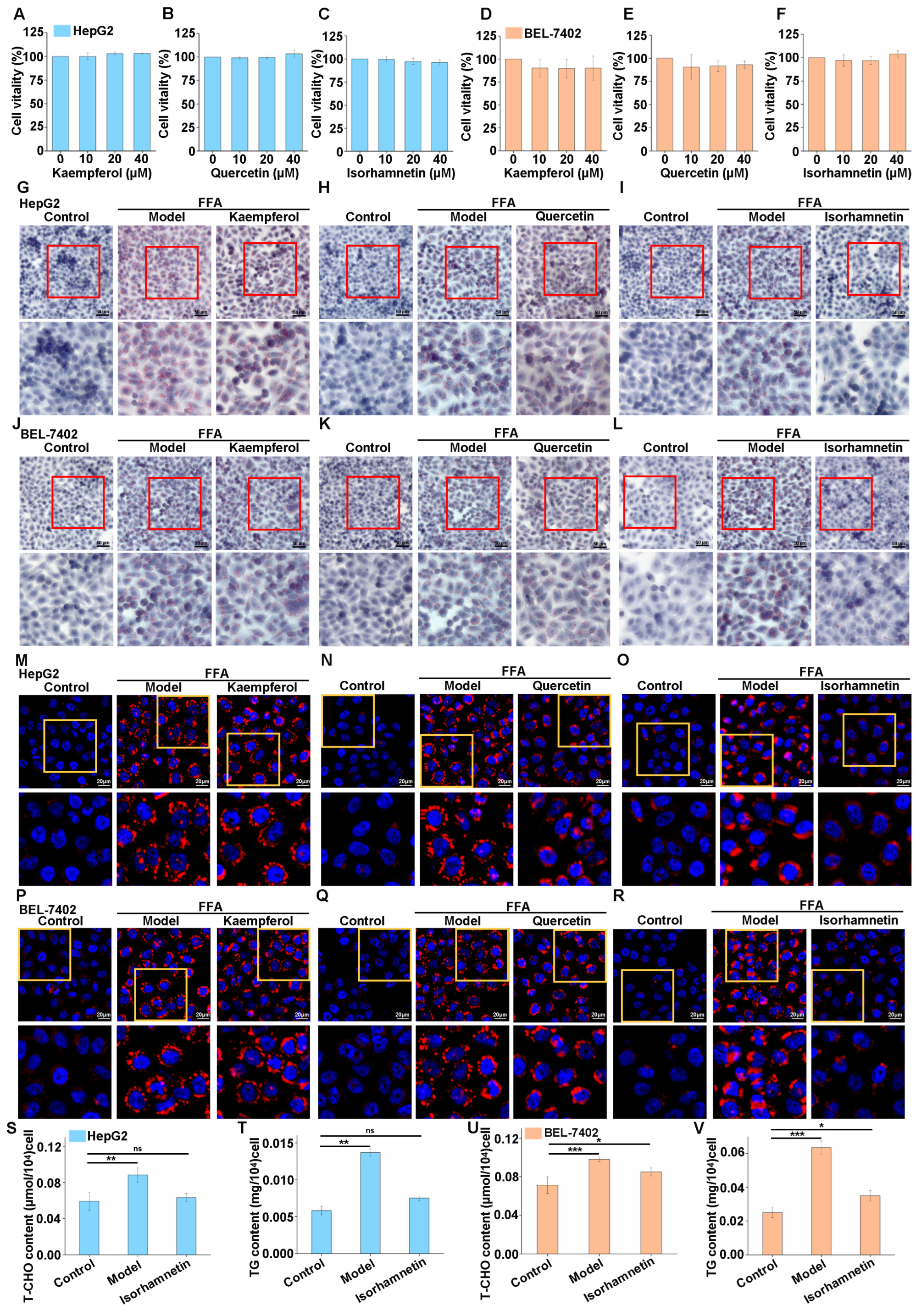
3.2. Isorhamnetin Can Inhibit Lipid Accumulation in High-Fat Cells Induced by FFA
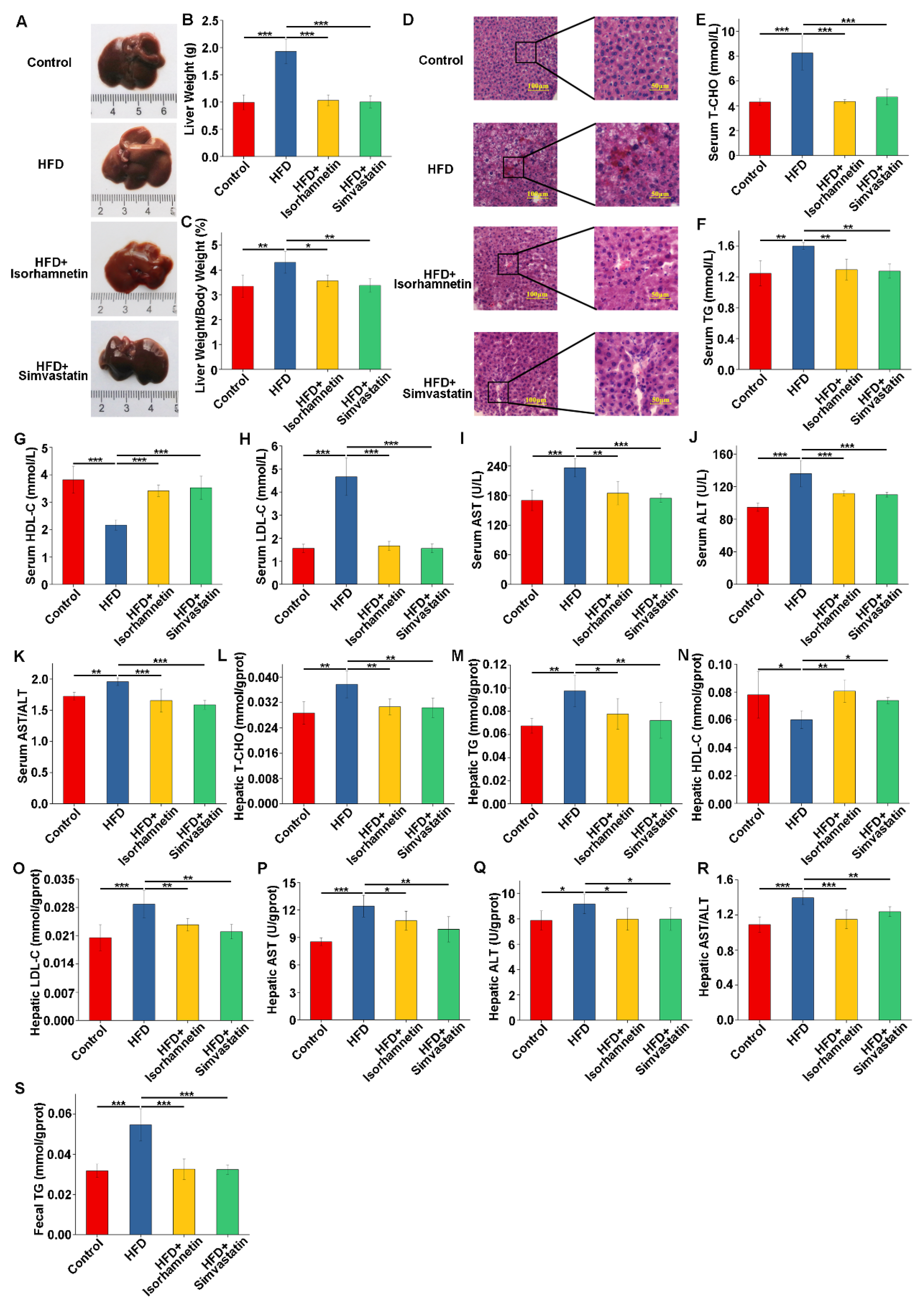
3.3. Isorhamnetin Derived from Quercetin Modulates the Weight of Mice on an HFD and Reduces Fat Accumulation
3.4. Isorhamnetin Intervention Alleviates HFD-Induced Liver Injury and Improves Related Indices in Mice
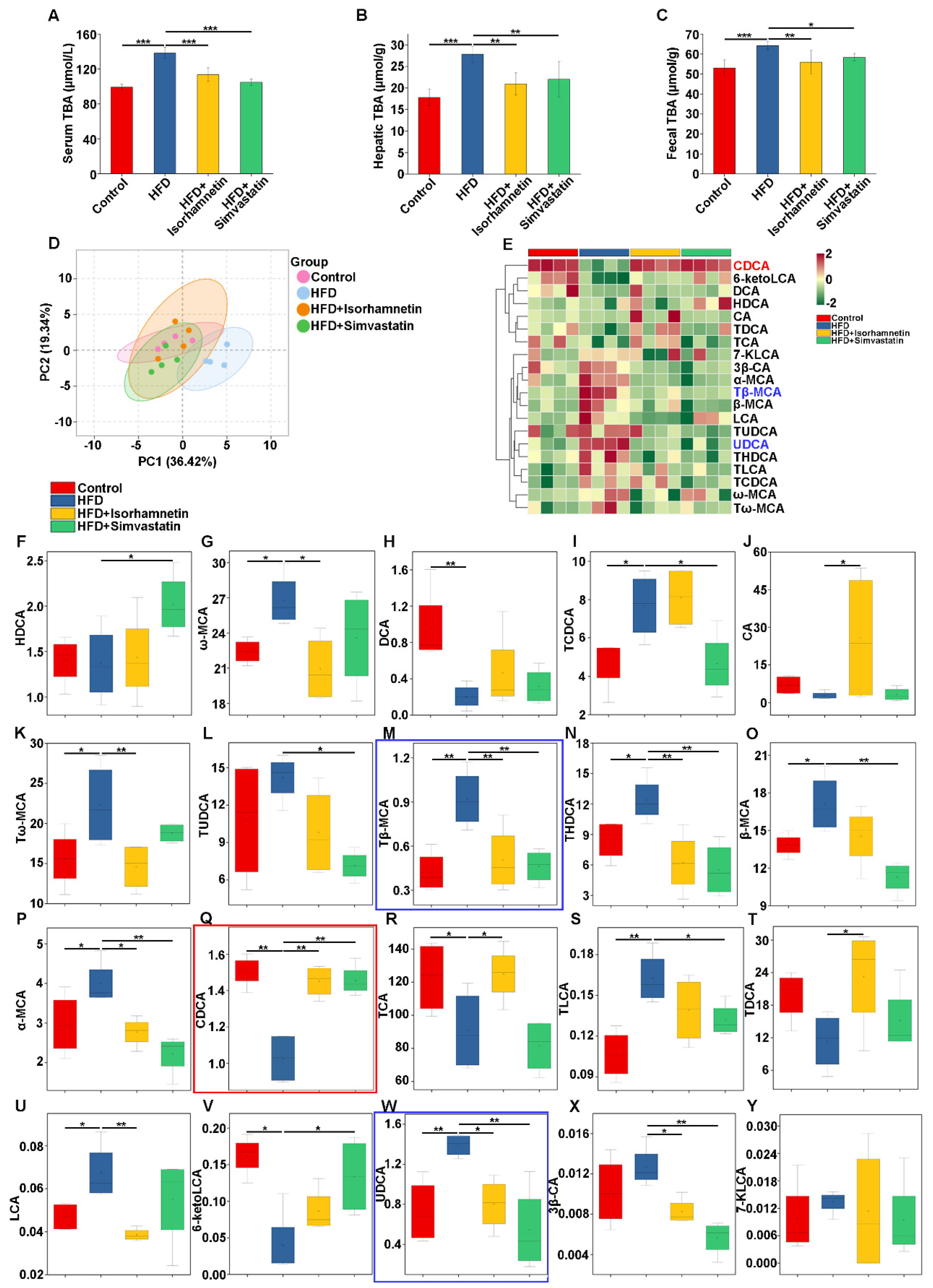
3.5. Isorhamnetin Alleviates NAFLD by Reducing Hepatic Bile Acid Accumulation
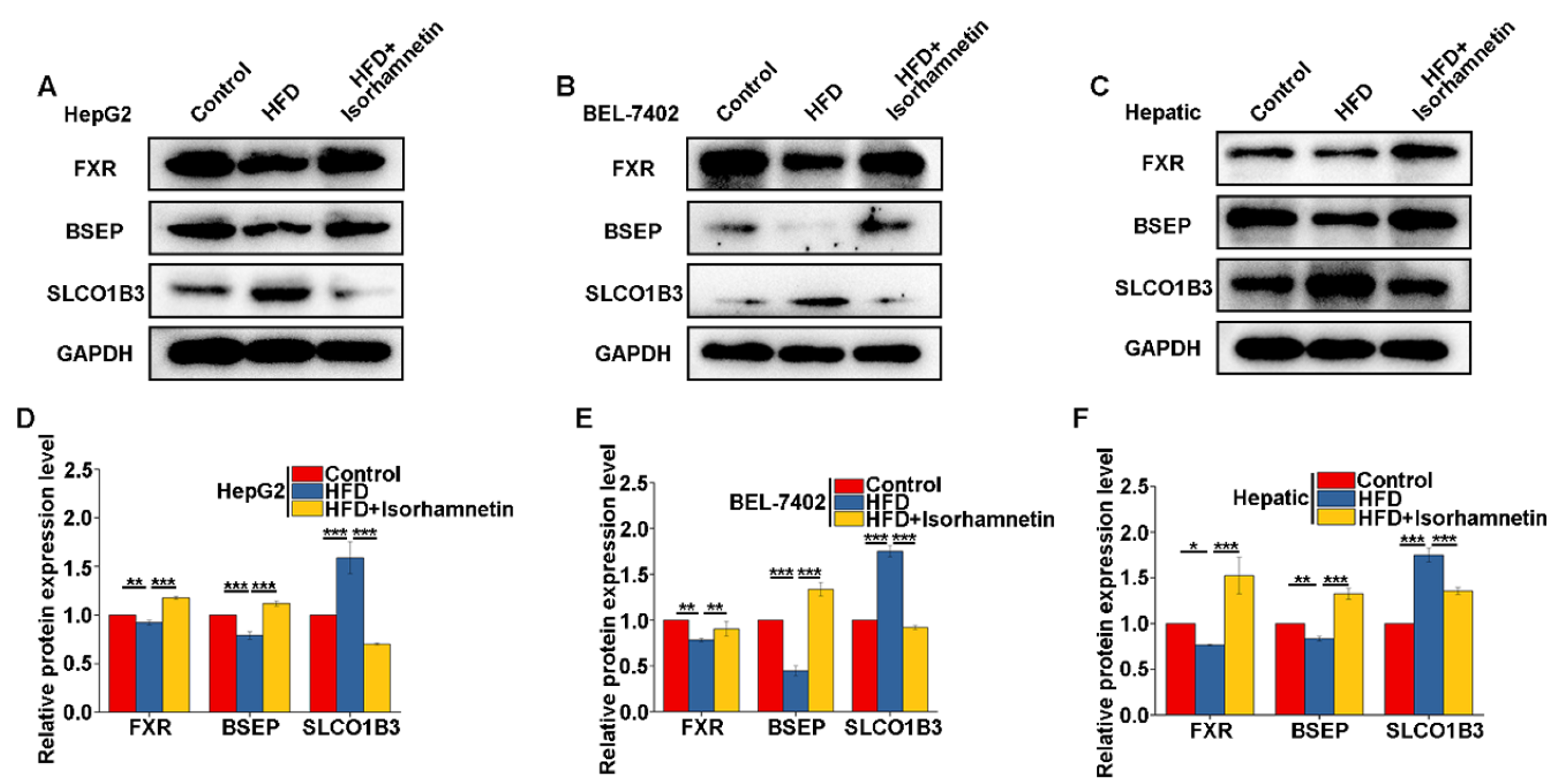
3.6. Isorhamnetin Regulates Bile Salt Metabolism in the Liver by Activating FXR to Alleviate NAFLD
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Song, K.; Kim, H.S.; Chae, H.W. Nonalcoholic fatty liver disease and insulin resistance in children. Clin. Exp. Pediatr. 2023, 66, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Paternostro, R.; Trauner, M. Current treatment of non-alcoholic fatty liver disease. J. Intern. Med. 2022, 292, 190–204. [Google Scholar] [CrossRef] [PubMed]
- Riazi, K.; Azhari, H.; Charette, J.H.; Underwood, F.E.; King, J.A.; Afshar, E.E.; Swain, M.G.; Congly, S.E.; Kaplan, G.G.; Shaheen, A.A. The prevalence and incidence of NAFLD worldwide: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2022, 7, 851–861. [Google Scholar] [CrossRef]
- Lee, E.; Korf, H.; Vidal-Puig, A. An adipocentric perspective on the development and progression of non-alcoholic fatty liver disease. J. Hepatol. 2023, 78, 1048–1062. [Google Scholar] [CrossRef]
- Yang, B.; Huang, S.; Yang, N.; Cao, A.; Zhao, L.; Zhang, J.; Zhao, G.; Ma, Q. Porcine bile acids promote the utilization of fat and vitamin A under low-fat diets. Front. Nutr. 2022, 9, 1005195. [Google Scholar] [CrossRef]
- Chow, M.D.; Lee, Y.H.; Guo, G.L. The role of bile acids in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Mol. Asp. Med. 2017, 56, 34–44. [Google Scholar] [CrossRef]
- Di Ciaula, A.; Garruti, G.; Lunardi Baccetto, R.; Molina-Molina, E.; Bonfrate, L.; Wang, D.Q.; Portincasa, P. Bile Acid Physiology. Ann. Hepatol. 2017, 16, s4–s14. [Google Scholar] [CrossRef]
- Watanabe, M.; Houten, S.M.; Wang, L.; Moschetta, A.; Mangelsdorf, D.J.; Heyman, R.A.; Moore, D.D.; Auwerx, J. Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c. J. Clin. Invest. 2004, 113, 1408–1418. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Feng, H.; Ding, X.; Meng, Q.H.; Zhang, S.R.; Li, J.; Chao, Y.; Ji, T.T.; Bi, Y.H.; Zhang, W.W.; et al. Alisol B 23-acetate adjusts bile acid metabolisim via hepatic FXR-BSEP signaling activation to alleviate atherosclerosis. Phytomedicine 2022, 101, 154120. [Google Scholar] [CrossRef]
- Xu, S.; Kong, L.; Li, L.; Wang, C.; Gu, J.; Luo, H.; Meng, Q. Farnesoid X receptor overexpression prevents hepatic steatosis through inhibiting AIM2 inflammasome activation in nonalcoholic fatty liver disease. Biochim. Biophys. Acta Mol. Basis Dis. 2024, 1870, 166930. [Google Scholar] [CrossRef]
- Xi, Y.; Li, H. Role of farnesoid X receptor in hepatic steatosis in nonalcoholic fatty liver disease. Biomed. Pharmacother. 2020, 121, 109609. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Li, F.; Zalzala, M.; Xu, J.; Gonzalez, F.J.; Adorini, L.; Lee, Y.K.; Yin, L.; Zhang, Y. Farnesoid X receptor activation increases reverse cholesterol transport by modulating bile acid composition and cholesterol absorption in mice. Hepatology 2016, 64, 1072–1085. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Zhu, G.; Xu, Z.; Zhu, J.; Ouyang, J.; Tong, Y.; Zhao, N.; Zhang, X.; Cheng, Y.; Zhang, L.; et al. Organic Anion Transporting Polypeptide (OATP) 1B3 is a Significant Transporter for Hepatic Uptake of Conjugated Bile Acids in Humans. Cell Mol. Gastroenterol. Hepatol. 2023, 16, 223–242. [Google Scholar] [CrossRef]
- Zhi, L.; Zhao, L.; Zhang, X.; Liu, W.; Gao, B.; Wang, F.; Wang, X.; Wang, G. SLCO1B3 promotes colorectal cancer tumorigenesis and metastasis through STAT3. Aging 2021, 13, 22164–22175. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Zelber-Sagi, S.; Henry, L.; Gerber, L.H. Lifestyle interventions in nonalcoholic fatty liver disease. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 708–722. [Google Scholar] [CrossRef]
- Barb, D.; Portillo-Sanchez, P.; Cusi, K. Pharmacological management of nonalcoholic fatty liver disease. Metabolism 2016, 65, 1183–1195. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.F.; Daglia, M.; Moghaddam, A.H.; Habtemariam, S.; Nabavi, S.M. Curcumin and Liver Disease: From Chemistry to Medicine. Compr. Rev. Food Sci. Food Saf. 2014, 13, 62–77. [Google Scholar] [CrossRef] [PubMed]
- Filho, A.M.; Pirozi, M.R.; Borges, J.T.; Pinheiro Sant’Ana, H.M.; Chaves, J.B.; Coimbra, J.S. Quinoa: Nutritional, functional, and antinutritional aspects. Crit. Rev. Food Sci. Nutr. 2017, 57, 1618–1630. [Google Scholar] [CrossRef]
- Tan, P.; Jin, L.; Qin, X.; He, B. Natural flavonoids: Potential therapeutic strategies for non-alcoholic fatty liver disease. Front. Pharmacol. 2022, 13, 1005312. [Google Scholar] [CrossRef]
- La, X.; Zhang, Z.; Liang, J.; Li, H.; Pang, Y.; He, X.; Kang, Y.; Wu, C.; Li, Z. Isolation and purification of flavonoids from quinoa whole grain and its inhibitory effect on lipid accumulation in nonalcoholic fatty liver disease by inhibiting the expression of CD36 and FASN. J. Sci. Food Agric. Online ahead of print.
- Bing, H.; Li, Y.L. The role of bile acid metabolism in the occurrence and development of NAFLD. Front. Mol. Biosci. 2022, 9, 1089359. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Trakooncharoenvit, A.; Nishikawa, M.; Ikushiro, S.; Hara, H. Comprehensive Analyses of Quercetin Conjugates by LC/MS/MS Revealed That Isorhamnetin-7-O-glucuronide-4’-O-sulfate Is a Major Metabolite in Plasma of Rats Fed with Quercetin Glucosides. J. Agric. Food Chem. 2019, 67, 4240–4249. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Jee, S.C.; Kim, K.S.; Kim, H.S.; Yu, K.N.; Sung, J.S. Quercetin and Isorhamnetin Attenuate Benzo[a]pyrene-Induced Toxicity by Modulating Detoxification Enzymes through the AhR and NRF2 Signaling Pathways. Antioxidants 2021, 10, 787. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Kroon, P.A.; Shao, H.; Needs, P.W.; Yang, X. Differential Effects of Quercetin and Two of Its Derivatives, Isorhamnetin and Isorhamnetin-3-glucuronide, in Inhibiting the Proliferation of Human Breast-Cancer MCF-7 Cells. J. Agric. Food Chem. 2018, 66, 7181–7189. [Google Scholar] [CrossRef]
- Buzzetti, E.; Pinzani, M.; Tsochatzis, E.A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism 2016, 65, 1038–1048. [Google Scholar] [CrossRef]
- Yu, Q.; Jiang, Z.; Zhang, L. Bile acid regulation: A novel therapeutic strategy in non-alcoholic fatty liver disease. Pharmacol. Ther. 2018, 190, 81–90. [Google Scholar] [CrossRef]
- Trauner, M.; Fuchs, C.D.; Halilbasic, E.; Paumgartner, G. New therapeutic concepts in bile acid transport and signaling for management of cholestasis. Hepatology 2017, 65, 1393–1404. [Google Scholar] [CrossRef]
- Chiang, J.Y.L.; Ferrell, J.M. Bile Acid Metabolism in Liver Pathobiology. Gene Expr. 2018, 18, 71–87. [Google Scholar] [CrossRef]
- Sekine, S.; Ogawa, R.; Ojima, H.; Kanai, Y. Expression of SLCO1B3 is associated with intratumoral cholestasis and CTNNB1 mutations in hepatocellular carcinoma. Cancer Sci. 2011, 102, 1742–1747. [Google Scholar] [CrossRef]


| No. | RT [min] | Formula | Class of Compound | Name | MW | MS/MS Fragment | Percentage of CQWF30 |
|---|---|---|---|---|---|---|---|
| 1 | 6.01 | C15H10O7 | Flavonoids | Quercetin | 302 | 303.0487 | 10.54 |
| 2 | 6.23 | C15H10O6 | Flavanoids | Kaempferol | 286 | 287.05435 | 8.83 |
| 3 | 6.26 | C27H30O15 | Flavanoids | Nictoflorin | 594 | 287.05447 | 7 |
| 4 | 6.49 | C27H30O16 | Flavanoids | Rutin | 610 | 303.04941 | 3.84 |
| 5 | 6.29 | C34H42O20 | Flavonoids | Typhaneoside | 770 | 317.06506 | 3.43 |
| 6 | 7.10 | C21H18O12 | Flavonoids | Scutellarin | 462 | 287.05441 | 2.73 |
| 7 | 6.01 | C21H20O12 | Flavanoids | Hyperoside | 464 | 303.04947 | 2.15 |
| 8 | 6.23 | C21H20O11 | Flavanoids | Orientin | 448 | 449.10721 | 1.91 |
| 9 | 6.29 | C16H12O7 | Flavonoids | Isorhamnetin | 316 | 317.06451 | 0.82 |
| 10 | 7.12 | C22H22O12 | Flavanoids | Isorhamnetin 3-galactoside | 478 | 301.03491 | 0.24 |
| 11 | 9.13 | C16H12O5 | Flavanoids | Glycitein | 284 | 268.03723 | 0.08 |
| 12 | 2.97 | C8H7NO2 | Flavanoids | 4-Hydroxymandelonitrile | 149 | 150.05487 | 0.08 |
| 13 | 11.33 | C16H12O5 | Flavanoids | 5-O-Methylgenistein | 284 | 285.07532 | 0.01 |
| Index | Compounds | Control | HFD | HFD+ Isorhamnetin | HFD+ Simvastatin |
|---|---|---|---|---|---|
| β-MCA | β-muricho lic acid | 13.84 ± 0.94 | 17.11 ± 2.36 # | 14.53 ± 2.43 | 11.28 ± 1.32 ** |
| 6-ketoLCA | 5-β-Cholanic Acid-3α-ol-6-one | 0.16 ± 0.03 | 0.04 ± 0.05 # | 0.09 ± 0.03 | 0.13 ± 0.05 * |
| α-MCA | α-muricho lic acid | 2.97 ± 0.78 | 4.00 ± 0.56 # | 2.77 ± 0.37 * | 2.22 ± 0.51 ** |
| UDCA | Ursodeoxycho lic acid | 0.73 ± 0.32 | 1.39 ± 0.11 ## | 0.80 ± 0.26 * | 0.54 ± 0.42 ** |
| ω-MCA | ω-muricho lic acid | 22.42 ± 1.05 | 26.77 ± 2.27 # | 20.94 ± 2.87 * | 23.59 ± 4.16 |
| 7-KLCA | 7-ketolithocho lic acid | 0.010 ± 0.01 | 0.013 ± 0.00 | 0.011 ± 0.01 | 0.009 ± 0.009 |
| HDCA | Hyodeoxycho lic acid | 1.40 ± 0.27 | 1.37 ± 0.41 | 1.43 ± 0.50 | 2.02 ± 0.35 * |
| LCA | Lithocho lic acid | 0.05 ± 0.01 | 0.07 ± 0.01 # | 0.04 ± 0.00 ** | 0.05 ± 0.02 |
| CDCA | Chenodeoxycho lic acid | 0.85 ± 0.07 | 1.24 ± 0.12 ## | 1.60 ± 0.11 ** | 1.49 ± 0.17 ** |
| 3β-CA | 3β-Cho lic Acid | 0.01 ± 0.00 | 0.013 ± 0.00 | 0.008 ± 0.00 * | 0.006 ± 0.00 ** |
| CA | Cho lic acid | 7.02 ± 3.80 | 2.76 ± 1.61 | 25.80 ± 26.68 * | 3.26 ± 2.68 |
| DCA | Deoxycho lic acid | 0.96 ± 0.43 | 0.20 ± 0.14 ## | 0.46 ± 0.46 | 0.31 ± 0.20 |
| Tω-MCA | Tauro-ω-muricho lic Acid sodium salt | 15.58 ± 3.63 | 22.31 ± 5.28 # | 14.59 ± 2.93 * | 18.81 ± 1.20 |
| TUDCA | Tauroursodeoxycho lic acid | 10.77 ± 4.90 | 14.20 ± 1.88 | 9.80 ± 3.63 | 7.14 ± 1.19 * |
| TLCA | Taurolithocho lic acid | 0.11 ± 0.02 | 0.16 ± 0.02 ## | 0.14 ± 0.02 | 0.13 ± 0.01 * |
| TDCA | Taurodeoxycho lic acid | 19.82 ± 4.65 | 11.37 ± 5.33 | 23.27 ± 9.57 * | 15.16 ± 6.29 |
| TCDCA | Taurochenodeoxycho lic acid | 4.70 ± 1.37 | 7.68 ± 1.73 # | 8.10 ± 1.61 | 4.64 ± 1.66 * |
| TCA | Taurocholic acid | 122.82 ± 22.07 | 90.77 ± 24.94 # | 124.96 ± 16.91 * | 81.42 ± 16.28 |
| THDCA | Taurohyodeoxycho lic Acid sodium salt | 8.47 ± 1.95 | 12.42 ± 2.30 # | 6.22 ± 3.02 ** | 5.53 ± 2.68 * |
| Tβ-MCA | Tauro-β-muricholic acid | 0.42 ± 0.14 | 0.92 ± 0.20 ## | 0.51 ± 0.22 ** | 0.46 ± 0.12 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
La, X.; Zhang, Z.; Dong, C.; Li, H.; He, X.; Kang, Y.; Wu, C.; Li, Z. Isorhamnetin in Quinoa Whole-Grain Flavonoids Intervenes in Non-Alcoholic Fatty Liver Disease by Modulating Bile Acid Metabolism through Regulation of FXR Expression. Foods 2024, 13, 3076. https://doi.org/10.3390/foods13193076
La X, Zhang Z, Dong C, Li H, He X, Kang Y, Wu C, Li Z. Isorhamnetin in Quinoa Whole-Grain Flavonoids Intervenes in Non-Alcoholic Fatty Liver Disease by Modulating Bile Acid Metabolism through Regulation of FXR Expression. Foods. 2024; 13(19):3076. https://doi.org/10.3390/foods13193076
Chicago/Turabian StyleLa, Xiaoqin, Zhaoyan Zhang, Cunli Dong, Hanqing Li, Xiaoting He, Yurui Kang, Changxin Wu, and Zhuoyu Li. 2024. "Isorhamnetin in Quinoa Whole-Grain Flavonoids Intervenes in Non-Alcoholic Fatty Liver Disease by Modulating Bile Acid Metabolism through Regulation of FXR Expression" Foods 13, no. 19: 3076. https://doi.org/10.3390/foods13193076




