Effect of Mixed Probiotics on Alleviating H1N1 Influenza Infection and Regulating Gut Microbiota
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Culture Conditions
2.2. Treatment of Mice In Vivo
2.3. Lung Tissue Cellular Injury
2.4. Histopathological Analysis of Lung Tissue
2.5. Viral Load Quantification and mRNA Expression Analysis of Antiviral Proteins
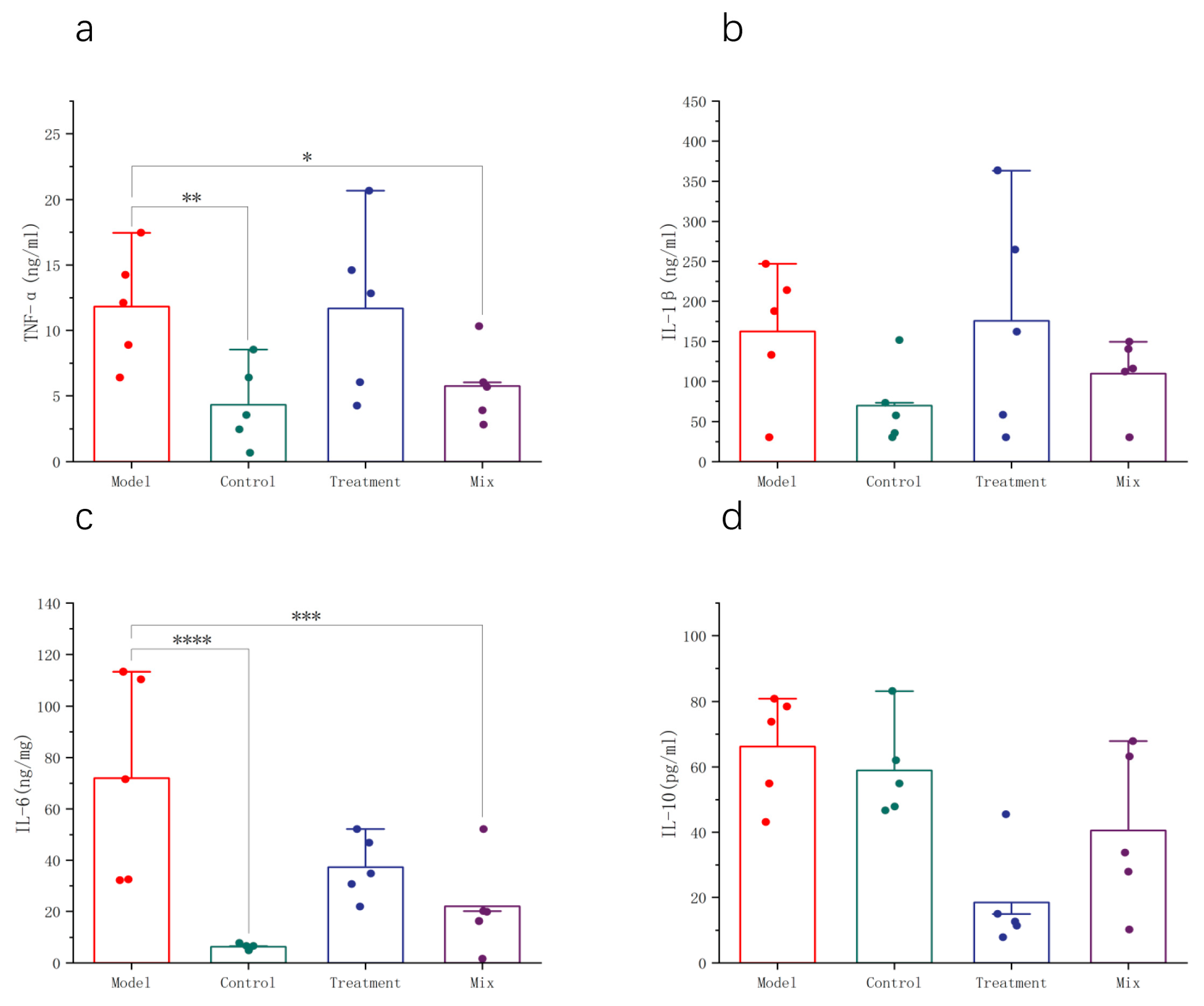
2.6. Cytokine Quantification
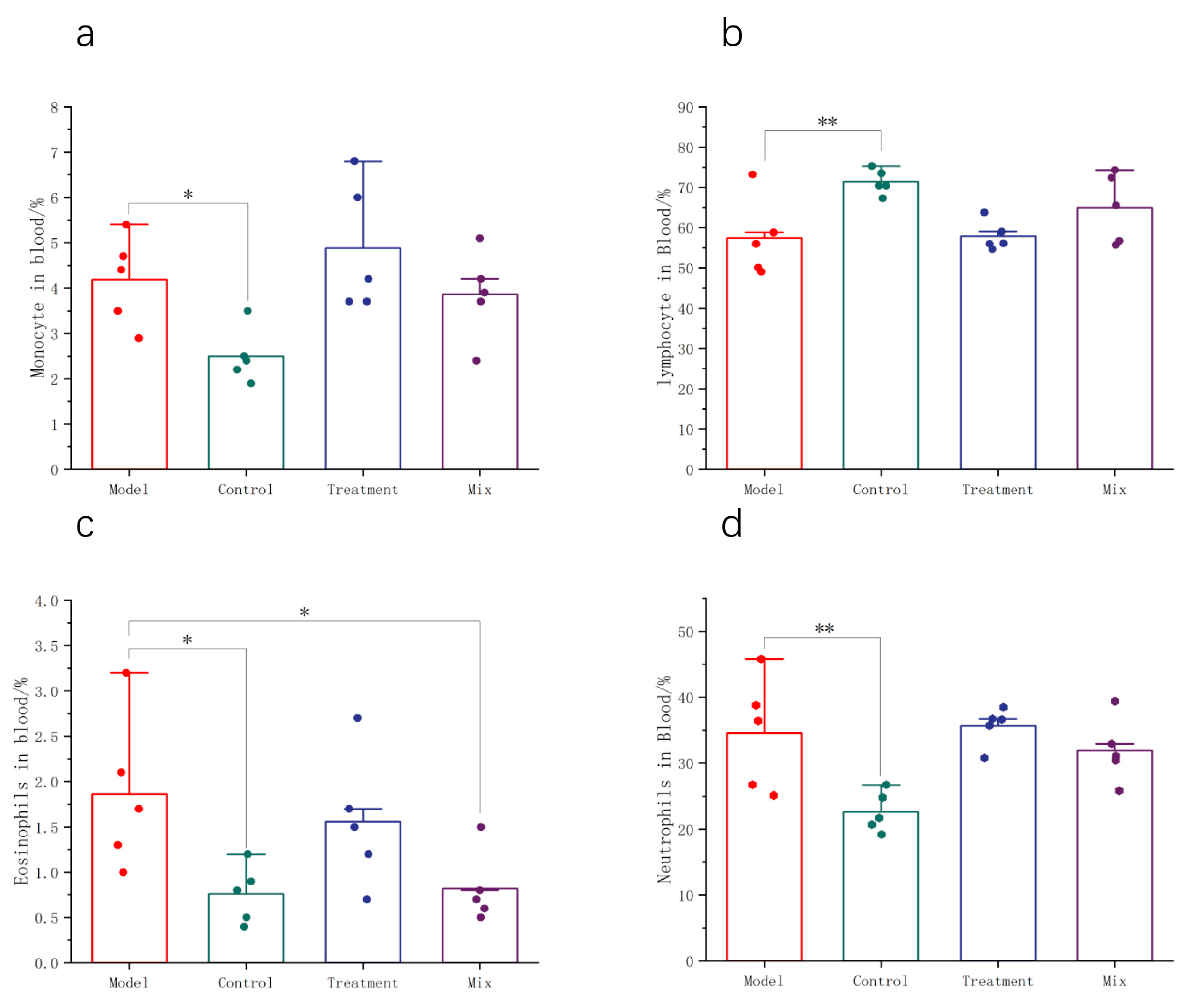
2.7. Blood Cell Analysis
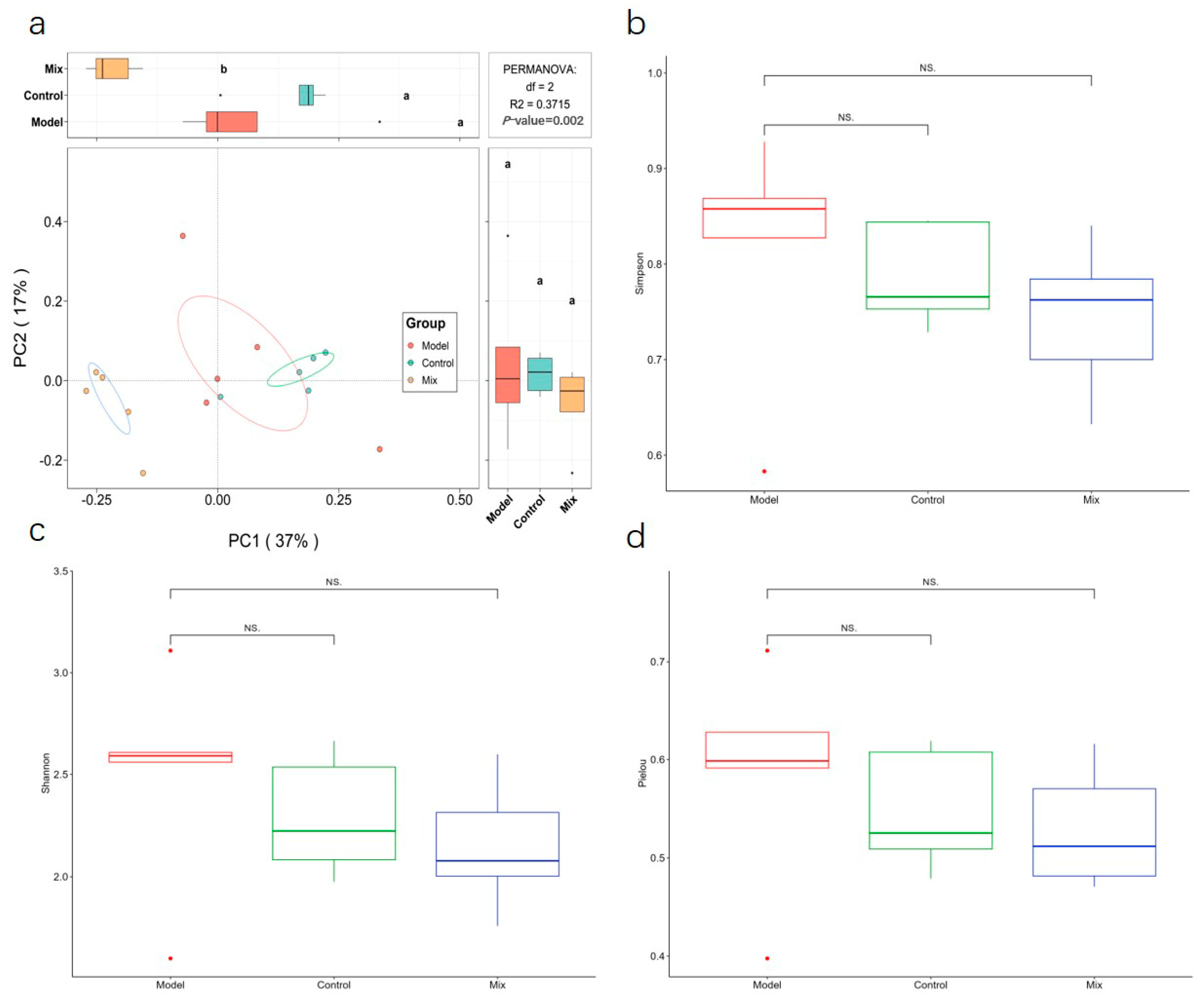
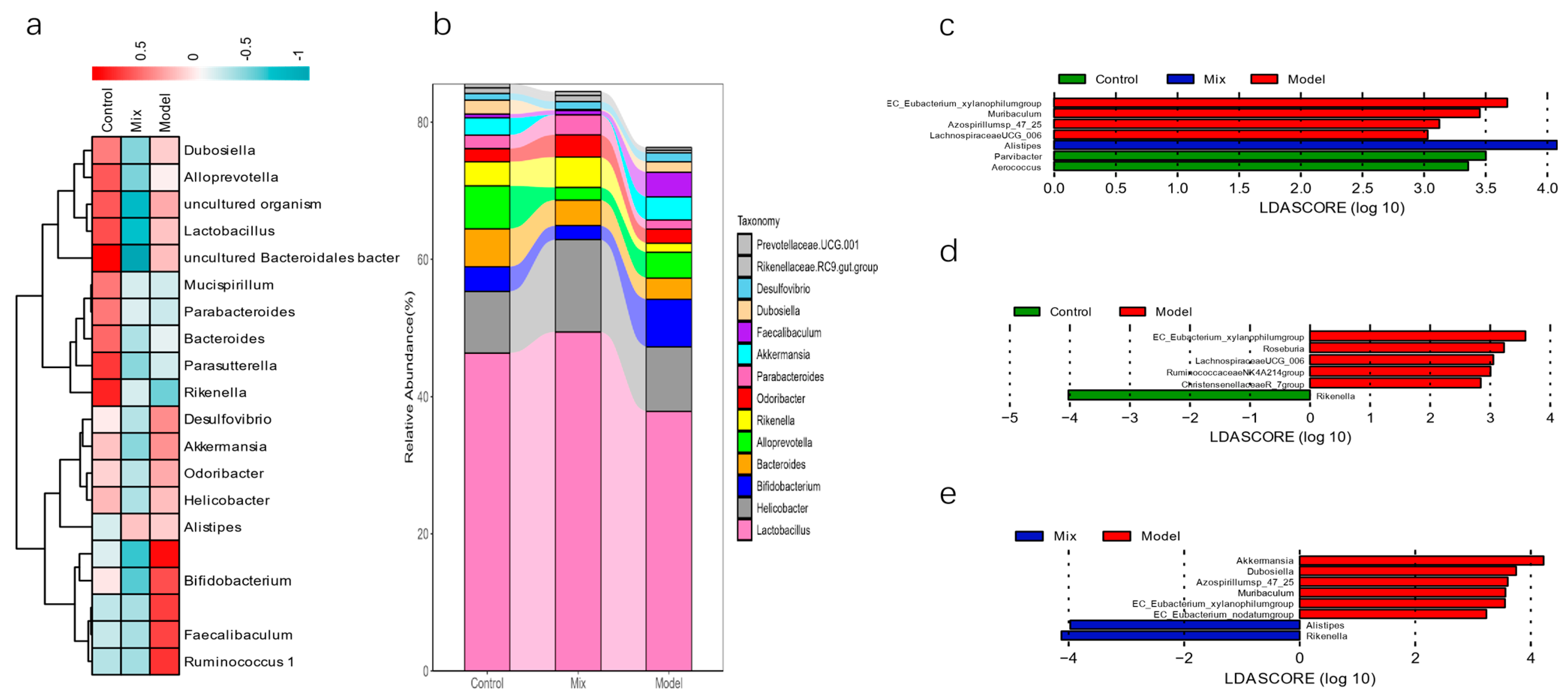
2.8. Gut Microbiota Analysis
2.9. Fecal Metabolomics
2.10. Statistic Analysis
3. Results
3.1. A Mixed Probiotics Solution Protects Mice from Influenza Virus Infection
3.2. Impact of Mixed Probiotics on the Systemic Immune Response in Mice
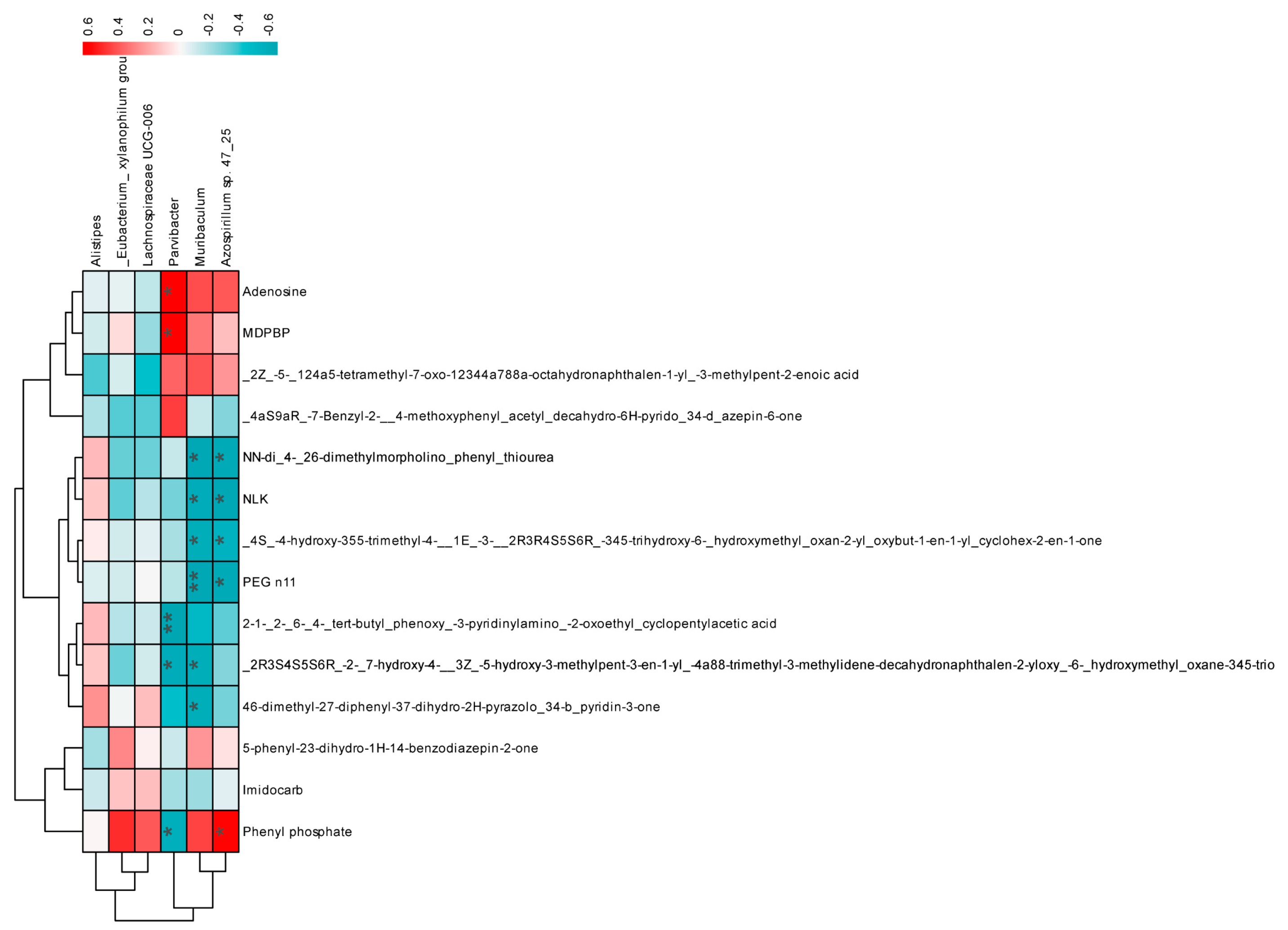
3.3. Impact of Mixed Probiotics Administration on the Gut Microbiota of Mice
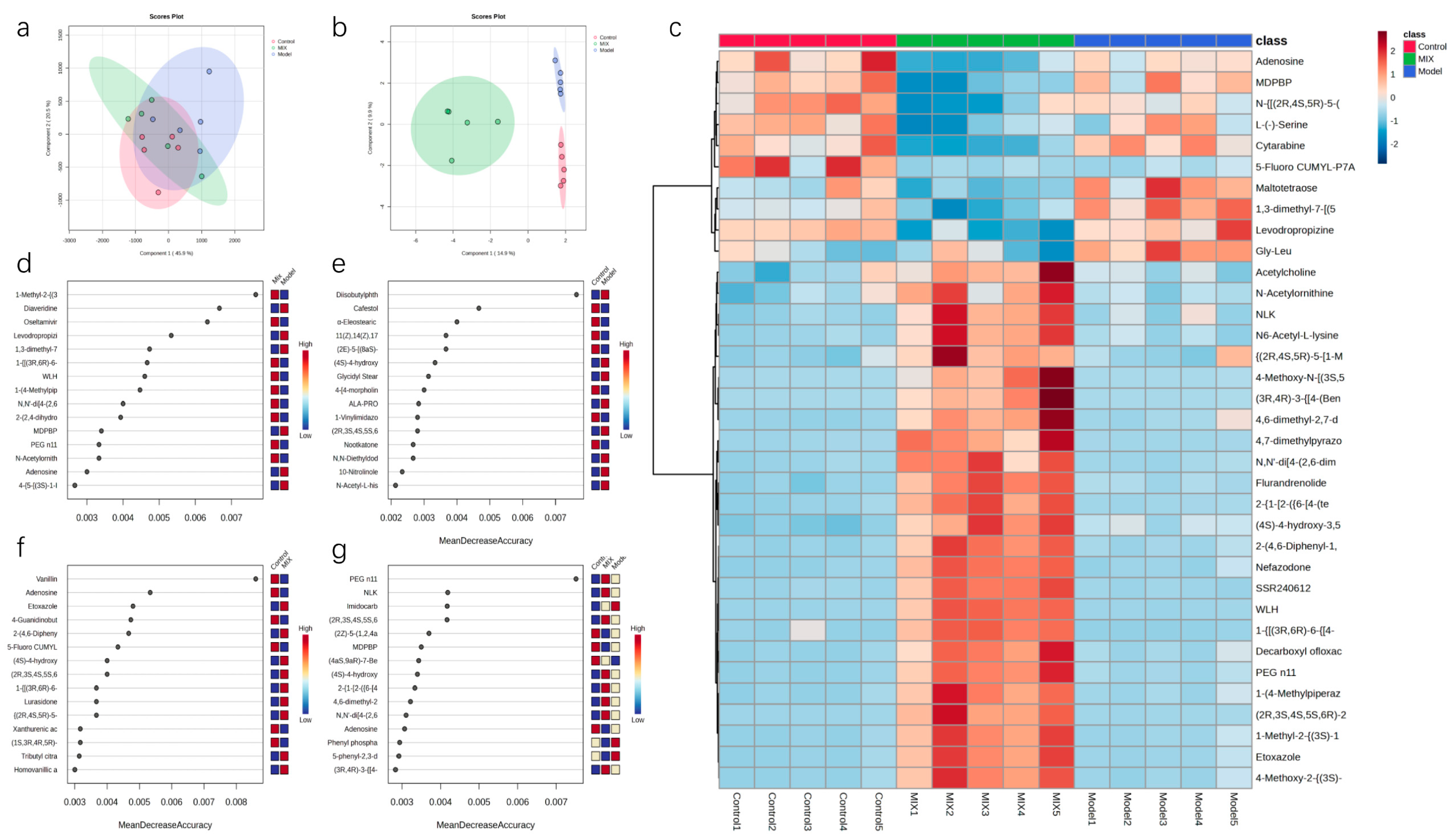
3.4. Effects of Mixed Probiotics on Mice Intestinal Metabolites
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tsai, S.-Y.; Segovia, J.A.; Chang, T.-H.; Morris, I.R.; Berton, M.T.; Tessier, P.A.; Tardif, M.R.; Cesaro, A.; Bose, S. DAMP molecule S100A9 acts as a molecular pattern to enhance inflammation during influenza A virus infection: Role of DDX21-TRIF-TLR4-MyD88 pathway. PLoS Pathog. 2014, 10, e1003848. [Google Scholar] [CrossRef] [PubMed]
- Herold, S.; Becker, C.; Ridge, K.M.; Budinger, G.S. Influenza virus-induced lung injury: Pathogenesis and implications for treatment. Eur. Respir. J. 2015, 45, 1463–1478. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wei, T.; Tianv, H.; Cheng, J.; Xiao, J.; Wang, M.; Hu, Y. Small intestinal injury in mice infected with respiratory influenza A virus: Evidence for virus induced gastroenteritis. Biotechnol. Lett. 2015, 37, 1585–1592. [Google Scholar] [CrossRef]
- Troeger, C.; Blacker, B.; Khalil, I.A.; Rao, P.C.; Cao, J.; Zimsen, S.R.M.; Albertson, S.B.; Deshpande, A.; Farag, T.; Abebe, Z.; et al. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect. Dis. 2018, 18, 1191–1210. [Google Scholar] [CrossRef]
- Vogel, A.B.; Lambert, L.; Kinnear, E.; Busse, D.; Erbar, S.; Reuter, K.C.; Wicke, L.; Perkovic, M.; Beissert, T.; Haas, H.; et al. Self-Amplifying RNA Vaccines Give Equivalent Protection against Influenza to mRNA Vaccines but at Much Lower Doses. Mol. Ther. 2018, 26, 446–455. [Google Scholar] [CrossRef]
- Wypych, T.P.; Wickramasinghe, L.C.; Marsland, B.J. The influence of the microbiome on respiratory health. Nat. Immunol. 2019, 20, 1279–1290. [Google Scholar] [CrossRef] [PubMed]
- Trompette, A.; Gollwitzer, E.S.; Yadava, K.; Sichelstiel, A.K.; Sprenger, N.; Ngom-Bru, C.; Blanchard, C.; Junt, T.; Nicod, L.P.; Harris, N.L.; et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat. Med. 2014, 20, 159–166. [Google Scholar] [CrossRef]
- Chen, M.-Y.; Li, H.; Lu, X.-X.; Ling, L.-J.; Weng, H.-B.; Sun, W.; Chen, D.-F.; Zhang, Y.-Y. Houttuynia cordata polysaccharide alleviated intestinal injury and modulated intestinal microbiota in H1N1 virus infected mice. Chin. J. Nat. Med. 2019, 17, 187–197. [Google Scholar] [CrossRef]
- Dang, A.T.; Marsland, B.J. Microbes, metabolites, and the gut-lung axis. Mucosal Immunol. 2019, 12, 843–850. [Google Scholar] [CrossRef]
- Rather, I.A.; Kamli, M.R.; Sabir, J.S.; Paray, B.A. Potential Antiviral Activity of Lactiplantibacillus plantarum KAU007 against Influenza Virus H1N1. Vaccines 2022, 10, 456. [Google Scholar] [CrossRef]
- Liu, X.; Fang, Z.; Deng, Y.; Lu, W.; Zhang, P.; Zhang, H.; Zhao, J.; Chen, W. Oral administration of probiotics protected mice from influenza virus infection. Food Biosci. 2020, 41, 100804. [Google Scholar] [CrossRef]
- Akatsu, H.; Nagafuchi, S.; Kurihara, R.; Okuda, K.; Kanesaka, T.; Ogawa, N.; Kanematsu, T.; Takasugi, S.; Yamaji, T.; Takami, M.; et al. Enhanced vaccination effect against influenza by prebiotics in elderly patients receiving enteral nutrition. Geriatr. Gerontol. Int. 2016, 16, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Wang, Z.; Stanton, C.; Ross, R.P.; Zhao, J.; Zhang, H.; Yang, B.; Chen, W. Lactobacillus rhamnosus FJSYC4-1 and Lactobacillus reuteri FGSZY33L6 alleviate metabolic syndrome via gut microbiota regulation. Food Funct. 2021, 12, 3919–3930. [Google Scholar] [CrossRef]
- Zhu, G.; Guo, M.; Zhao, J.; Zhang, H.; Wang, G.; Chen, W. Integrative Metabolomic Characterization Reveals the Mediating Effect of Bifidobacterium breve on Amino Acid Metabolism in a Mouse Model of Alzheimer’s Disease. Nutrients 2022, 14, 735. [Google Scholar] [CrossRef]
- Nouria, B.; Raphaëlle, B.-S.; Muhamed-Kkeir, T. Lactobacillus paracasei feeding improves the control of secondary experimental meningococcal infection in flu-infected mice. BMC Infect. Dis. 2018, 18, 167. [Google Scholar]
- Codo, A.C.; Davanzo, G.G.; de Brito Monteiro, L.; de Souza, G.F.; Muraro, S.P.; Virgilio-da-Silva, J.V.; Prodonoff, J.S.; Carregari, V.C.; de Biagi Junior, C.A.; Crunfli, F.; et al. Elevated Glucose Levels Favor SARS-CoV-2 Infection and Monocyte Response through a HIF-1α/Glycolysis-Dependent Axis. Cell Metab. 2020, 32, 437–446.e435. [Google Scholar] [CrossRef]
- Treon, S.P.; Castillo, J.J.; Skarbnik, A.P.; Soumerai, J.D.; Ghobrial, I.M.; Guerrera, M.L.; Meid, K.; Yang, G. The BTK inhibitor ibrutinib may protect against pulmonary injury in COVID-19-infected patients. Blood 2020, 135, 1912–1915. [Google Scholar] [CrossRef]
- Atyeo, C.G.; Shook, L.L.; Brigida, S.; De Guzman, R.M.; Demidkin, S.; Muir, C.; Akinwunmi, B.; Baez, A.M.; Sheehan, M.L.; McSweeney, E.; et al. Maternal immune response and placental antibody transfer after COVID-19 vaccination across trimester and platforms. Nat. Commun. 2022, 13, 3571. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Gu, Y.; Chakarov, S.; Bleriot, C.; Kwok, I.; Chen, X.; Shin, A.; Huang, W.; Dress, R.J.; Dutertre, C.A.; et al. Fate Mapping via Ms4a3-Expression History Traces Monocyte-Derived Cells. Cell 2019, 178, 1509–1525.e1519. [Google Scholar] [CrossRef]
- García-Sastre, A. Induction and evasion of type I interferon responses by influenza viruses. Virus Res. 2011, 162, 12–18. [Google Scholar] [CrossRef]
- Chang, L.A.; Choi, A.; Rathnasinghe, R.; Warang, P.; Noureddine, M.; Jangra, S.; Chen, Y.; De Geest, B.G.; Schotsaert, M. Influenza breakthrough infection in vaccinated mice is characterized by non-pathological lung eosinophilia. Front. Immunol. 2023, 14, 1217181. [Google Scholar] [CrossRef]
- Shi, N.; Li, N.; Duan, X.; Niu, H. Interaction between the gut microbiome and mucosal immune system. Mil. Med. Res. 2017, 4, 14. [Google Scholar] [CrossRef]
- Kuppalli, K.; Rasmussen, A.L. A glimpse into the eye of the COVID-19 cytokine storm. EBioMedicine 2020, 55, 102789. [Google Scholar] [CrossRef]
- Monsalvo, A.C.; Batalle, J.P.; Lopez, M.F.; Krause, J.C.; Klemenc, J.; Hernandez, J.Z.; Maskin, B.; Bugna, J.; Rubinstein, C.; Aguilar, L.; et al. Severe pandemic 2009 H1N1 influenza disease due to pathogenic immune complexes. Nat. Med. 2011, 17, 195–199. [Google Scholar] [CrossRef]
- Bermejo-Martin, J.F.; Ortiz de Lejarazu, R.; Pumarola, T.; Rello, J.; Almansa, R.; Ramírez, P.; Martin-Loeches, I.; Varillas, D.; Gallegos, M.C.; Serón, C.; et al. Th1 and Th17 hypercytokinemia as early host response signature in severe pandemic influenza. Crit. Care 2009, 13, R201. [Google Scholar] [CrossRef]
- To, K.K.; Hung, I.F.; Li, I.W.; Lee, K.L.; Koo, C.K.; Yan, W.W.; Liu, R.; Ho, K.Y.; Chu, K.H.; Watt, C.L.; et al. Delayed clearance of viral load and marked cytokine activation in severe cases of pandemic H1N1 2009 influenza virus infection. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2010, 50, 850–859. [Google Scholar] [CrossRef]
- Qin, T.; Chen, Y.; Huangfu, D.; Yin, Y.; Miao, X.; Yin, Y.; Chen, S.; Peng, D.; Liu, X. PA-X Protein of H1N1 Subtype Influenza Virus Disables the Nasal Mucosal Dendritic Cells for Strengthening Virulence. Virulence 2022, 13, 1928–1942. [Google Scholar] [CrossRef]
- Steed, A.L.; Christophi, G.P.; Kaiko, G.E.; Sun, L.; Goodwin, V.M.; Jain, U.; Esaulova, E.; Artyomov, M.N.; Morales, D.J.; Holtzman, M.J.; et al. The microbial metabolite desaminotyrosine protects from influenza through type I interferon. Science 2017, 357, 498–502. [Google Scholar] [CrossRef]
- Wu, X.; Li, R.F.; Lin, Z.S.; Xiao, C.; Liu, B.; Mai, K.L.; Zhou, H.X.; Zeng, D.Y.; Cheng, S.; Weng, Y.C.; et al. Coinfection with influenza virus and non-typeable Haemophilus influenzae aggregates inflammatory lung injury and alters gut microbiota in COPD mice. Front. Microbiol. 2023, 14, 1137369. [Google Scholar] [CrossRef] [PubMed]
- Trachootham, D.; Chupeerach, C.; Tuntipopipat, S.; Pathomyok, L.; Boonnak, K.; Praengam, K.; Promkam, C.; Santivarangkna, C. Drinking fermented milk containing Lactobacillus paracasei 431 (IMULUS™) improves immune response against H1N1 and cross-reactive H3N2 viruses after influenza vaccination: A pilot randomized triple-blinded placebo controlled trial. J. Funct. Foods 2017, 33, 1–10. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Majumder, R.; Rather, I.A.; Nam, G.-J.; Park, Y.-H. Molecular Characterization of Lactobacillus plantarum YML016 with Anti-Diabetic, Anti-Melanogenic and Anti-Viral Efficacy. Natl. Acad. Sci. Lett. 2018, 41, 301–305. [Google Scholar] [CrossRef]
- Rau, M.; Rehman, A.; Dittrich, M.; Groen, A.K.; Hermanns, H.M.; Seyfried, F.; Beyersdorf, N.; Dandekar, T.; Rosenstiel, P.; Geier, A. Fecal SCFAs and SCFA-producing bacteria in gut microbiome of human NAFLD as a putative link to systemic T-cell activation and advanced disease. United Eur. Gastroenterol. J. 2018, 6, 1496–1507. [Google Scholar] [CrossRef]
- Lezutekong, J.N.; Nikhanj, A.; Oudit, G.Y. Imbalance of gut microbiome and intestinal epithelial barrier dysfunction in cardiovascular disease. Clin. Sci. 2018, 132, 901–904. [Google Scholar] [CrossRef]
- Wu, M.; Li, P.; An, Y.; Ren, J.; Yan, D.; Cui, J.; Li, D.; Li, M.; Wang, M.; Zhong, G. Phloretin ameliorates dextran sulfate sodium-induced ulcerative colitis in mice by regulating the gut microbiota. Pharmacol. Res. 2019, 150, 104489. [Google Scholar] [CrossRef]
- Walker, A.; Pfitzner, B.; Harir, M.; Schaubeck, M.; Calasan, J.; Heinzmann, S.S.; Turaev, D.; Rattei, T.; Endesfelder, D.; Castell, W.Z.; et al. Sulfonolipids as novel metabolite markers of Alistipes and Odoribacter affected by high-fat diets. Sci. Rep. 2017, 7, 11047. [Google Scholar] [CrossRef]
- Rabinowitz, M.H.; Andrews, R.C.; Becherer, J.D.; Bickett, D.M.; Bubacz, D.G.; Conway, J.G.; Cowan, D.J.; Gaul, M.; Glennon, K.; Lambert, M.H.; et al. Design of Selective and Soluble Inhibitors of Tumor Necrosis Factor-α Converting Enzyme (TACE). J. Med. Chem. 2001, 44, 4252–4267. [Google Scholar] [CrossRef]
- Lee, S.; Nam, K.Y.; Oh, J.; Lee, S.; Cho, S.M.; Choi, Y.W.; Cho, J.Y.; Lee, B.J.; Hong, J.H. Evaluation of the effects of food on levodropropizine controlled-release tablet and its pharmacokinetic profile in comparison to that of immediate-release tablet. Drug Des. Dev. Ther. 2018, 12, 1413–1420. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.H.; Cho, Y.J.; Jeong, S.H. Pharmacokinetic Analysis of Levodropropizine and Its Potential Therapeutic Advantages Considering Eosinophil Levels and Clinical Indications. Pharmaceuticals 2024, 17, 234. [Google Scholar] [CrossRef]
- Di Virgilio, F.; Adinolfi, E. Extracellular purines, purinergic receptors and tumor growth. Oncogene 2017, 36, 293–303. [Google Scholar] [CrossRef]
- Elham, S.; Elahe, A.; Mehdi, M.; Shakila, Y.; Amene, S.; Nima, R. Inosine, Gut Microbiota, and Cancer Immunometabolism. Am. J. Physiology. Endocrinol. Metab. 2022, 324, E1–E8. [Google Scholar]
- Liang, C.; Jinling, F.; Eong, O.E.; Hou, L.Y. Serial Metabolome Changes in a Prospective Cohort of Subjects with Influenza Viral Infection and Comparison with Dengue Fever. J. Proteome Res. 2017, 16, 2614–2622. [Google Scholar]


| Gene | Forward/Reverse | Sequence (5′ to 3′) |
|---|---|---|
| GAPDH | Forward | AATGGTGAAGGTCGGTGTGAAC |
| Reverse | GCCTTGACTGTGCCGTTGAA | |
| NP | Forward | GGCACCAAACGGTCTTACGA |
| Reverse | TCACCTGATCAACTCCATTACCA | |
| MxA | Forward | CCAACTGGAATCCTCCTGGAA |
| Reverse | GCCGCACCTTCTCCTCATAG | |
| Oas1 | Forward | GAAGAGGCTGATGTGTGGCT |
| Reverse | TGTCCAGTTCTCTTCTACCTGC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Zhao, Y.; Pei, Z.; Zhao, J.; Zhang, P.; Zhang, X.; Zhang, Z.; Lu, W. Effect of Mixed Probiotics on Alleviating H1N1 Influenza Infection and Regulating Gut Microbiota. Foods 2024, 13, 3079. https://doi.org/10.3390/foods13193079
Wang H, Zhao Y, Pei Z, Zhao J, Zhang P, Zhang X, Zhang Z, Lu W. Effect of Mixed Probiotics on Alleviating H1N1 Influenza Infection and Regulating Gut Microbiota. Foods. 2024; 13(19):3079. https://doi.org/10.3390/foods13193079
Chicago/Turabian StyleWang, Hongchao, Yuhao Zhao, Zhangming Pei, Jianxin Zhao, Pinghu Zhang, Xinyue Zhang, Zhijian Zhang, and Wenwei Lu. 2024. "Effect of Mixed Probiotics on Alleviating H1N1 Influenza Infection and Regulating Gut Microbiota" Foods 13, no. 19: 3079. https://doi.org/10.3390/foods13193079








