Effects of Variable-Temperature Roasting on the Flavor Compounds of Xinjiang Tannur-Roasted Mutton
Abstract
:1. Introduction
2. Materials and Methods
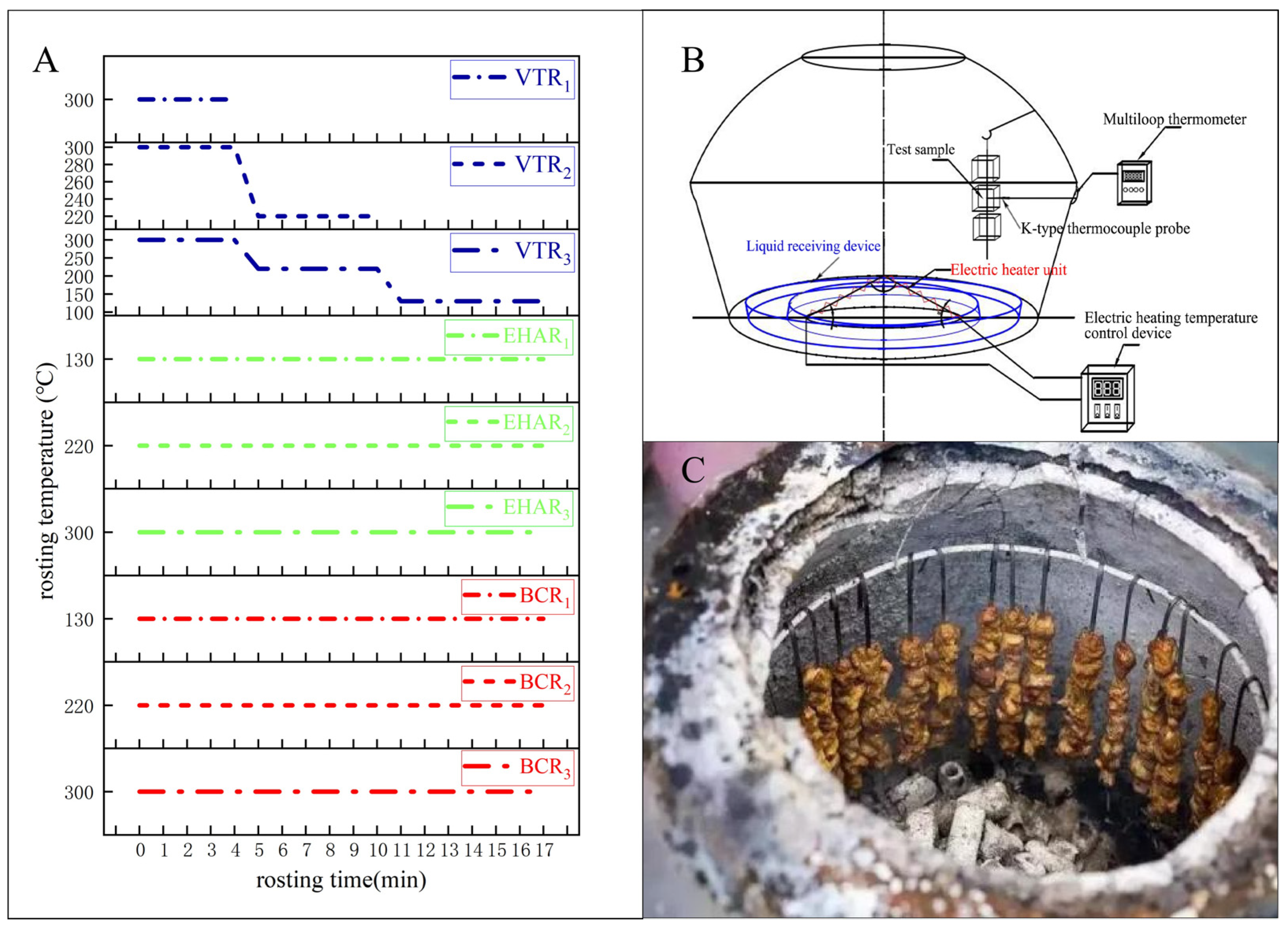
2.1. Sample Preparation
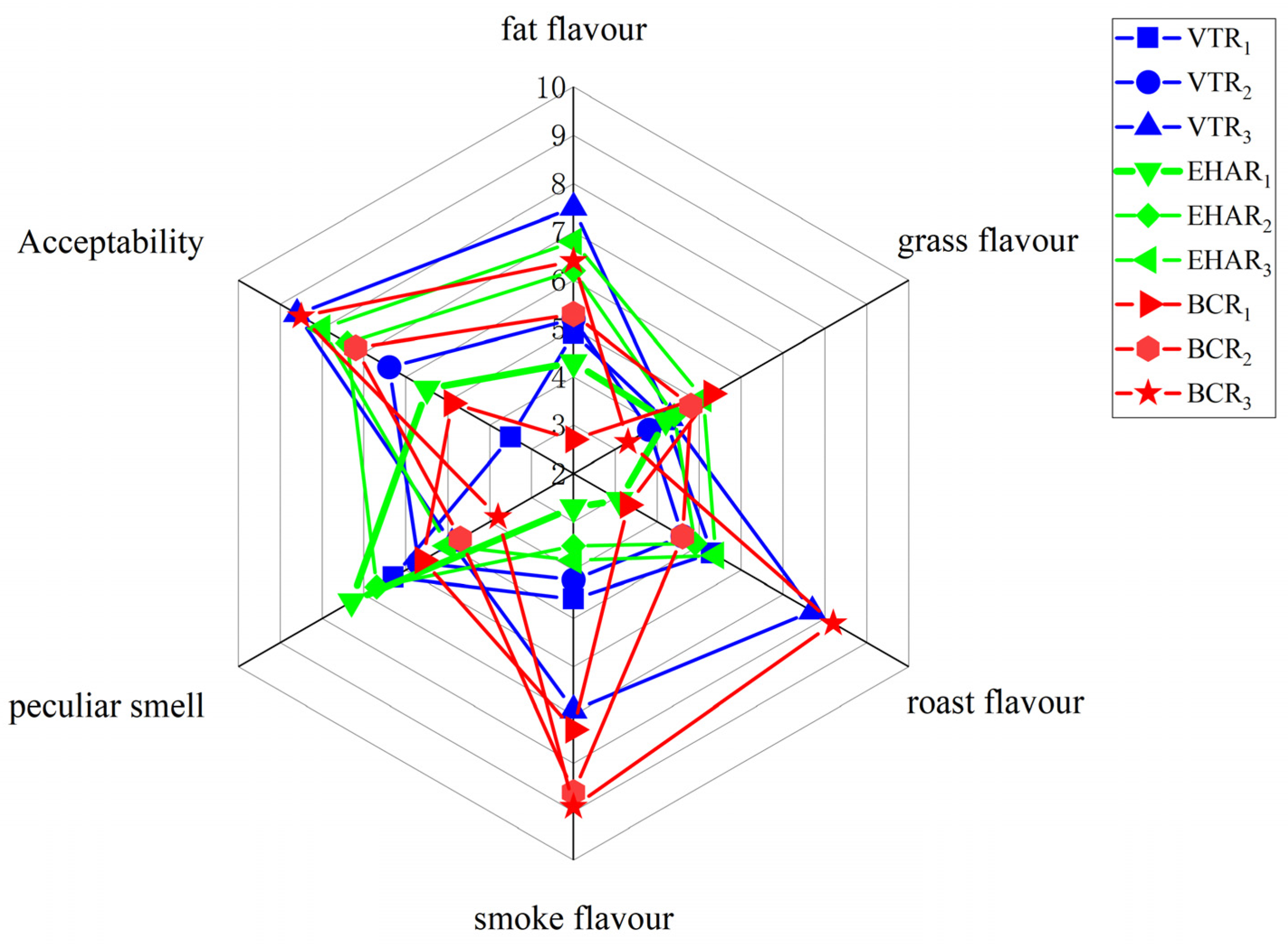
2.2. Sensory Evaluation
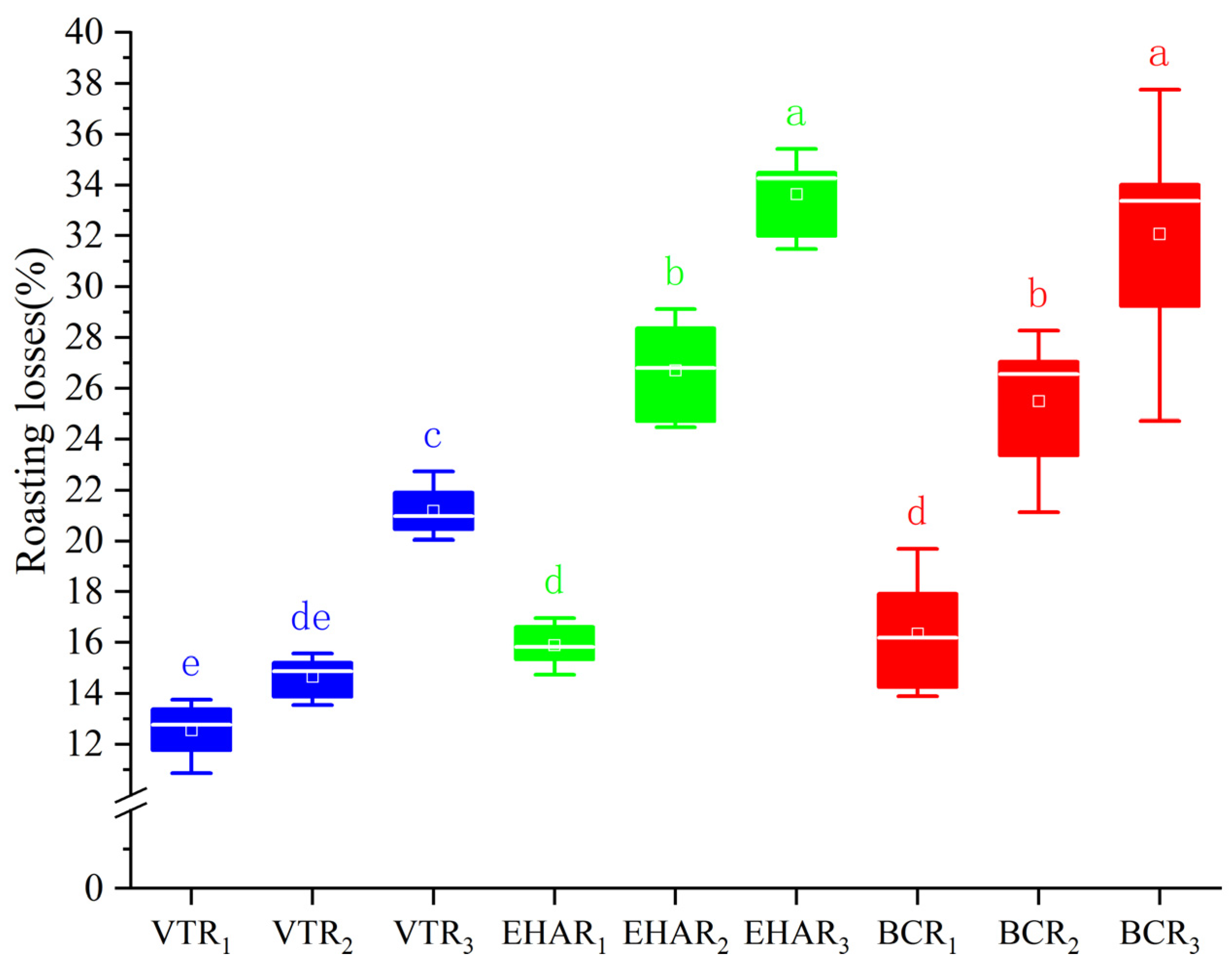
2.3. Roasting Losses
2.4. Differential Scanning Calorimetry (DSC)
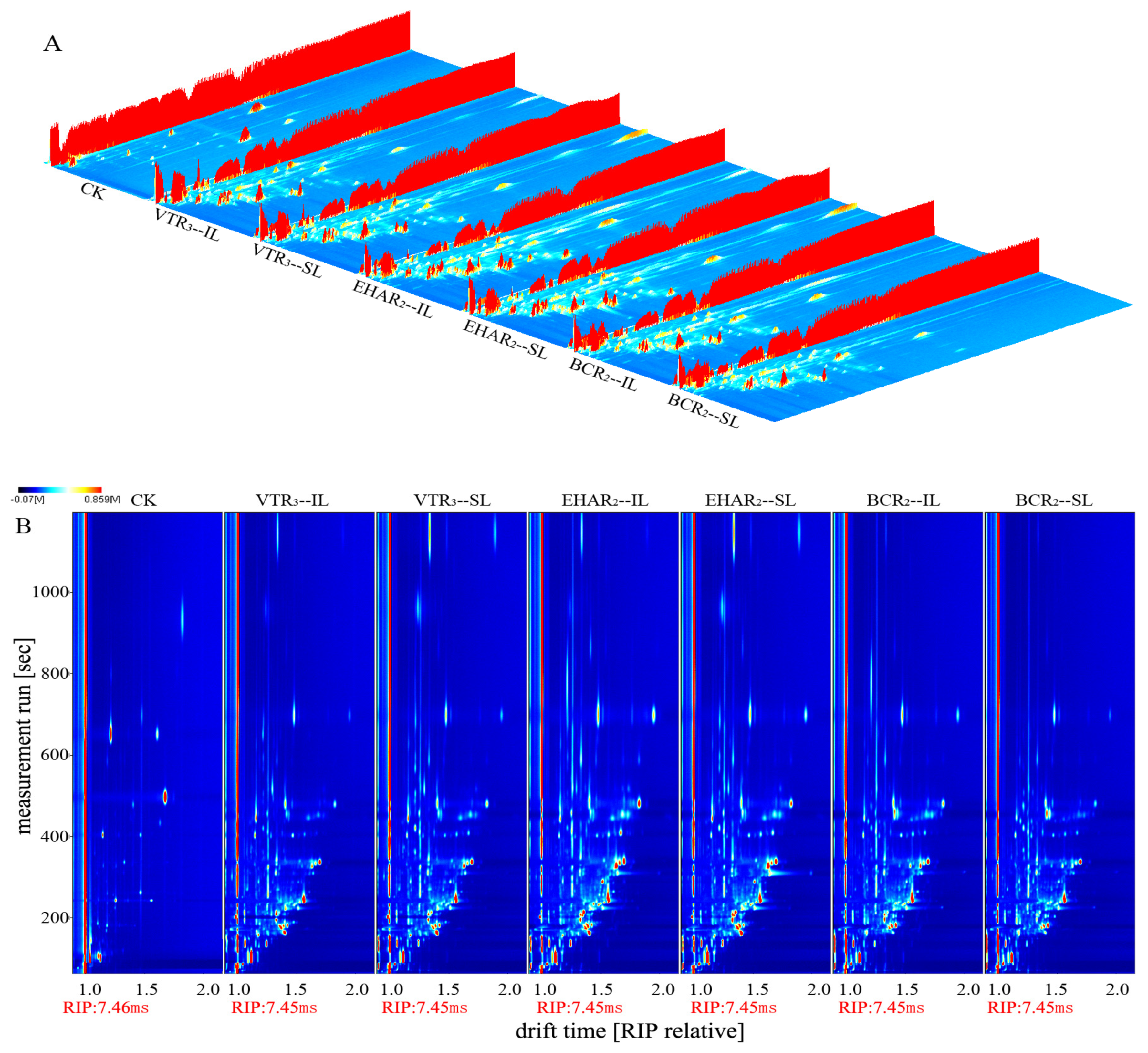
2.5. Gas Chromatography–Ion Mobility Spectrometry (GC-IMS)
2.6. Free Fatty Acids Analysis
2.7. Free Amino Acids Analysis
2.8. Statistical Analysis
3. Results and Discussion
3.1. Effects of Variable-Temperature Roasting Methods on Roasting Loss
3.2. DSC Thermogram of Tannur-Roasted Mutton
3.3. Analysis of Volatile Flavor Compounds and Sensory Evaluation
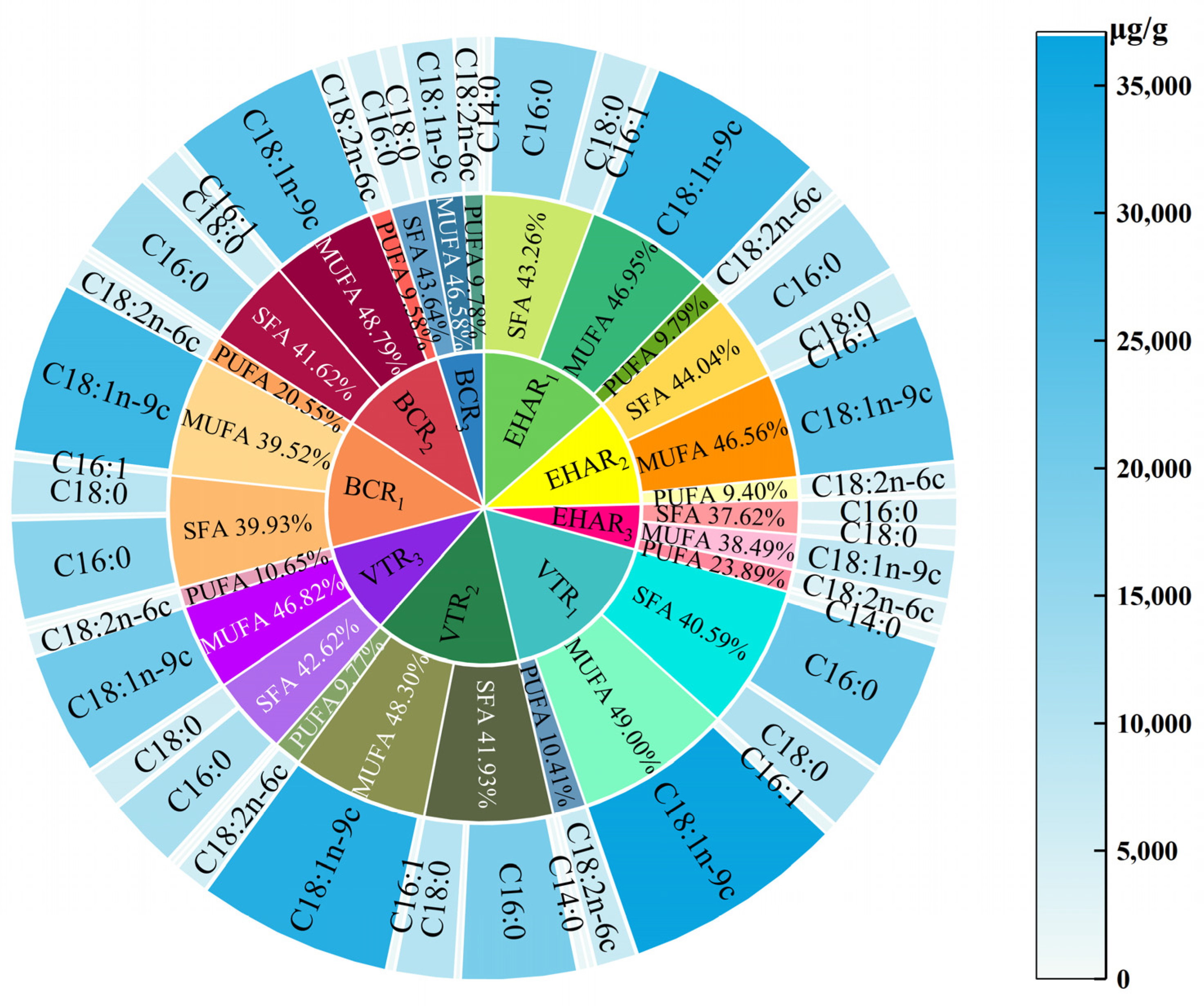
3.4. Effects of Variable-Temperature Roasting Methods on Free Fatty Acids
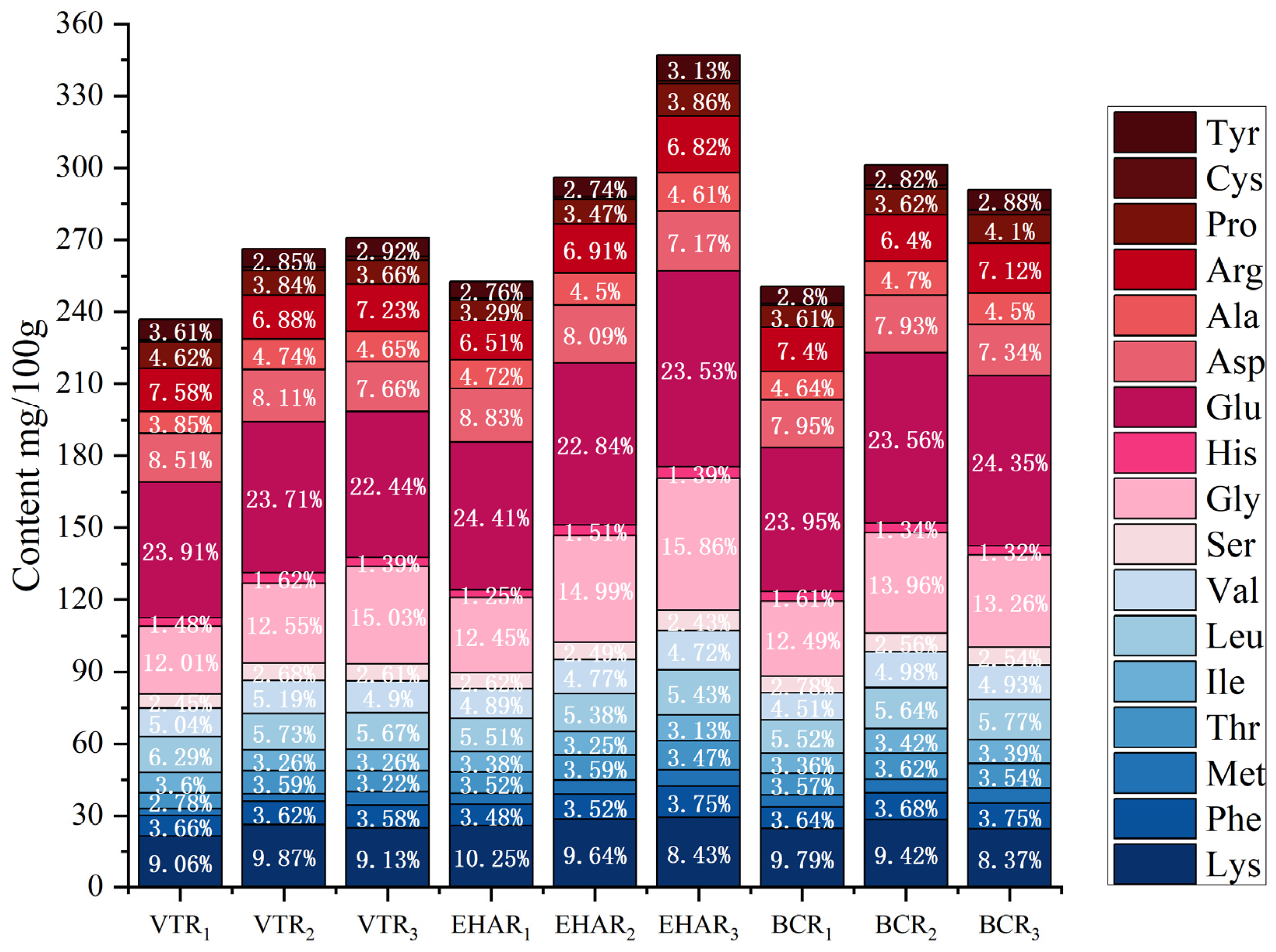
3.5. Effects of Variable-Temperature Roasting Methods on Free Amino Acids
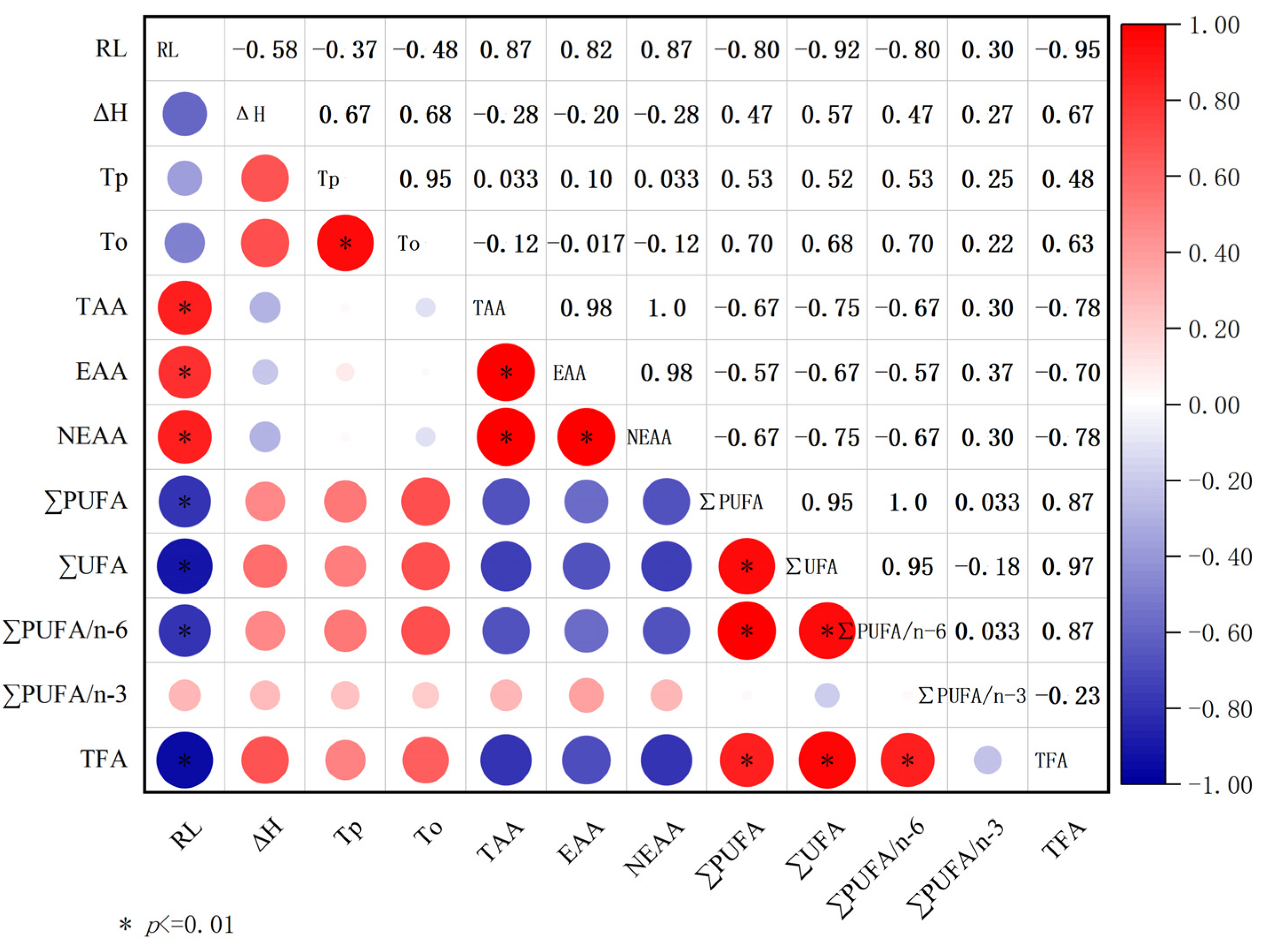
3.6. Correlation between Roasting Loss, Dsc, Free Fatty Acids, and Free Amino Acids
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wei, J.; Wang, L.; Liu, F.; Xu, Z.; Ma, X.; Wang, Z. Effect of variable-temperature roasting on the color, tenderness, heterocyclic amine and polycyclic aromatic hydrocarbon contents of Xinjiang Nang pit-roasted lamb meat. Food Sci. 2023, 44, 121–130, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Ismail, I.; Hwang, Y.H.; Joo, S.T. Interventions of two-stage thermal sous-vide cooking on the toughness of beef semitendinosus. Meat Sci. 2019, 157, 107882. [Google Scholar] [CrossRef]
- Uttaro, B.; Zawadski, S.; McLeod, B. Efficacy of multi-stage sous-vide cooking on tenderness of low value beef muscles. Meat Sci. 2019, 149, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Ma, J.; Pan, T.; Suleman, R.; Wang, Z.; Zhang, D. Effects of roasting by charcoal, electric, microwave and superheated steam methods on (non) volatile compounds in oyster cuts of roasted lamb. Meat Sci. 2021, 172, 108324. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, D.; Liu, H.; Wang, Z.; Hui, T.; Sun, J. Comprehensive Evaluation of Volatile and Nonvolatile Compounds in Oyster Cuts of Roasted Lamb at Different Processing Stages Using Traditional Nang Roasting. Foods 2021, 10, 1508. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, D.; Chen, R.; Yang, X.; Liu, H.; Wang, Z.; Hui, T. Comprehensive Evaluation of Flavor in Charcoal and Electric-Roasted Tamarix Lamb by HS-SPME/GC-MS Combined with Electronic Tongue and Electronic Nose. Foods 2021, 10, 2676. [Google Scholar] [CrossRef]
- Shahidi, F.; Hossain, A. Role of lipids in food flavor generation. Molecules 2022, 27, 5014. [Google Scholar] [CrossRef]
- Liu, H.; Hui, T.; Zheng, X.; Li, S.; Wei, X.; Li, P.; Wang, Z. Characterization of key lipids for binding and generating aroma compounds in roasted mutton by UPLC-ESI-MS/MS and Orbitrap Exploris GC. Food Chem. 2022, 374, 131723. [Google Scholar] [CrossRef]
- Xiao, X.; Hou, C.; Zhang, D.; Li, X.; Ren, C.; Ijaz, M.; Hussain, Z.; Liu, D. Effect of pre- and post-rigor on texture, flavor, heterocyclic aromatic amines and sensory evaluation of roasted lamb. Meat Sci. 2020, 169, 108220. [Google Scholar] [CrossRef]
- Matsui, K.; Sasahara, S.; Akakabe, Y.; Kajiwara, T. Linoleic acid 10-hydroperoxide as an intermediate during formation of 1-octen-3-ol from linoleic acid in Lentinus decadetes. Biosci. Biotechnol. Biochem. 2003, 67, 2280–2282. [Google Scholar] [CrossRef]
- Zou, Y.; Kang, D.; Liu, R.; Qi, J.; Zhou, G.; Zhang, W. Effects of ultrasonic assisted cooking on the chemical profiles of taste and flavor of spiced beef. Ultrason. Sonochemistry 2018, 46, 36–45. [Google Scholar] [CrossRef]
- Xi, J.; Zhan, P.; Tian, H.; Wang, P. Effect of Spices on the Formation of VOCs in Roasted Mutton Based on GC-MS and Principal Component Analysis. J. Food Qual. 2019, 2019, 8568920. [Google Scholar] [CrossRef]
- Aheto, J.H.; Huang, X.; Tian, X.; Lv, R.; Dai, C.; Bonah, E.; Chang, X. Evaluation of lipid oxidation and volatile compounds of traditional dry-cured pork belly: The hyperspectral imaging and multi-gas-sensory approaches. J. Food Process Eng. 2020, 43, e13092. [Google Scholar] [CrossRef]
- Flores, M. The Eating Quality of Meat: III—Flavor//Lawrie’s Meat Science; Woodhead Publishing: Cambridge, UK, 2023; pp. 421–455. [Google Scholar] [CrossRef]
- Liu, H.; Hui, T.; Fang, F.; Li, S.; Wang, Z.; Zhang, D. The formation of key aroma compounds in roasted mutton during the traditional charcoal process. Meat Sci. 2022, 184, 108689. [Google Scholar] [CrossRef] [PubMed]
- DB 65/T 3788; Nanjiang Mutton Sheep Backyard Production Technology Procedures. Local Standards of Xinjiang Uygur Autonomous Region. Administration of Quality and Technology Supervision of Xinjiang Uygur Autonomous Region. Local Information Service Platform: Xinjiang, China, 2015; pp. 1–5.
- GB/T 4356; Code of practice for livestock and poultry slaughtering operation-Sheep and goat.State Administration for Market Regulation of China and National Standardization Administration of the People’s Republic of China. Standard Press of China: Beijing, China, 2023; pp. 1–4.
- Petričević, S.; Marušić, R.N.; Lukić, K.; Listeš, E.; Medić, H. Differentiation of dry-cured hams from different processing methods by means of volatile compounds, physico-chemical and sensory analysis. Meat Sci. 2018, 137, 217–227. [Google Scholar] [CrossRef]
- GB/T 39625; Sensory analysis-MethodologyGeneral guidance for establishing a sensory profile. National Standards of the People’s Republic of China. State Administration for Market Regulation and National Standardization Administration of the People’s Republic of China: Beijing, China, 2020; pp. 4–7.
- GB/T 13868; Sensory analysis-General guidance for the design of test rooms. National Standards of the People’s Republic of China. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China and National Standardization Administration of the People’s Republic of China: Beijing, China, 2009; pp. 2–5.
- ISO 11132:2021; Sensory Analysis—Methodology—Guidelines for the Measurement of the Performance of a Quantitative Descriptive Sensory Panel. International Organization for Standardization: Geneva, Switzerland, 2021.
- Vaskoska, R.; Vénien, A.; Ha, M.; White, J.D.; Unnithan, R.R.; Astruc, T.; Warner, R.D. Thermal denaturation of proteins in the muscle fibre and connective tissue from bovine muscles composed of type I (masseter) or type II (cutaneous trunci) fibres: DSC and FTIR microspectroscopy study. Food Chem. 2021, 343, 128544. [Google Scholar] [CrossRef]
- Wang, F.; Gao, Y.; Wang, H.; Xi, B.; He, X.; Yang, X.; Li, W. Analysis of volatile compounds and flavor fingerprint in Jingyuan lamb of different ages using gas chromatography-ion mobility spectrometry (GC-IMS). Meat Sci. 2021, 175, 108449. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wang, G.; Yin, X.; Ge, C.; Liao, G. Effects of different cooking methods on free fatty acid profile, water-soluble compounds and flavor compounds in Chinese Piao chicken meat. Food Res. Int. 2021, 149, 110696. [Google Scholar] [CrossRef] [PubMed]
- Sabikun, N.; Bakhsh, A.; Rahman, M.S.; Hwang, Y.H.; Joo, S.T. Volatile and nonvolatile taste compounds and their correlation with umami and flavor characteristics of chicken nuggets added with milkfat and potato mash. Food Chem. 2021, 343, 128499. [Google Scholar] [CrossRef]
- Purslow, P.P. Contribution of collagen and connective tissue to cooked meat toughness; some paradigms reviewed. Meat Sci. 2018, 144, 127–134. [Google Scholar] [CrossRef]
- Vaskoska, R.; Ha, M.; Ong, L.; Chen, G.; White, J.; Gras, S.; Warner, R. Myosin sensitivity to thermal denaturation explains differences in water loss and shrinkage during cooking in muscles of distinct fibre types. Meat Sci. 2021, 179, 108521. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Wu, Q.; Xue, W.; Wu, R.; Huang, Y.; Chen, L.; Han, Y.; Wu, J.; Borjigin, G.; Sha, R. Characterization of Type I and Type III Collagen in the Intramuscular Connective Tissue of Wuzhumuqin Sheep. Animals 2023, 24, 395. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.I.; Lee, E.J.; Hong, G.P. Effects of Temperature and Time on the Cookery Properties of Sous-vide Processed Pork Loin. Food Sci. Anim. Resour. 2019, 39, 65–72. [Google Scholar] [CrossRef]
- Kang, J.; Solis Rueda, K.A. Enthalpy-entropy compensation in the denaturation of proteins of bovine masseter and cutaneous trunci. Meat Sci. 2022, 184, 108688. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Cao, S.; Yang, L.; Li, Z. Flavor formation based on lipid in meat and meat products: A review. J. Food Biochem. 2022, 46, e14439. [Google Scholar] [CrossRef] [PubMed]
- Calkins, C.R.; Hodgen, J.M. A fresh look at meat flavor. Meat Sci. 2007, 77, 63. [Google Scholar] [CrossRef]
- Marco, A.; Navarro, J.L.; Flores, M. Quantitation of selected odor-active constituents in dry fermented sausages prepared with different curing salts. J. Agric. Food Chem. 2007, 55, 3058–3065. [Google Scholar] [CrossRef]
- Casaburi, A.; Piombino, P.; Nychas, G.J.; Villani, F.; Ercolini, D. Bacterial populations and the volatilome associated to meat spoilage. Food Microbiol. 2015, 45, 83–102. [Google Scholar] [CrossRef] [PubMed]
- Bleicher, J.; Ebner, E.E.; Bak, K.H. Formation and Analysis of Volatile and Odor Compounds in Meat-A Review. Molecules 2022, 8, 6703. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Macleod, G.; Ames, J.M. The effect of heat on beef aroma: Comparisons of chemical composition and sensory properties. Flavour Fragr. J. 1986, 1, 91–104. [Google Scholar] [CrossRef]
- García-González, D.L.; Tena, N.; Aparicio-Ruiz, R.; Morales, M.T. Relationship between sensory attributes and volatile compounds qualifying dry-cured hams. Meat Sci. 2008, 80, 315–325. [Google Scholar] [CrossRef] [PubMed]
- McGorrin, R.J. The significance of volatile sulfur compounds in food flavors: An overview. Volatile Sulfur Compd. Food 2011, 1068, 3–31. [Google Scholar] [CrossRef]
- Han, D.; Zhang, C.-H.; Fauconnier, M.-L.; Mi, S. Characterization and differentiation of boiled pork from Tibetan, Sanmenxia and Duroc × (Landrac × Yorkshire) pigs by volatiles profiling and chemometrics analysis. Food Res. Int. 2020, 130, 108910. [Google Scholar] [CrossRef] [PubMed]
- Resconi, V.C.; Campo, M.M.; Montossi, F.; Ferreira, V.; Sañudo, C.; Escudero, A. Relationship between odour-active compounds and flavour perception in meat from lambs fed different diets. Meat Sci. 2010, 85, 700–706. [Google Scholar] [CrossRef]
- Perea-Sanz, L.; López-Díez, J.J.; Belloch, C.; Flores, M. Counteracting the effect of reducing nitrate/nitrite levels on dry fermented sausage aroma by Debaryomyces hansenii inoculation. Meat Sci. 2020, 164, 108103. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.; Mercier, F.; Tournayre, P.; Martin, J.L.; Berdagué, J.L. Identification and origin of odorous sulfur compounds in cooked ham. Food Chem. 2014, 155, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Brewer, M.S. Irradiation effects on meat flavor: A review. Meat Sci. 2009, 81, 1–14. [Google Scholar] [CrossRef]
- Sohail, A.; Al-Dalali, S.; Wang, J.; Xie, J.; Shakoor, A.; Asimi, S.; Shah, H.; Patil, P. Aroma compounds identified in cooked meat: A review. Food Res. Int. 2022, 157, 111385. [Google Scholar] [CrossRef] [PubMed]
- Bi, J.; Li, Y.; Yang, Z.; Lin, Z.; Chen, F.; Liu, S.; Li, C. Effect of different cooking times on the fat flavor compounds of pork belly. J. Food Biochem. 2022, 46, e14184. [Google Scholar] [CrossRef]
- Del Bianco, S.; Natalello, A.; Luciano, G.; Valenti, B.; Campidonico, L.; Gkarane, V.; Monahan, F.; Biondi, L.; Favotto, S.; Sepulcri, A.; et al. Influence of dietary inclusion of tanninextracts from mimosa, chestnut and tara on volatile compounds and flavour in lamb meat. Meat Sci. 2020, 172, 108336. [Google Scholar] [CrossRef] [PubMed]
- Conte, F.; Cincotta, F.; Condurso, C.; Verzera, A.; Panebianco, A. Odor Emissions from Raw Meat of Freshly Slaughtered Cattle during Inspection. Foods 2021, 10, 2411. [Google Scholar] [CrossRef] [PubMed]
- Machiels, D.; van Ruth, S.M.; Posthumus, M.A.; Istasse, L. Gas chromatography-olfactometry analysis of the volatile compounds of two commercial Irish beef meats. Talanta 2003, 60, 755–764. [Google Scholar] [CrossRef] [PubMed]
- García-González, D.L.; Tena, N.; Aparicio-Ruiz, R.; Aparicio, R. Sensor responses to fat food aroma: A comprehensive study of dry-cured ham typicality. Talanta 2014, 120, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Kerler, J.; Grosch, W. Odorants contributing to warmed-over flavor (WOF) of refrigerated cooked beef. J. Food Sci. 1996, 61, 1271–1274+1284. [Google Scholar] [CrossRef]
- Corral, S.; Leitner, E.; Siegmund, B.; Flores, M. Determination of sulfur and nitrogen compounds during the processing of dry fermented sausages and their relation to amino acid generation. Food Chem. 2016, 190, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Hui, T.; Fang, F.; Ma, Q.; Li, S.; Zhang, D.; Wang, Z. Characterization and discrimination of key aroma compounds in pre-and postrigor roasted mutton by GC-O-MS, GC E-Nose and aroma recombination experiments. Foods 2021, 10, 2387. [Google Scholar] [CrossRef] [PubMed]
- Lunn, J.; Theobald, H.E. The health effects of dietary unsaturated fatty acids. Nutr. Bull. 2006, 31, 178–224. [Google Scholar] [CrossRef]
- Janiszewski, P.; Grześkowiak, E.; Lisiak, D.; Borys, B.; Borzuta, K.; Pospiech, E.; Poławska, E. The influence of thermal processing on the fatty acid profile of pork and lamb meat fed diet with increased levels of unsaturated fatty acids. Meat Sci. 2016, 111, 161–167. [Google Scholar] [CrossRef]
- Ortuño, J.; Mateo, L.; Rodríguez-Estrada, M.T.; Bañón, S. Effects of sous vide vs grilling methods on lamb meat colour and lipid stability during cooking and heated display. Meat Sci. 2021, 171, 108287. [Google Scholar] [CrossRef]
- Gerber, N.; Scheeder, M.R.L.; Wenk, C. The influence of cooking and fat trimmingon the actual nutrient intake from meat. Meat Sci. 2009, 81, 148–154. [Google Scholar] [CrossRef]
- Campo, M.M.; Muela, E.; Olleta, J.L.; Moreno, L.A.; Santaliestra-Pasias, A.M.; Mesana, M.I.; Saňudo, C. Influence of cooking method on the nutrient composition of Spanish light lamb. J. Food Compos. Anal. 2013, 31, 185–190. [Google Scholar] [CrossRef]
- Corino, C.; Rossi, R.; Cannata, S.; Ratti, S. Effect of dietary linseed on the nutritional value and quality of pork and pork products: Systematic review and meta analysis. Meat Sci. 2014, 98, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Fedorov, F.S.; Yaqin, A.; Krasnikov, D.V.; Kondrashov, V.A.; Ovchinnikov, G.; Kostyukevich, Y.; Nasibulin, A.G. Detecting cooking state of grilled chicken by electronic nose and computer vision techniques. Food Chem. 2021, 345, 128747. [Google Scholar] [CrossRef] [PubMed]
- Elmore, J.S.; Mottram, D.S. Formation of 2-alkyl-(2H)-thiapyrans and 2-alkylthiophenes in cooked beef and lamb. J. Agric. Food Chem. 2000, 48, 2420. [Google Scholar] [CrossRef]

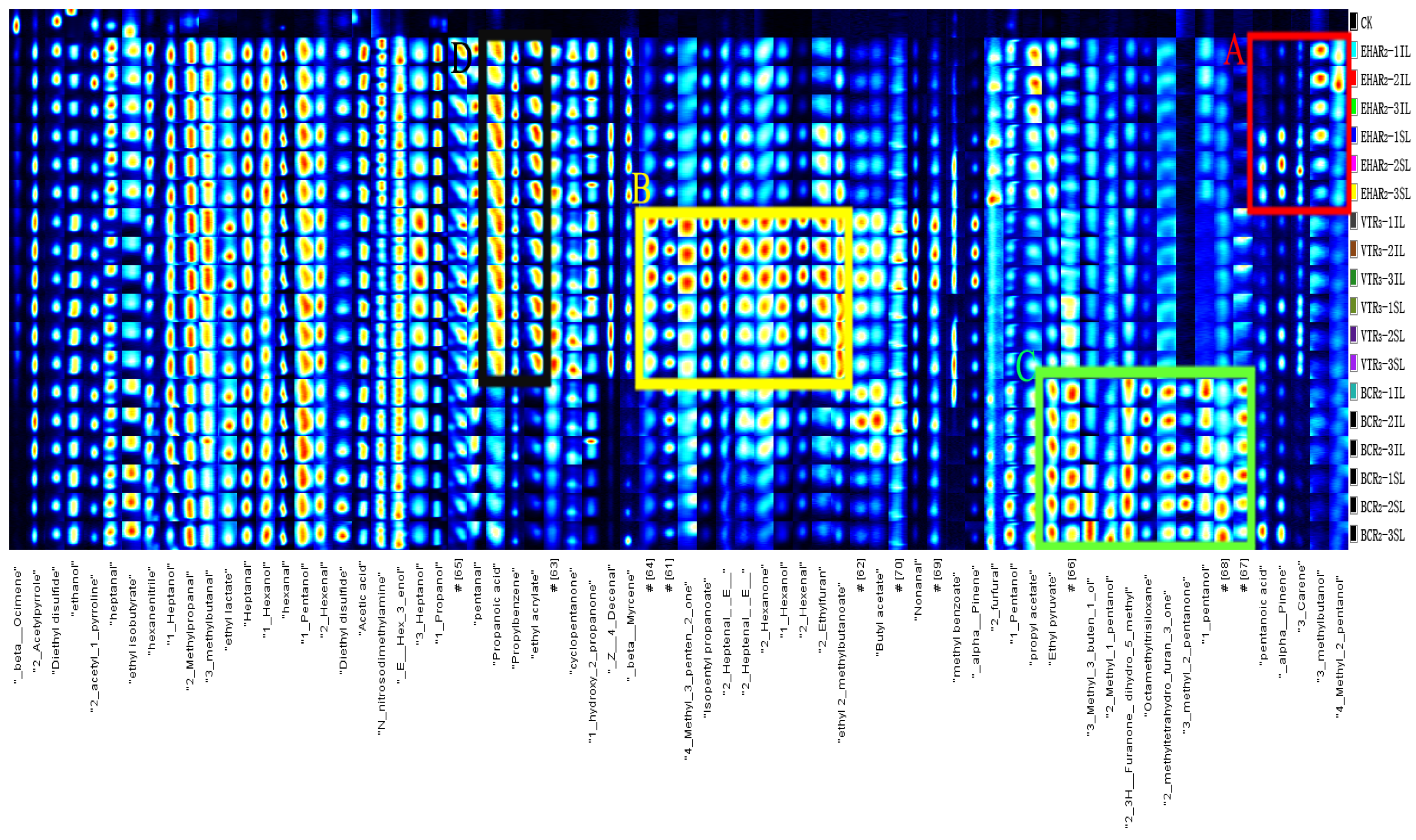
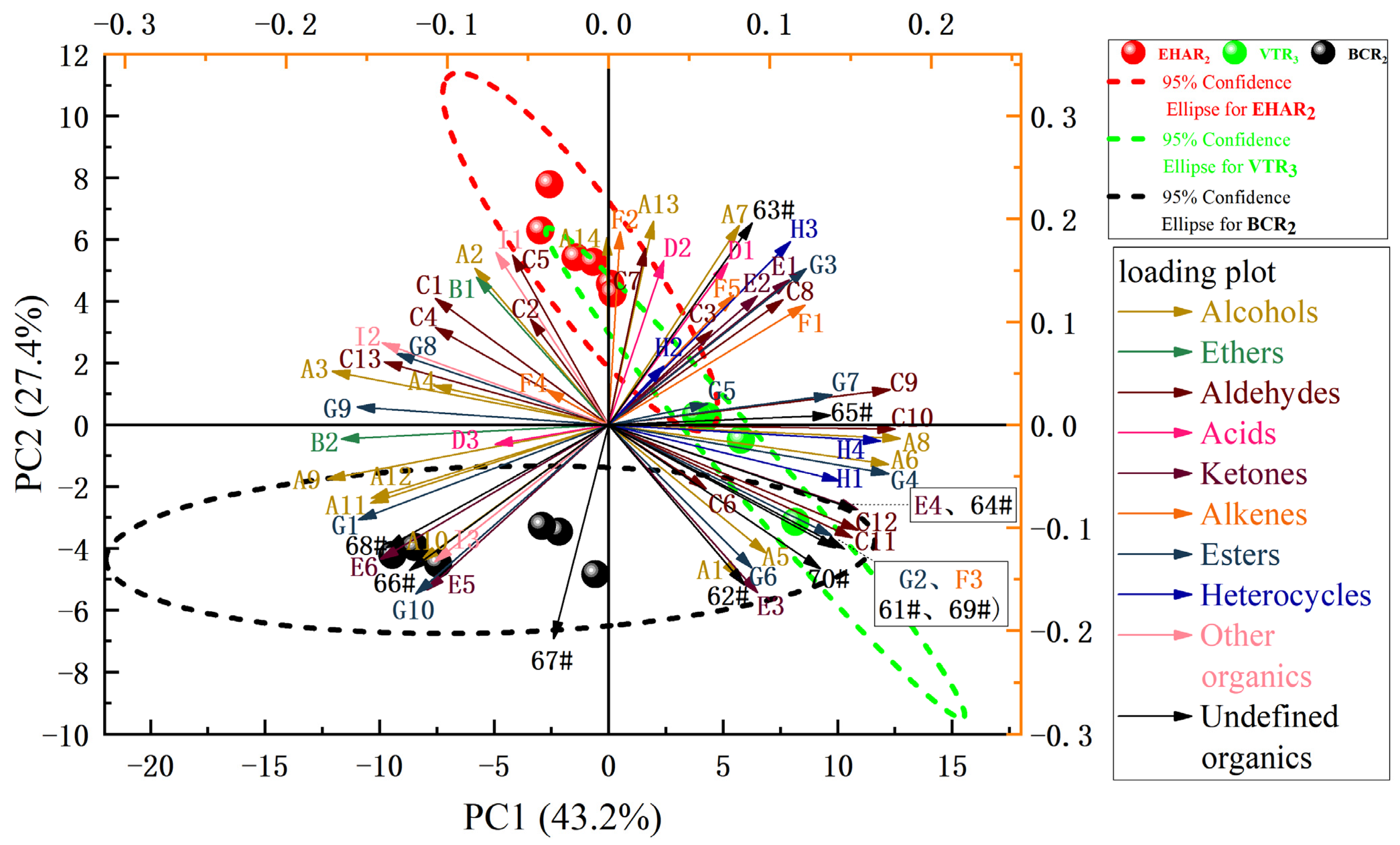
| Volatiles | Serial Number | Compound | Flavor Description | Characteristic Peak Number | CAS# | RI | Rt(s) | Dt (ms) | References |
|---|---|---|---|---|---|---|---|---|---|
| Alcohols | A1 | Ethanol | / | 4 | C64175 | 487.1 | 86.96 | 1.0505 | |
| A2 | 1-Heptanol | green, musty, pungent | 9 | C111706 | 968.7 | 484.533 | 1.4068 | [31] | |
| A3 | 1-Hexanol-D | woody, cut grass, chemical–winey, fatty, fruity, weak metallic | 41 | C111273 | 877.8 | 327.663 | 1.6299 | [32] | |
| A4 | 1-Pentanol-M | roasted | 63 | C71410 | 778.4 | 222.813 | 1.5179 | [33] | |
| A5 | (E)-Hex-3-enol | / | 21 | C928972 | 830 | 272.228 | 1.2607 | ||
| A6 | 3-Heptanol | fresh, grassy, fatty, rancid | 22 | C589822 | 884.1 | 335.798 | 1.6625 | [34] | |
| A7 | 1-Propanol | / | 23 | C71238 | 541.8 | 101.915 | 1.1163 | ||
| A8 | 1-Hexanol-M | woody, cut grass, chemical–winey, fatty, fruity, weak metallic | 14 | C111273 | 863.9 | 310.489 | 1.3263 | ||
| A9 | 1-Pentanol-D | roasted | 16 | C71410 | 785.4 | 229.037 | 1.2543 | ||
| A10 | 3-Methyl-3-buten-1-ol | sweet fruity | 57 | C763326 | 737.2 | 189.156 | 1.1598 | [35] | |
| A11 | 2-Methyl-1-pentanol | caramel | 58 | C105306 | 845 | 288.529 | 1.3005 | [36] | |
| A12 | 1-Pentanol-D | roasted | 53 | C71410 | 766.5 | 212.484 | 1.2549 | ||
| A13 | 3-Methylbutanol | woody, acorn, pleasant green | 69 | C123513 | 738.7 | 190.334 | 1.4782 | [37] | |
| A14 | 4-Methyl-2-pentanol | / | 70 | C108112 | 764.8 | 211.091 | 1.2882 | ||
| Ethers | B1 | Diethyl disulfide-M | moldy, sulfur | 3 | C110816 | 926.2 | 402.129 | 1.1526 | [38] |
| B2 | Diethyl disulfide-D | moldy, sulfur | 18 | C110816 | 926.2 | 402.129 | 1.287 | [38] | |
| Aldehydes | C1 | Heptanal-M | fatty, putty | 6 | C111717 | 891 | 344.837 | 1.3342 | [39] |
| C2 | 2-Methylpropanal | fresh, aldehydic, floral, pungent | 10 | C78842 | 563.1 | 108.396 | 1.0887 | [40] | |
| C3 | 3-Methylbutanal | caramel, chocolate | 11 | C590863 | 647.1 | 138.307 | 1.1577 | [41] | |
| C4 | Heptanal-D | fatty, putty | 13 | C111717 | 886.9 | 339.413 | 1.6977 | ||
| C5 | Hexanal | fruity, fatty, green | 15 | C66251 | 804.1 | 246.314 | 1.5661 | [42] | |
| C6 | 2-Hexenal-M | salty meat, dry ham | 17 | C505577 | 846.6 | 290.355 | 1.1842 | [33] | |
| C7 | Pentanal | salty, musty odors | 25 | C110623 | 689.8 | 156.752 | 1.1789 | [43] | |
| C8 | (Z)-4-Decenal | fruity, heavy, sweet | 32 | C21662099 | 1201.3 | 1142.345 | 1.3425 | [44] | |
| C9 | (E)-2-Heptenal-M | fatty, fruity | 38 | C18829555 | 955.6 | 457.538 | 1.2534 | [45] | |
| C10 | (E)-2-Heptenal-D | fatty, fruity | 39 | C18829555 | 954.2 | 454.697 | 1.6843 | [45] | |
| C11 | 2-Hexenal-D | salty meat, dry ham | 42 | C505577 | 845.3 | 288.859 | 1.5154 | ||
| C12 | Nonanal | citrus, green, citronella grass | 48 | C124196 | 1064.1 | 697.868 | 1.9507 | [46] | |
| C13 | 2-Furfural | almond, woody | 52 | C98011 | 828.8 | 271.033 | 1.3369 | [44] | |
| Acids | D1 | Acetic acid | pungent, acidic, cheesy, vinegar | 19 | C64197 | 643.4 | 136.812 | 1.0675 | [47] |
| D2 | Propanoic acid | / | 26 | C79094 | 696.9 | 161.239 | 1.2638 | ||
| D3 | Pentanoic acid | sweaty | 66 | C109524 | 902.5 | 362.42 | 1.2203 | [44] | |
| Ketones | E1 | Cyclopentanone | / | 30 | C120923 | 767.8 | 213.583 | 1.3296 | |
| E2 | 1-Hydroxy-2-propanone | butter, sweet | 31 | C116096 | 652 | 140.301 | 1.25 | [44] | |
| E3 | 4-Methyl-3-penten-2-one | sweet, chemical | 36 | C141797 | 792.7 | 235.604 | 1.4431 | [48] | |
| E4 | 2-Hexanone | / | 40 | C591786 | 790.1 | 233.242 | 1.4895 | [48] | |
| E5 | 2-Methyltetrahydro-furan-3-one | caramel, green | 61 | C3188009 | 794 | 236.865 | 1.4242 | [36] | |
| E6 | 3-Methyl-2-pentanone | / | 62 | C565617 | 756.6 | 204.357 | 1.4817 | ||
| Alkenes | F1 | Beta-Ocimene | / | 1 | C13877913 | 1044.6 | 650.762 | 1.2183 | |
| F2 | Beta-Myrcene | green | 33 | C123353 | 984.2 | 518.631 | 1.2987 | [41] | |
| F3 | Alpha-Pinene-M | sharp, pine | 67 | C80568 | 930.4 | 409.598 | 1.6718 | ||
| F4 | Alpha-Pinene-D | sharp, pine | 51 | C80568 | 935.9 | 419.76 | 1.2189 | ||
| F5 | 3-Carene | earthy, green, fresh | 68 | C13466789 | 1007 | 568.552 | 1.2189 | [42] | |
| Esters | G1 | Ethyl isobutyrate | / | 7 | C97621 | 734.5 | 187.162 | 1.1906 | |
| G2 | Ethyl lactate | / | 12 | C97643 | 798.4 | 240.891 | 1.527 | ||
| G3 | Ethyl acrylate | / | 27 | C140885 | 703.1 | 165.227 | 1.3997 | ||
| G4 | Isopentyl propanoate | / | 37 | C105680 | 967.3 | 481.691 | 1.8275 | ||
| G5 | Ethyl 2-methylbutanoate | fruity, sweet | 44 | C7452791 | 864.2 | 310.818 | 1.6465 | [44] | |
| G6 | Butyl acetate | fruity | 46 | C123864 | 824 | 266.014 | 1.6138 | [49] | |
| G7 | Methyl benzoate | / | 50 | C93583 | 1095.1 | 780.014 | 1.216 | ||
| G8 | Propyl acetate | / | 54 | C109604 | 708.3 | 168.683 | 1.1636 | ||
| G9 | Ethyl pyruvate | / | 55 | C617356 | 727 | 181.678 | 1.422 | ||
| G10 | 2(3H)-Furanone, dihydro-5-methyl | cardboard and metallic | 59 | C108292 | 954.2 | 454.697 | 1.417 | [50] | |
| Heterocycles | H1 | 2-Acetylpyrrole | toasted | 2 | C1072839 | 1066.3 | 703.33 | 1.4798 | [51] |
| H2 | 2-Acetyl-1-pyrroline | green, strange, coffee, sweet, fried corn | 5 | C85213225 | 927.5 | 404.493 | 1.4723 | [51] | |
| H3 | Propylbenzene | / | 27 | C103651 | 948.4 | 443.331 | 1.1599 | ||
| H4 | 2-Ethylfuran | fruity, floral | 43 | C3208160 | 698.1 | 161.991 | 1.3142 | [39] | |
| Other organics | I1 | hexanenitrile | / | 8 | C628739 | 875.6 | 324.951 | 1.2625 | |
| I2 | N-nitrosodimethylamine | / | 20 | C62759 | 747.6 | 197.132 | 1.0632 | ||
| I3 | Octamethyltrisiloxane | / | 60 | C107517 | 877.1 | 326.847 | 1.5509 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, J.; Wang, L.; Ma, X.; Xu, Z.; Wang, Z. Effects of Variable-Temperature Roasting on the Flavor Compounds of Xinjiang Tannur-Roasted Mutton. Foods 2024, 13, 3077. https://doi.org/10.3390/foods13193077
Wei J, Wang L, Ma X, Xu Z, Wang Z. Effects of Variable-Temperature Roasting on the Flavor Compounds of Xinjiang Tannur-Roasted Mutton. Foods. 2024; 13(19):3077. https://doi.org/10.3390/foods13193077
Chicago/Turabian StyleWei, Jian, Li Wang, Xin Ma, Zequan Xu, and Zirong Wang. 2024. "Effects of Variable-Temperature Roasting on the Flavor Compounds of Xinjiang Tannur-Roasted Mutton" Foods 13, no. 19: 3077. https://doi.org/10.3390/foods13193077






