Validation of a Passive Solar Drying System Using Pineapple
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
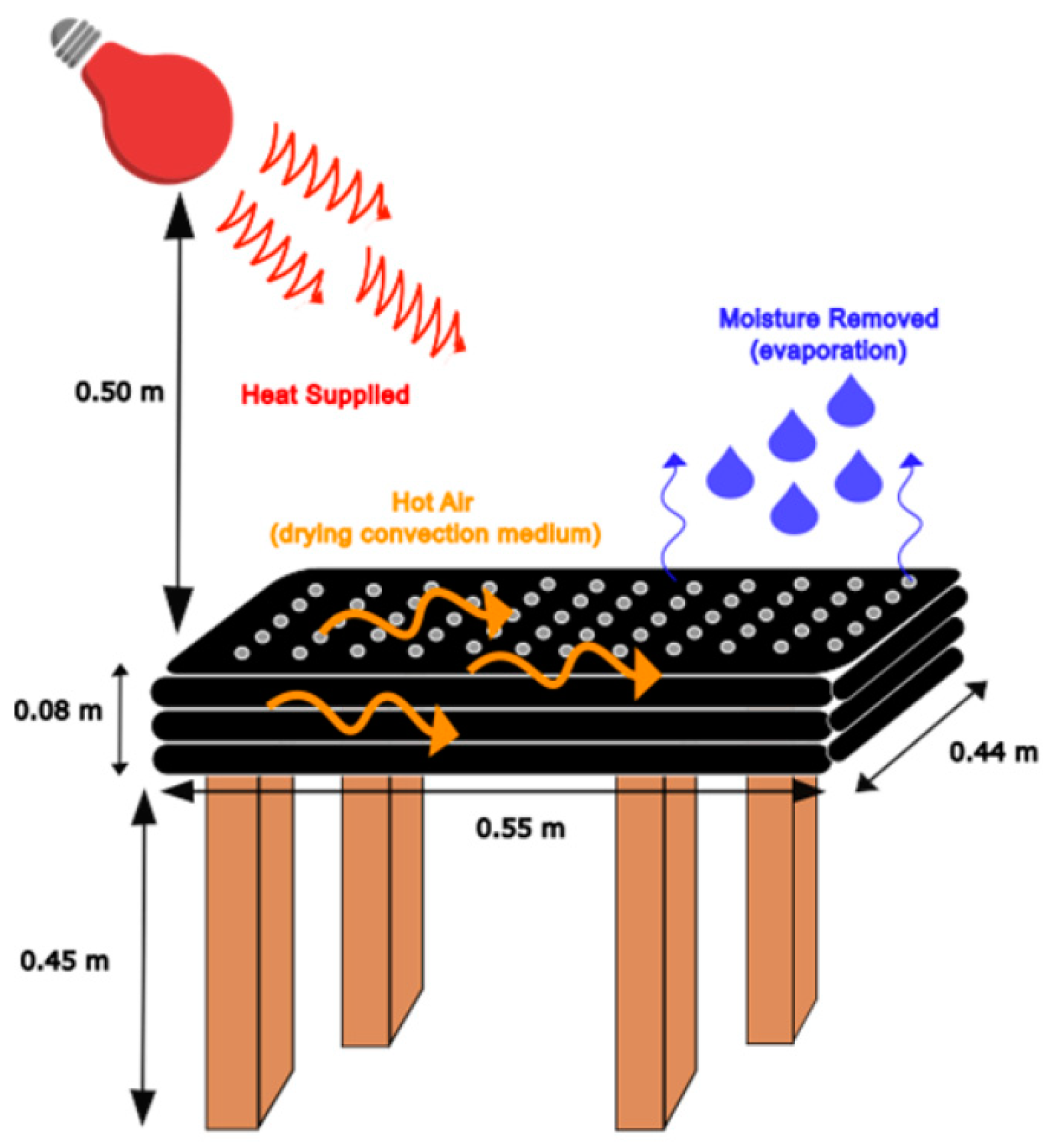
2.2. Passive Solar Drying and Freeze-Drying Systems’ Setup
2.3. Water Content Analysis
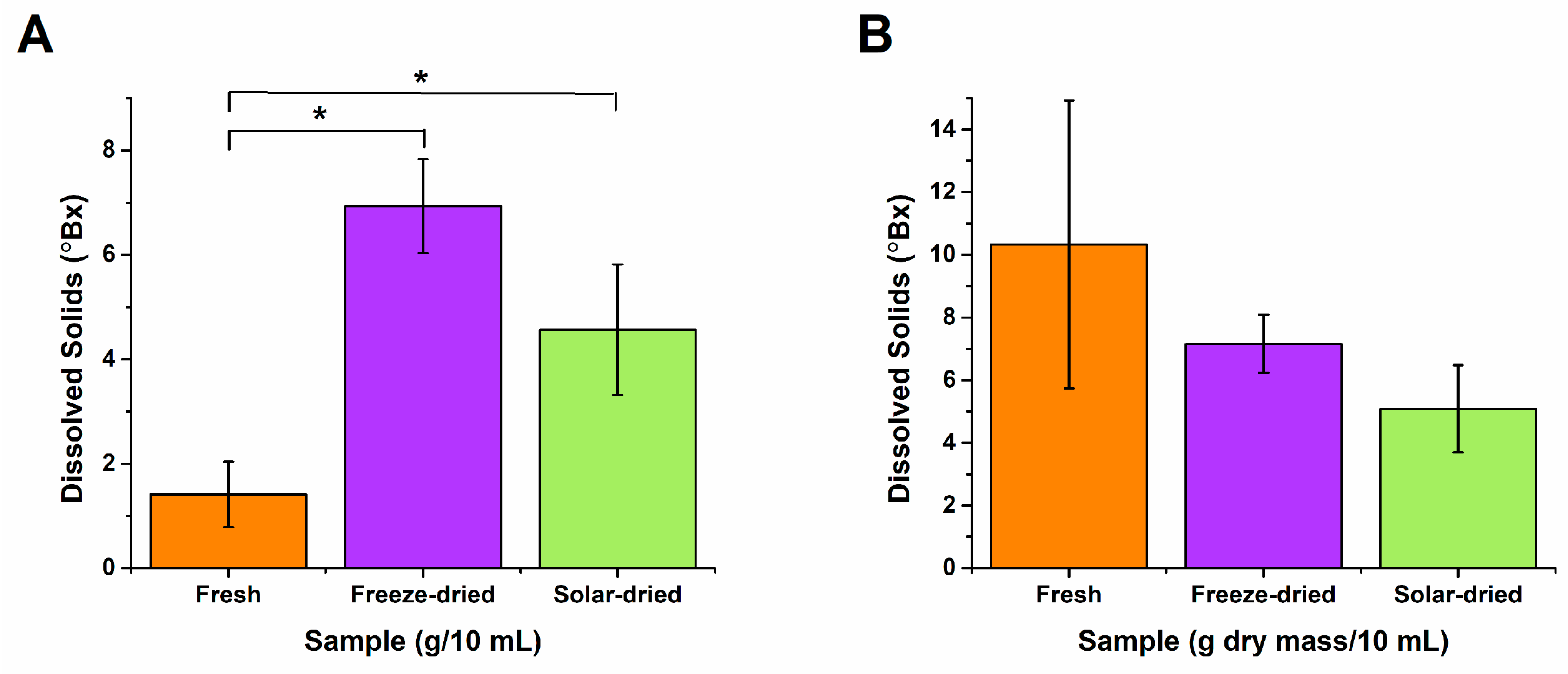
2.4. Vitamin C, Total Phenolic Contents, Iron, and Dissolved Solids Analysis
2.5. Microbiological Analysis
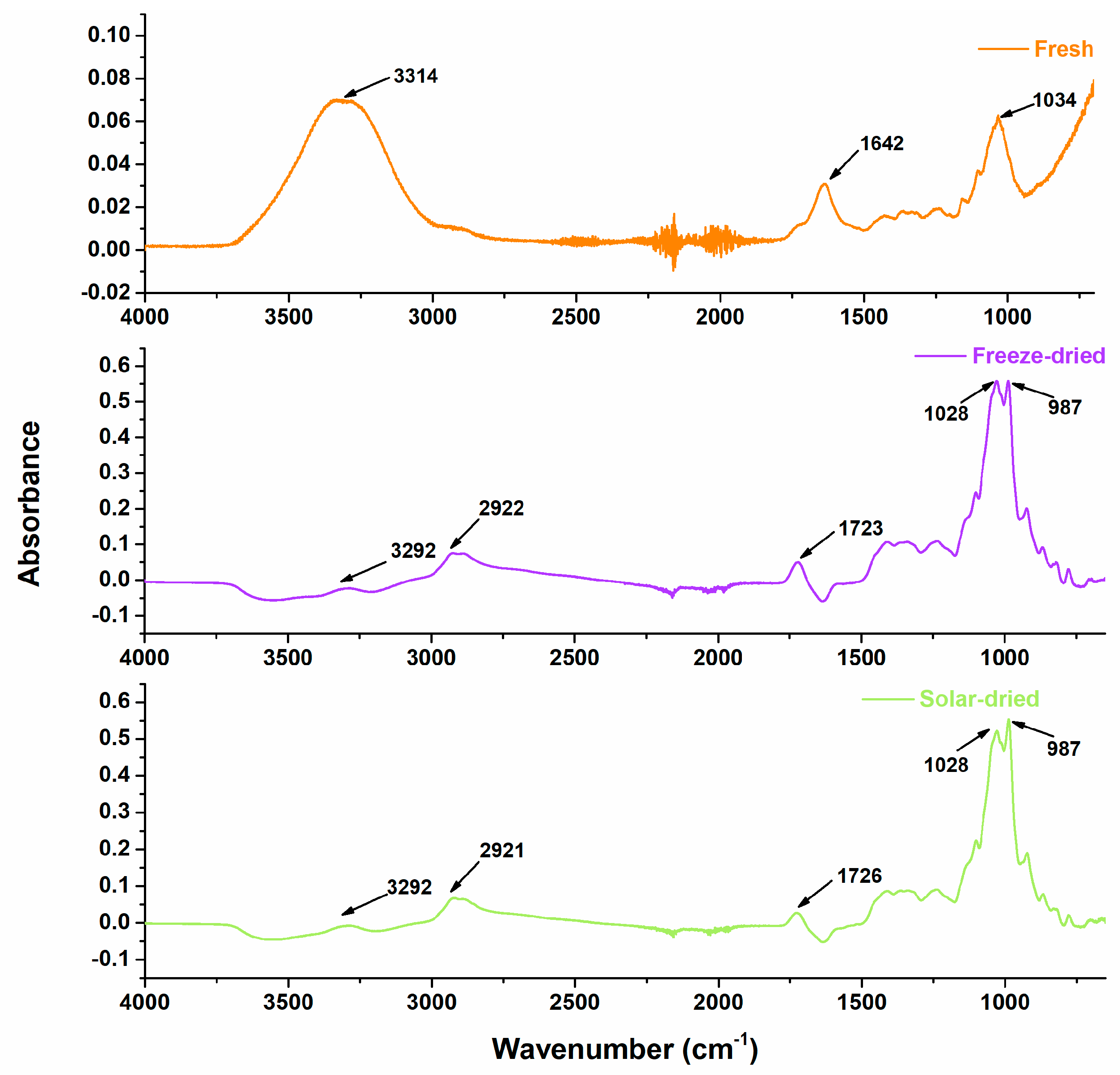
2.6. Fourier Transform Infrared (FTIR) Spectroscopy
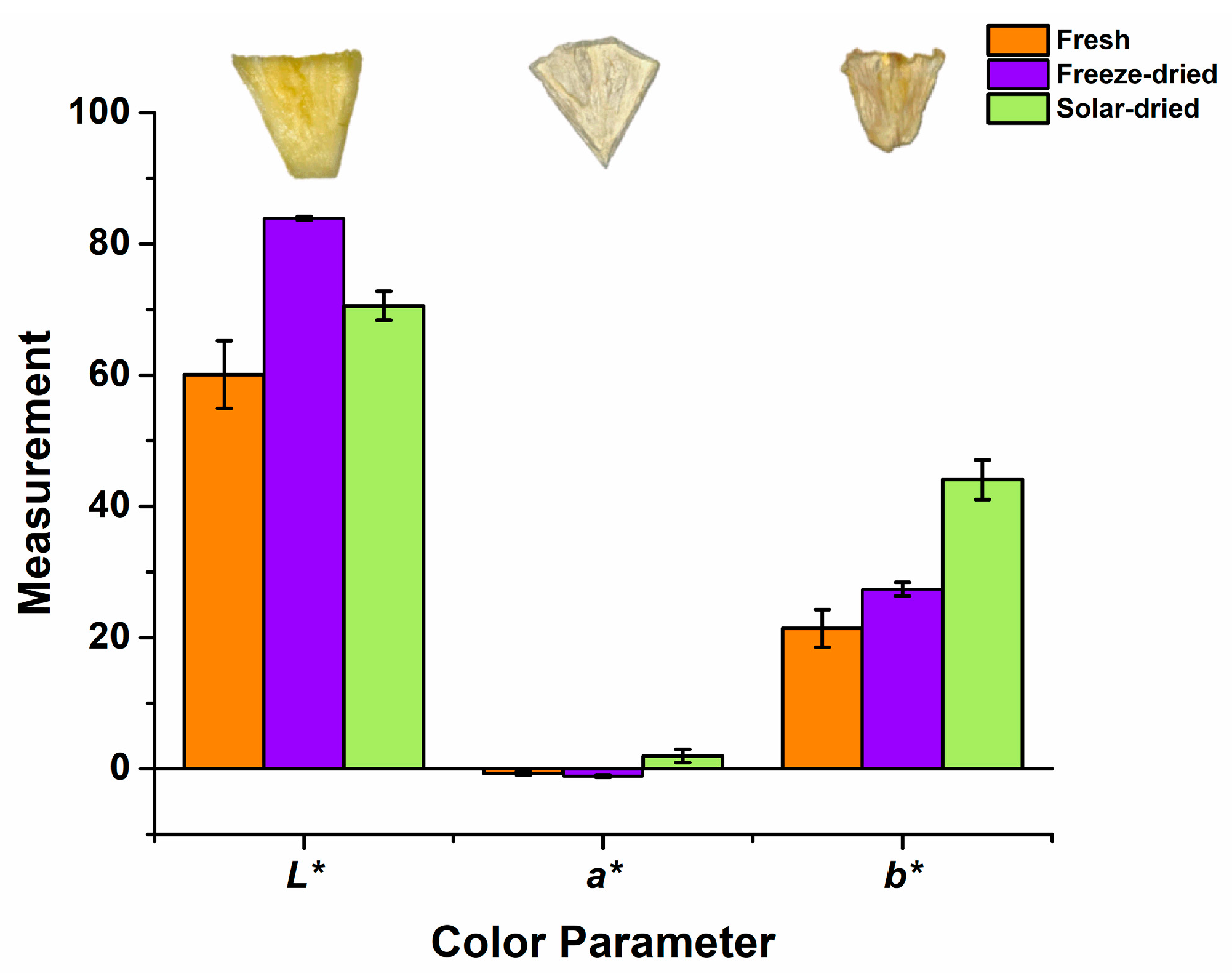
2.7. Color Analysis
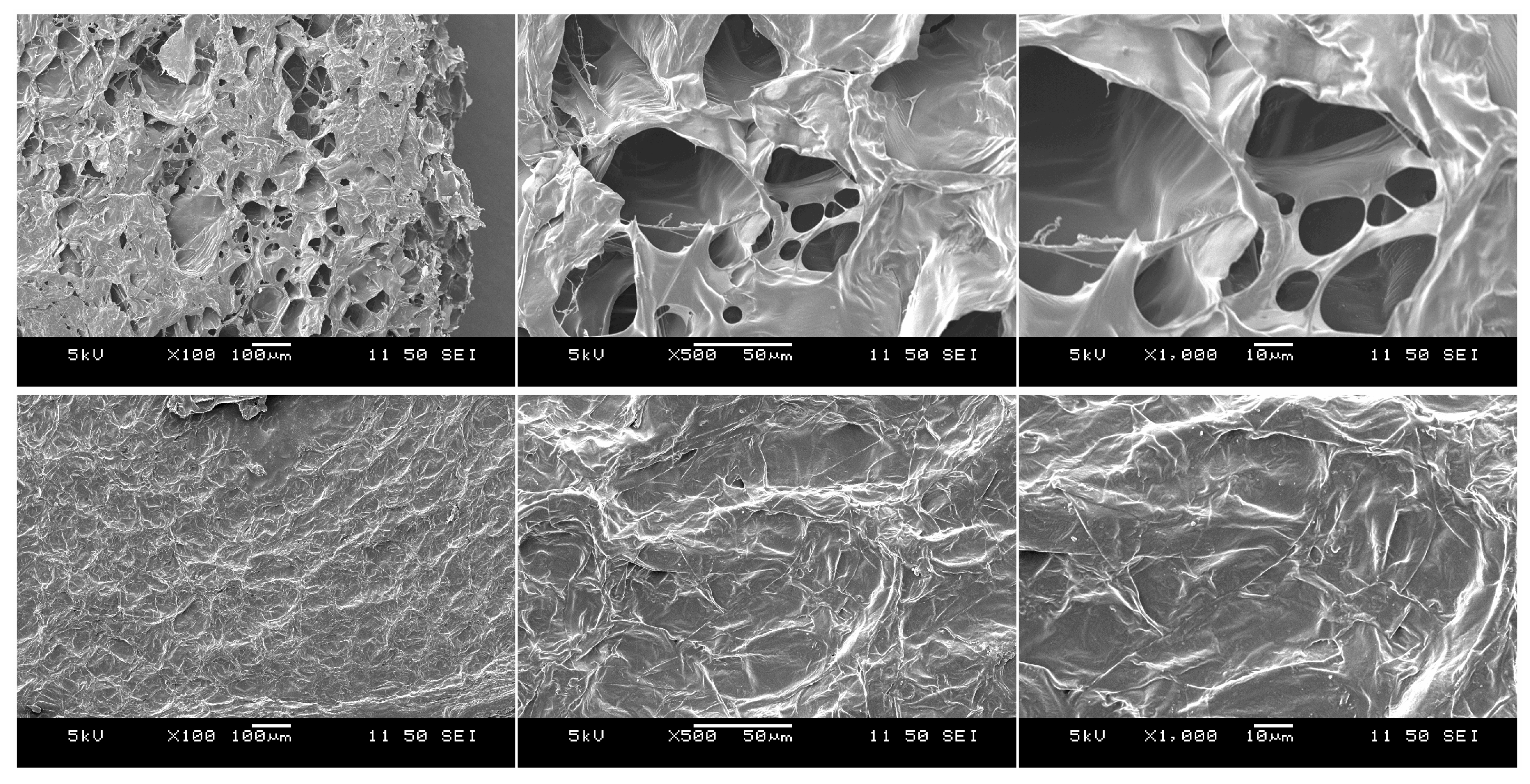
2.8. Microstructure Analysis
2.9. Statistical Analysis
3. Results and Discussion
3.1. Simulation of Sub-Saharan African Climate
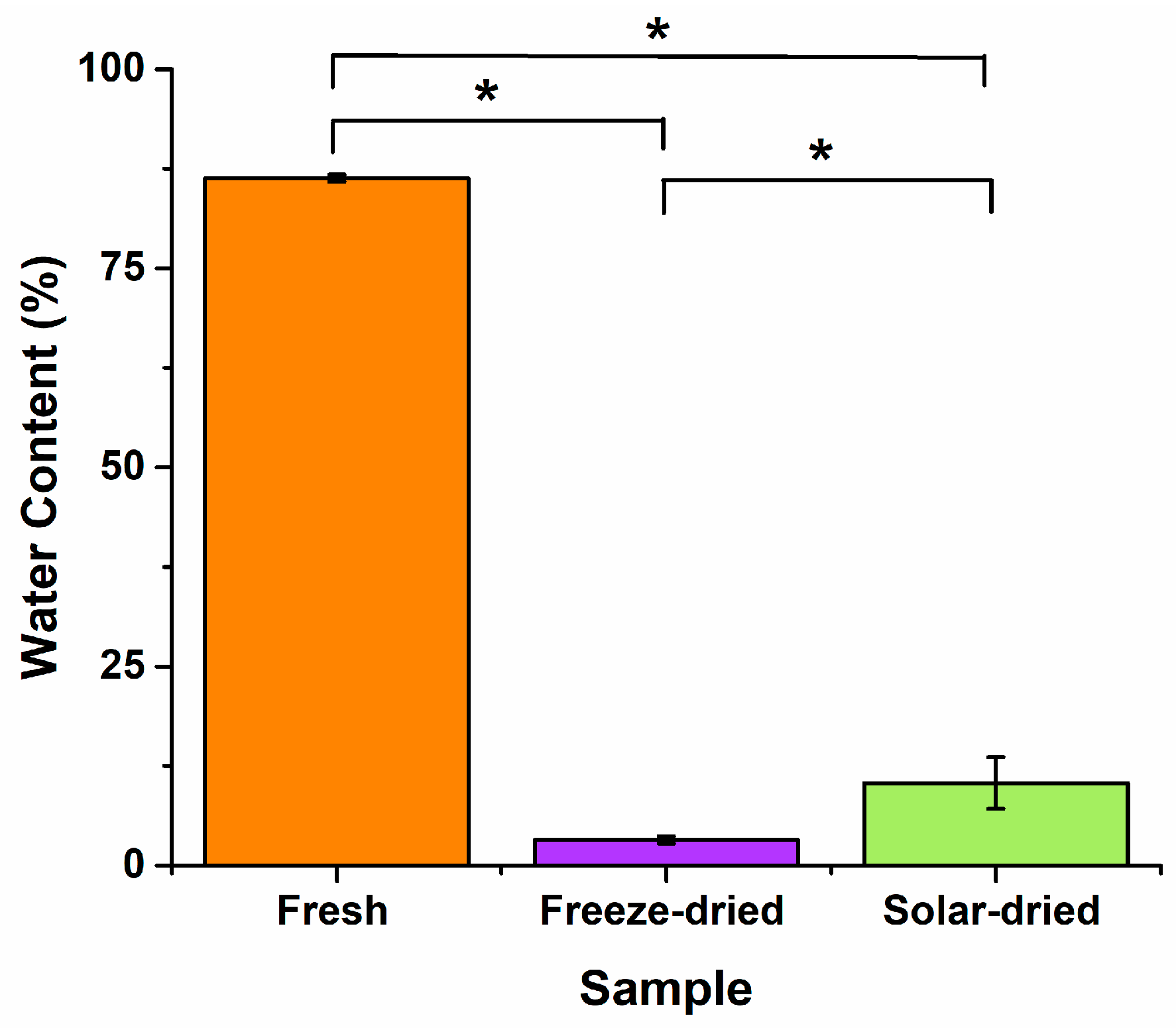
3.2. Water Content Post-Drying
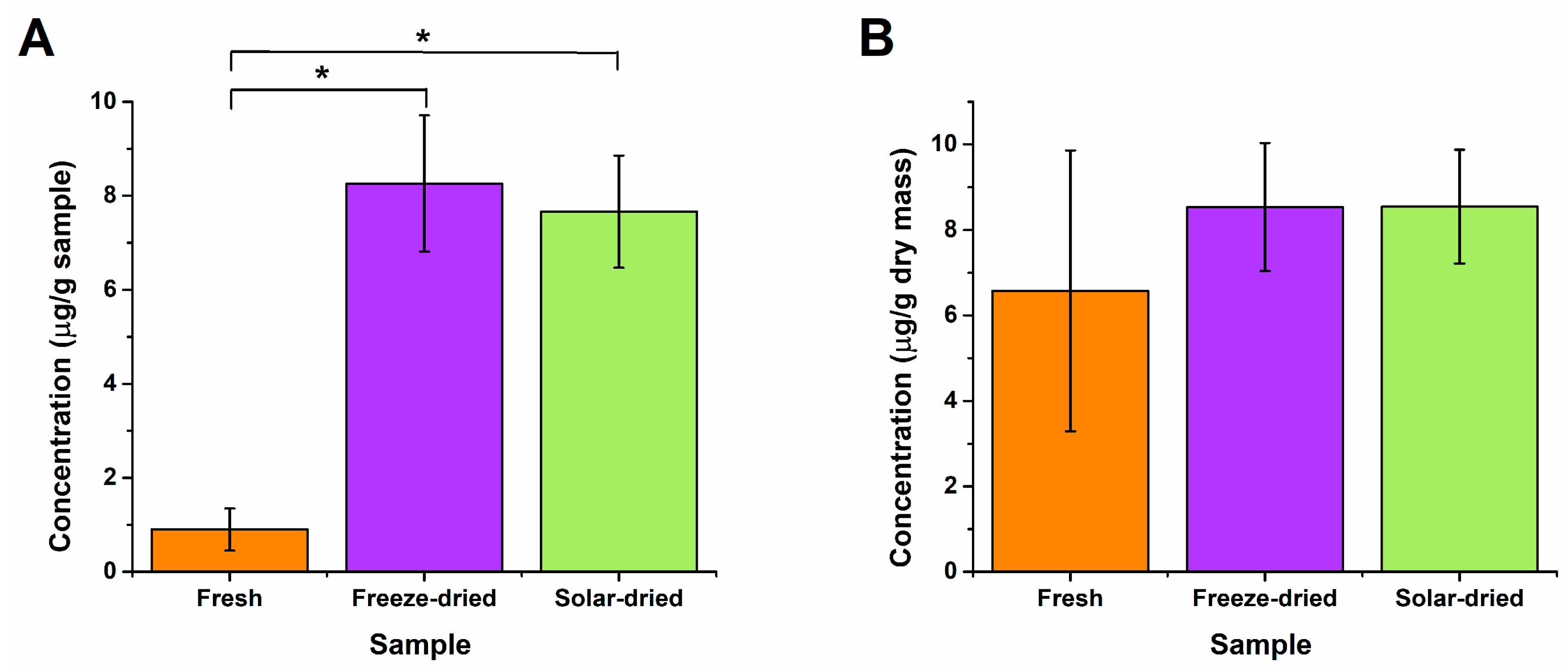
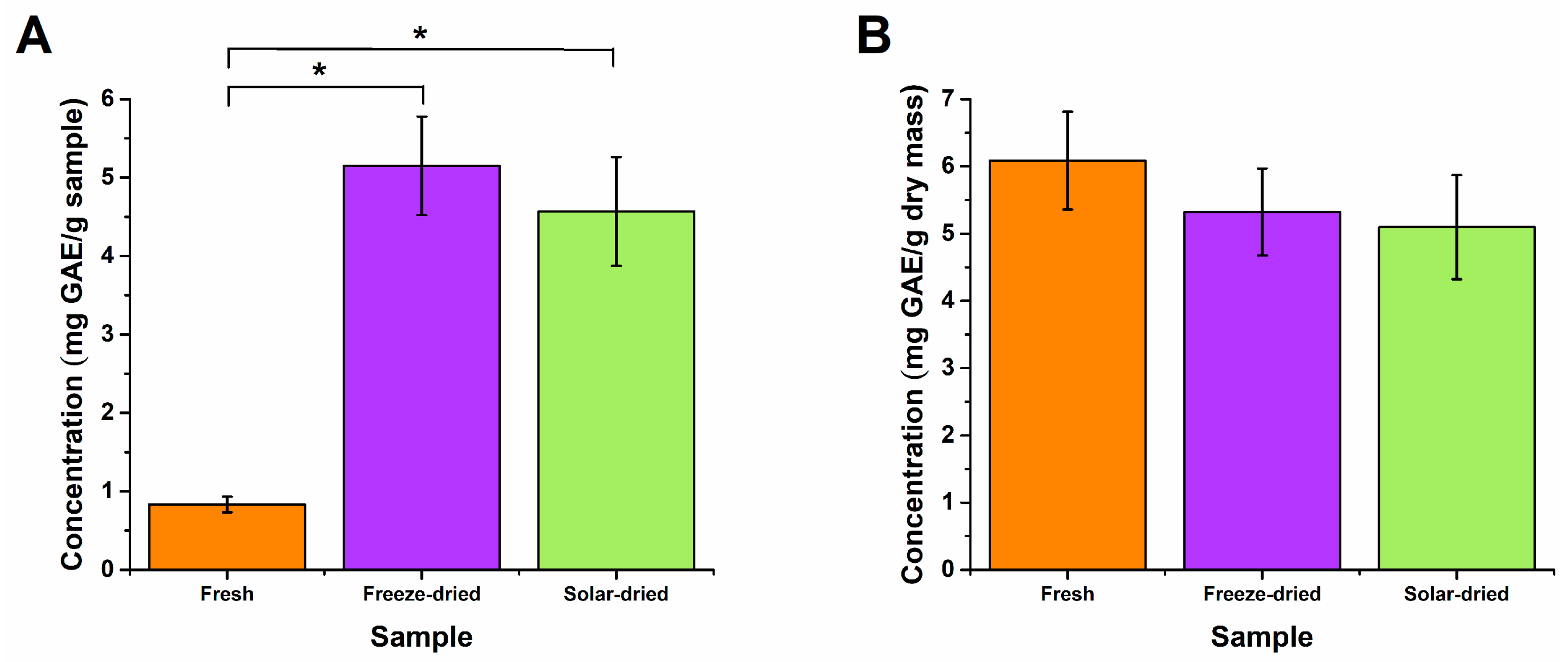
3.3. Post-Drying Nutritional Content
3.4. Post-Drying Microbiological Content
3.5. Post-Drying Color and Microstructure
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations Environment Programme. Why the Global Fight to Tackle Food Waste Has Only Just Begun. 2022. Available online: https://www.unep.org/news-and-stories/story/why-global-fight-tackle-food-waste-has-only-just-begun (accessed on 23 September 2024).
- Zereyesus, Y.A.; Cardell, L. Global food insecurity grows in 2022 amid backdrop of higher prices, Black Sea Conflict. Amber Waves Econ. Food Farming Nat. Resour. Rural. Am. 2022, 2022. Available online: https://www.ers.usda.gov/amber-waves/2022/november/global-food-insecurity-grows-in-2022-amid-backdrop-of-higher-prices-black-sea-conflict/ (accessed on 23 September 2024).
- Feng, S.; Bi, J.; Laaksonen, T.; Laurén, P.; Yi, J. Texture of freeze-dried intact and restructured fruits: Formation mechanisms and control technologies. Trends Food Sci. Technol. 2024, 143, 104267. [Google Scholar] [CrossRef]
- Pondie, T.M.; Engwali, F.D.; Nkoa, B.E.O.; Domguia, E.N. Does energy poverty increases starvation? Evidence from Sub-Saharan Africa. Environ. Sci. Pollut. Res. 2023, 30, 48721–48738. [Google Scholar] [CrossRef] [PubMed]
- Ingham, J.; Kanungo, M.; Beauchamp, B.; Korbut, M.; Swedish, M.; Navin, M.; Zhang, W. Validation of solar dehydrator for food drying applications: A Granny Smith apple study. J. Chem. Eng. Res. Updates 2022, 9, 13–21. [Google Scholar] [CrossRef]
- Han, L.-c.; Jin, T.-x. Effects of combined vacuum and heat pump drying on drying characteristics and physicochemical properties of pineapple. LWT 2024, 192, 115727. [Google Scholar] [CrossRef]
- Chokngamvong, S.; Suvanjumrat, C. Study of drying kinetics and activation energy for drying a pineapple piece in the crossflow dehydrator. Case Stud. Therm. Eng. 2023, 49, 103351. [Google Scholar] [CrossRef]
- Chokngamvong, S.; Suvanjumrat, C. Development of conjugate heat- and moisture-transfer model for pineapple drying using OpenFOAM. Case Stud. Therm. Eng. 2024, 60, 104770. [Google Scholar] [CrossRef]
- Bhatta, S.; Stevanovic Janezic, T.; Ratti, C. Freeze-drying of plant-based foods. Foods 2020, 9, 87. [Google Scholar] [CrossRef]
- Bhuvaneswari, S.; Joshi, M.; D’Souza, A. Quantitative analysis of iron and ascorbic acid contents in locally consumed fruits and vegetables. Int. Res. J. Biol. Sci. 2015, 4, 42–47. [Google Scholar]
- Elgailani, I.E.; Gad-Elkareem, M.; Noh, E.; Adam, O.; Alghamdi, A. Comparison of two methods for the determination of vitamin C (ascorbic acid) in some fruits. Am. J. Chem. 2017, 2, 1–7. [Google Scholar] [CrossRef]
- Hutchinson, N.; Wu, Y.; Wang, Y.; Kanungo, M.; DeBruine, A.; Kroll, E.; Gilmore, D.J.; Eckrose, Z.; Gaston, S.; Matel, P.; et al. Green synthesis of gold nanoparticles using upland cress and their biochemical characterization and assessment. Nanomaterials 2022, 12, 28. [Google Scholar] [CrossRef] [PubMed]
- Adams, P.E. Determining iron content in foods by spectrophotometry. J. Chem. Educ. 1995, 72, 649–651. [Google Scholar] [CrossRef]
- Chai, A.; Schmidt, K.; Brewster, G.; Xiong, L.S.P.; Church, B.; Wahl, T.; Sadabadi, H.; Kumpaty, S.; Zhang, W. Design of pectin-based hydrogel microspheres for targeted pulmonary delivery. Gels 2023, 9, 707. [Google Scholar] [CrossRef] [PubMed]
- Di Egidio, V.; Sinelli, N.; Limbo, S.; Torri, L.; Franzetti, L.; Casiraghi, E. Evaluation of shelf-life of fresh-cut pineapple using FT-NIR and FT-IR spectroscopy. Postharvest Biol. Technol. 2009, 54, 87–92. [Google Scholar] [CrossRef]
- Liu, C.; Lv, M.; Du, H.; Deng, H.; Zhou, L.; Li, P.; Li, X.; Li, B. Effect of preliminary treatment by pulsed electric fields and blanching on the quality of fried sweet potato chips. Foods 2023, 12, 2147. [Google Scholar] [CrossRef]
- Jiang, D.; Li, C.; Lin, Z.; Wu, Y.; Pei, H. Effects of pulsation ratio on center temperature and drying characteristics of pineapple slices with pulsed vacuum drying. Int. J. Agric. Biol. Eng. 2022, 15, 242–253. [Google Scholar] [CrossRef]
- Middleton, J.F.M.; Clarke, J.I.; Gardiner, R.K.A.; Kröner, A.; Mabogunje, A.L.; McMaster, D.N.; Nicol, D.S.H.W.; Smedley, A.; Steel, R.W. Climate of Africa. Encycl. Britanica. 2024. Available online: https://www.britannica.com/place/Africa/Climate (accessed on 23 September 2024).
- Nijhuis, H.H.; Torringa, H.M.; Muresan, S.; Yuksel, D.; Leguijt, C.; Kloek, W. Approaches to improving the quality of dried fruit and vegetables. Trends Food Sci. Technol. 1998, 9, 13–20. [Google Scholar] [CrossRef]
- Witthuhn, R.C.; Engelbrecht, S.; Joubert, E.; Britz, T.J. Microbial content of commercial South African high-moisture dried fruits. J. Appl. Microbiol. 2005, 98, 722–726. [Google Scholar] [CrossRef]
- UNECE Standard DDP-28; Concerning the Marketing and Commercial Quality Control of Dried Pineapple. United Nations Economic Commission for Europe: Geneva, Switzerland, 2014. Available online: https://unece.org/fileadmin/DAM/trade/agr/standard/dry/Standards/DDP28_DriedPineapples_2014_e.pdf (accessed on 23 September 2024).
- United States Department of Agriculture. Pineapple, Raw, All Varieties. 2019. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/169124/nutrients (accessed on 23 September 2024).
- Herbig, A.-L.; Renard, C.M.G.C. Factors that impact the stability of vitamin C at intermediate temperatures in a food matrix. Food Chem. 2017, 220, 444–451. [Google Scholar] [CrossRef]
- Verbeyst, L.; Bogaerts, R.; Van der Plancken, I.; Hendrickx, M.; Van Loey, A. Modelling of vitamin C degradation during thermal and high-pressure treatments of red fruit. Food Bioprocess Technol. 2013, 6, 1015–1023. [Google Scholar] [CrossRef]
- Burdurlu, H.S.; Koca, N.; Karadeniz, F. Degradation of vitamin C in citrus juice concentrates during storage. J. Food Eng. 2006, 74, 211–216. [Google Scholar] [CrossRef]
- Silva-Espinoza, M.A.; Ayed, C.; Foster, T.; Camacho, M.D.M.; Martínez-Navarrete, N. The Impact of Freeze-Drying Conditions on the Physico-Chemical Properties and Bioactive Compounds of a Freeze-Dried Orange Puree. Foods 2019, 9, 32. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, S.; Edna, M.; Siraj, K. The effect of traditional and improved solar drying methods on the sensory quality and nutritional composition of fruits: A case of mangoes and pineapples. Heliyon 2020, 6, e04163. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Batool, A.; Yaqub, S.; Iqbal, A.; Kauser, S.; Arif, M.R.; Ali, S.; Gorsi, F.I.; Nisar, R.; Hussain, A.; et al. Effects of spray drying and ultrasonic assisted extraction on the phytochemicals, antioxidant and antimicrobial activities of strawberry fruit. Food Chem. Adv. 2024, 5, 100755. [Google Scholar] [CrossRef]
- Watrelot, A.; Savits, J.; Moroney, M. Estimating grape maturity by total soluble solids. Iowa State Univ. Ext. Outreach Grape Matur. Ser. 2020. Available online: https://store.extension.iastate.edu/product/16075 (accessed on 23 September 2024).
- Al-Dairi, M.; Pathare, P.B.; Al-Yahyai, R. Chemical and nutritional quality changes of tomato during postharvest transportation and storage. J. Saudi Soc. Agric. Sci. 2021, 20, 401–408. [Google Scholar] [CrossRef]
- Mohd Ali, M.; Hashim, N.; Abd Aziz, S.; Lasekan, O. Shelf life prediction and kinetics of quality changes in pineapple (Ananas comosus) varieties at different storage temperatures. Horticulturae 2022, 8, 992. [Google Scholar] [CrossRef]
- MacKenzie, S.J.; Chandler, C.K.; Hasing, T.; Whitaker, V.M. The role of temperature in the late-season decline in soluble solids content of strawberry fruit in a subtropical production system. HortScience 2011, 46, 1562–1566. [Google Scholar] [CrossRef]
- Asim, M.; Abdan, K.; Jawaid, M.; Nasir, M.; Dashtizadeh, Z.; Ishak, M.R.; Hoque, M.E. A review on pineapple leaves fibre and its composites. Int. J. Polym. Sci. 2015, 2015, 950567. [Google Scholar] [CrossRef]
- Chaves, D.M.; Araújo, J.C.; Gomes, C.V.; Gonçalves, S.P.; Fangueiro, R.; Ferreira, D.P. Extraction, characterization and properties evaluation of pineapple leaf fibers from Azores pineapple. Heliyon 2024, 10, e26698. [Google Scholar] [CrossRef]
- Malakar, S.; Arora, V.K.; Nema, P.K.; Yadav, D.K. Development of infrared-assisted hybrid solar dryer for drying pineapple slices: Investigation of drying characteristics, mass transfer parameters, and quality attributes. Innov. Food Sci. Emerg. Technol. 2023, 88, 103437. [Google Scholar] [CrossRef]
- Alp, D.; Bulantekin, Ö. The microbiological quality of various foods dried by applying different drying methods: A review. Eur. Food Res. Technol. 2021, 247, 1333–1343. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Liao, X.; Wu, H.; Qiu, J.; Wan, R.; Wang, X.; Yi, R.; Xu, Q.; Liu, X. Comparison of different varieties on quality characteristics and microbial activity of fresh-cut pineapple during storage. Foods 2022, 11, 2788. [Google Scholar] [CrossRef] [PubMed]
- Breijyeh, Z.; Jubeh, B.; Karaman, R. Resistance of Gram-negative bacteria to current antibacterial agents and approaches to resolve it. Molecules 2020, 25, 1340. [Google Scholar] [CrossRef]
- Leff, J.W.; Fierer, N. Bacterial communities associated with the surfaces of fresh fruits and vegetables. PLoS ONE 2013, 8, e59310. [Google Scholar] [CrossRef]
- Gomez, S.; Kuruvila, B.; Maneesha, P.K.; Joseph, M. Variation in physico-chemical, organoleptic and microbial qualities of intermediate moisture pineapple (Ananas comosus (L.) Merr.) slices during storage. Food Prod. Process. Nutr. 2022, 4, 5. [Google Scholar] [CrossRef]
- Alegbeleye, O.; Odeyemi, O.A.; Strateva, M.; Stratev, D. Microbial spoilage of vegetables, fruits and cereals. Appl. Food Res. 2022, 2, 100122. [Google Scholar] [CrossRef]
- United Kingdom Health Protection Agency. Guidelines for Assessing the Microbiological Safety of Ready-to-Eat Foods. 2009. Available online: https://assets.publishing.service.gov.uk/media/5a7efde0e5274a2e8ab497a4/Guidelines_for_assessing_the_microbiological_safety_of_ready-to-eat_foods_on_the_market.pdf (accessed on 23 September 2024).
- National Institute for Communicable Diseases. Guidelines for the Microbiological Examination of Ready-to-Eat Foods. 2001. Available online: https://www.nicd.ac.za/wp-content/uploads/2018/05/Guidelines_for_Microbiological_examination_of_ready-to-eat_foods.pdf (accessed on 23 September 2024).
- Bassey, E.J.; Cheng, J.-H.; Sun, D.-W. Enhancing infrared drying of red dragon fruit by novel and innovative thermoultrasound and microwave-mediated freeze-thaw pretreatments. LWT 2024, 202, 116225. [Google Scholar] [CrossRef]
- Savaş, E.; Manav, M. Process optimization and characterization of physicochemical properties of freeze-dried pineapple snacks enriched with propolis. Int. J. Food Prop. 2023, 26, 2693–2715. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuhn, K.; Strnad, C.; Bowman, P.; Young, K.; Kroll, E.; DeBruine, A.; Knudson, I.; Navin, M.; Cheng, Q.; Swedish, M.; et al. Validation of a Passive Solar Drying System Using Pineapple. Foods 2024, 13, 3081. https://doi.org/10.3390/foods13193081
Kuhn K, Strnad C, Bowman P, Young K, Kroll E, DeBruine A, Knudson I, Navin M, Cheng Q, Swedish M, et al. Validation of a Passive Solar Drying System Using Pineapple. Foods. 2024; 13(19):3081. https://doi.org/10.3390/foods13193081
Chicago/Turabian StyleKuhn, Katie, Charles Strnad, Paige Bowman, Keoni Young, Emma Kroll, Anna DeBruine, Ian Knudson, Michael Navin, Qingsu Cheng, Michael Swedish, and et al. 2024. "Validation of a Passive Solar Drying System Using Pineapple" Foods 13, no. 19: 3081. https://doi.org/10.3390/foods13193081






