Food Safety and Nutraceutical Potential of Caramel Colour Class IV Using In Vivo and In Vitro Assays
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. In Vivo Fly Stocks
2.3. In Vitro Cell Culture Conditions
2.4. In Vivo Safety Studies
2.4.1. Toxicity Assays
2.4.2. Genotoxicity Assay
2.5. In Vivo Evaluation of Nutraceutical Potential
2.5.1. Antitoxicity Assay
2.5.2. Antigenotoxicity Assay
2.5.3. Chronic Treatments: Lifespan and Healthspan Assays
2.6. In Vitro Evaluation of Nutraceutical Potential
2.6.1. Cytotoxicity Assay
2.6.2. DNA Fragmentation Status
2.6.3. Clastogenicity: SCGE (Comet Assay)
2.6.4. Methylation Status of HL-60 Cells
3. Results
3.1. In Vivo Assays
3.2. In vitro Assays
4. Discussion
4.1. Food Safety Assays: Toxicity and Genotoxicity
4.2. Nutraceutical Potential Assays
4.3. In vivo Assays
4.4. In vitro Assays
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fairweather, F.A.; Swann, C.A. Food additives and cancer. Proc. Nutr. Soc. 1981, 40, 21–30. [Google Scholar] [CrossRef]
- Trewavas, A.; Stewart, D. Paradoxical effects of chemicals in the diet on health. Curr. Opin. Plant Biol. 2003, 6, 185–190. [Google Scholar] [CrossRef]
- Redmon, T. Assessing the attitudes and bahaviors of pedestrians and drivers in traffic situations. Inst. Transp. Eng. 2003, 73, 26. [Google Scholar]
- Willett, W.C. Diet and health: What should we eat? Science (New York, NY) 1994, 264, 532–537. [Google Scholar] [CrossRef] [PubMed]
- Ishidate, M., Jr.; Sofuni, T.; Yoshikawa, K.; Hayashi, M.; Nohmi, T.; Sawada, M.; Matsuoka, A. Primary mutagenicity screening of food additives currently used in Japan. Food Chem. Toxicol. 1984, 22, 623–636. [Google Scholar] [CrossRef]
- Tsubono, Y.; Ogawa, K.; Watanabe, Y.; Nishino, Y.; Tsuji, I.; Watanabe, T.; Nakatsuka, H.; Takahashi, N.; Kawamura, M.; Hisamichi, S. Food frequency questionnaire as a screening test. Nutr. Cancer 2001, 39, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Licht, B.; Shaw, K.; Smith, C.; Mendoza, M.; Orr, J.; Myers, D. Characterization of caramel colour iv. Food Chem. Toxicol. 1992, 30, 365–373. [Google Scholar] [CrossRef]
- Golon, A.; Kuhnert, N. Unraveling the chemical composition of caramel. J. Agric. Food Chem. 2012, 60, 3266–3274. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Vargas, F.; Paredes-López, O. Chemicals and colorants as nutraceuticals. In Natural Colorants for Food and Nutraceuticals Uses; CRC Press: Boca Raton, FL, USA, 2003; pp. 257–305. [Google Scholar]
- Sengar, G.; Sharma, H.K. Food caramels: A review. J. Food Sci. Technol. 2014, 51, 1686–1696. [Google Scholar] [CrossRef]
- Graf, U.; Wurgler, F.E.; Katz, A.J.; Frei, H.; Juon, H.; Hall, C.B.; Kale, P.G. Somatic mutation and recombination test in drosophila melanogaster. Environ. Mutagen. 1984, 6, 153–188. [Google Scholar] [CrossRef]
- Reiter, L.T.; Potocki, L.; Chien, S.; Gribskov, M.; Bier, E. A systematic analysis of human disease-associated gene sequences in drosophila melanogaster. Genome Res. 2001, 11, 1114–1125. [Google Scholar] [CrossRef]
- Anter, J.; Fernandez-Bedmar, Z.; Villatoro-Pulido, M.; Demyda-Peyras, S.; Moreno-Millan, M.; Alonso-Moraga, A.; Munoz-Serrano, A.; Luque de Castro, M.D. A pilot study on the DNA-protective, cytotoxic, and apoptosis-inducing properties of olive-leaf extracts. Mutat. Res. 2011, 723, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Merinas-Amo, T.; Tasset-Cuevas, I.; Díaz-Carretero, A.M.; Alonso-Moraga, Á.; Calahorro, F. In vivo and in vitro studies of the role of lyophilised blond lager beer and some bioactive components in the modulation of degenerative processes. J. Funct. Foods 2016, 27, 274–294. [Google Scholar] [CrossRef]
- Mateo-Fernández, M.; Merinas-Amo, T.; Moreno-Millán, M.; Alonso-Moraga, Á.; Demyda-Peyrás, S. In vivo and in vitro genotoxic and epigenetic effects of two types of cola beverages and caffeine: A multiassay approach. BioMed Res. Int. 2016, 2016, 7574843. [Google Scholar] [CrossRef]
- Fleming, J.E.; Reveillaud, I.; Niedzwiecki, A. Role of oxidative stress in drosophila aging. Mutat. Res. 1992, 275, 267–279. [Google Scholar] [CrossRef]
- Li, S.; Chen, K.; Li, X.; Zhang, X.; Liu, S.V. A new cultivation system for studying chemical effects on the lifespan of the fruit fly. Exp. Gerontol. 2010, 45, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Beckingham, K.M.; Armstrong, J.D.; Texada, M.J.; Munjaal, R.; Baker, D.A. Drosophila melanogaster-the model organism of choice for the complex biology of multi-cellular organisms. Gravit. Space Res. Bull. 2007, 18, 17–29. [Google Scholar]
- Balls, M. Progressing toward the reduction, refinement and replacement of laboratory animal procedures: Thoughts on some encounters with dr iain purchase. Toxicol. Vitr. 2004, 18, 165–170. [Google Scholar] [CrossRef]
- Leszczyniecka, M.; Roberts, T.; Dent, P.; Grant, S.; Fisher, P.B. Differentiation therapy of human cancer: Basic science and clinical applications. Pharmacol. Ther. 2001, 90, 105–156. [Google Scholar] [CrossRef]
- Fesus, L.; Szondy, Z.; Uray, I. Probing the molecular program of apoptosis by cancer chemopreventive agents. J. Cell. Biochem. Suppl. 1995, 22, 151–161. [Google Scholar] [CrossRef]
- Hong, W.K.; Sporn, M.B. Recent advances in chemoprevention of cancer. Science (New York, NY) 1997, 278, 1073–1077. [Google Scholar] [CrossRef]
- Suzuki, M.M.; Bird, A. DNA methylation landscapes: Provocative insights from epigenomics. Nat. Rev. Genet. 2008, 9, 465. [Google Scholar] [CrossRef]
- Roman-Gomez, J.; Jimenez-Velasco, A.; Agirre, X.; Castillejo, J.A.; Navarro, G.; San Jose-Eneriz, E.; Garate, L.; Cordeu, L.; Cervantes, F.; Prosper, F.; et al. Repetitive DNA hypomethylation in the advanced phase of chronic myeloid leukemia. Leuk. Res. 2008, 32, 487–490. [Google Scholar] [CrossRef]
- Jirtle, R.L.; Skinner, M.K. Environmental epigenomics and disease susceptibility. Nat. Rev. Genet. 2007, 8, 253–262. [Google Scholar] [CrossRef]
- Yan, J.; Huen, D.; Morely, T.; Johnson, G.; Gubb, D.; Roote, J.; Adler, P.N. The multiple-wing-hairs gene encodes a novel gbd-fh3 domain-containing protein that functions both prior to and after wing hair initiation. Genetics 2008, 180, 219–228. [Google Scholar] [CrossRef]
- Ren, N.; Charlton, J.; Adler, P.N. The flare gene, which encodes the aip1 protein of drosophila, functions to regulate f-actin disassembly in pupal epidermal cells. Genetics 2007, 176, 2223–2234. [Google Scholar] [CrossRef]
- Maes, P.; Monakhova, Y.B.; Kuballa, T.; Reusch, H.; Lachenmeier, D.W. Qualitative and quantitative control of carbonated cola beverages using 1h nmr spectroscopy. J. Agric. Food Chem. 2012, 60, 2778–2784. [Google Scholar] [CrossRef]
- Frei, H.; Wurgler, F.E. Optimal experimental design and sample size for the statistical evaluation of data from somatic mutation and recombination tests (smart) in drosophila. Mutat. Res. 1995, 334, 247–258. [Google Scholar] [CrossRef]
- Fernández-Bedmar, Z.; Arenas-Chaparro, R.; Merinas-Amo, T.; Mateo-Fernández, M.; Tasset-Cuevas, I.; Lozano-Baena, M.; de Haro-Bailón, A.; Campos-Sánchez, J. Modulator role of trilinolein/triolein and resveratrol on the health promoting effects of processed foods: Edible oils and red wine. Toxicol. Lett. 2016, 258, S159. [Google Scholar] [CrossRef]
- Tasset-Cuevas, I.; Fernandez-Bedmar, Z.; Dolores Lozano-Baena, M.; Campos-Sanchez, J.; de Haro-Bailon, A.; Munoz-Serrano, A.; Alonso-Moraga, A. Protective effect of borage seed oil and gamma linolenic acid on DNA: In vivo and in vitro studies. PLoS ONE 2013, 8, e56986. [Google Scholar] [CrossRef]
- Abraham, S.K.; Singh, S.P. Anti-genotoxicity and glutathione s-transferase activity in mice pretreated with caffeinated and decaffeinated coffee. Food Chem. Toxicol. 1999, 37, 733–739. [Google Scholar] [CrossRef]
- Fernandez-Bedmar, Z.; Anter, J.; de La Cruz-Ares, S.; Munoz-Serrano, A.; Alonso-Moraga, A.; Perez-Guisado, J. Role of citrus juices and distinctive components in the modulation of degenerative processes: Genotoxicity, antigenotoxicity, cytotoxicity, and longevity in drosophila. J. Toxicol. Environ. Health A 2011, 74, 1052–1066. [Google Scholar] [CrossRef]
- Anter, J.; Tasset, I.; Demyda-Peyrás, S.; Ranchal, I.; Moreno-Millán, M.; Romero-Jimenez, M.; Muntané, J.; Luque de Castro, M.D.; Muñoz-Serrano, A.; Alonso-Moraga, Á. Evaluation of potential antigenotoxic, cytotoxic and proapoptotic effects of the olive oil by-product “alperujo”, hydroxytyrosol, tyrosol and verbascoside. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2014, 772, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Gyori, B.M.; Venkatachalam, G.; Thiagarajan, P.S.; Hsu, D.; Clement, M.V. Opencomet: An automated tool for comet assay image analysis. Redox Biol. 2014, 2, 457–465. [Google Scholar] [CrossRef]
- Nikolaidis, G.; Raji, O.Y.; Markopoulou, S.; Gosney, J.R.; Bryan, J.; Warburton, C.; Walshaw, M.; Sheard, J.; Field, J.K.; Liloglou, T. DNA methylation biomarkers offer improved diagnostic efficiency in lung cancer. Cancer Res. 2012, 72, 5692–5701. [Google Scholar] [CrossRef]
- Liloglou, T.; Bediaga, N.G.; Brown, B.R.; Field, J.K.; Davies, M.P. Epigenetic biomarkers in lung cancer. Cancer Lett. 2014, 342, 200–212. [Google Scholar] [CrossRef]
- Weisenberger, D.J.; Campan, M.; Long, T.I.; Kim, M.; Woods, C.; Fiala, E.; Ehrlich, M.; Laird, P.W. Analysis of repetitive element DNA methylation by methylight. Nucleic Acids Res. 2005, 33, 6823–6836. [Google Scholar] [CrossRef] [PubMed]
- Frei, H.; Wurgler, F.E. Statistical methods to decide whether mutagenicity test data from drosophila assays indicate a positive, negative, or inconclusive result. Mutat. Res. 1988, 203, 297–308. [Google Scholar] [CrossRef]
- Vartanian, L.R.; Schwartz, M.B.; Brownell, K.D. Effects of soft drink consumption on nutrition and health: A systematic review and meta-analysis. Am. J. Public Health 2007, 97, 667–675. [Google Scholar] [CrossRef]
- MacKenzie, K.; Boysen, B.; Field, W.; Petsel, S.; Chappel, C.; Emerson, J.; Stanley, J. Toxicity studies of caramel colour iii and 2-acetyl-4 (5)-tetrahydroxybutylimidazole in f344 rats. Food Chem. Toxicol. 1992, 30, 417–425. [Google Scholar] [CrossRef]
- Hargreaves, M.B.; Jones, B.C.; Smith, D.A.; Gescher, A. Inhibition of p-nitrophenol hydroxylase in rat liver microsomes by small aromatic and heterocyclic molecules. Drug Metab. Dispos. 1994, 22, 806–810. [Google Scholar]
- Moretton, C.; Crétier, G.; Nigay, H.; Rocca, J.L. Quantification of 4-methylimidazole in class iii and iv caramel colors: Validation of a new method based on heart-cutting two-dimensional liquid chromatography (lc-lc). J. Agric. Food Chem. 2011, 59, 3544–3550. [Google Scholar] [CrossRef]
- Cunha, S.; Barrado, A.; Faria, M.; Fernandes, J. Assessment of 4-(5-) methylimidazole in soft drinks and dark beer. J. Food Compos. Anal. 2011, 24, 609–614. [Google Scholar] [CrossRef]
- Brusick, D.; Jagannath, D.; Galloway, S.; Nestmann, E. Genotoxicity hazard assessment of caramel colours iii and iv. Food Chem. Toxicol. 1992, 30, 403–410. [Google Scholar] [CrossRef]
- Norizadeh Tazehkand, M.; Topaktas, M.; Yilmaz, M.B. Assessment of chromosomal aberration in the bone marrow cells of swiss albino mice treated by 4-methylimidazole. Drug Chem. Toxicol. 2016, 39, 307–311. [Google Scholar] [CrossRef]
- Vollmuth, T.A. Caramel color safety–an update. Food Chem. Toxicol. 2018, 111, 578–596. [Google Scholar] [CrossRef]
- Batista, C.; Barros, L.; Carvalho, A.M.; Ferreira, I.C. Nutritional and nutraceutical potential of rape (brassica napus l. Var. Napus) and “tronchuda” cabbage (brassica oleraceae l. Var. Costata) inflorescences. Food Chem. Toxicol. 2011, 49, 1208–1214. [Google Scholar] [CrossRef]
- Bell, R.; Hubbard, A.; Chettier, R.; Chen, D.; Miller, J.P.; Kapahi, P.; Tarnopolsky, M.; Sahasrabuhde, S.; Melov, S.; Hughes, R.E. A human protein interaction network shows conservation of aging processes between human and invertebrate species. PLoS Genet. 2009, 5, e1000414. [Google Scholar] [CrossRef]
- Tsai, P.J.; Yu, T.Y.; Chen, S.H.; Liu, C.C.; Sun, Y.F. Interactive role of color and antioxidant capacity in caramels. Food Res. Int. 2009, 42, 380–386. [Google Scholar] [CrossRef]
- Suocheng, W.; Huining, L.; Haoqin, L.; Luju, L.; Zhu, G. Coca-cola and pepsi-cola affect ovaries and follicles development. Biomed. Res. 2016, 27, 710–717. [Google Scholar]
- Forchhammer, L.; Ersson, C.; Loft, S.; Möller, L.; Godschalk, R.W.; van Schooten, F.J.; Jones, G.D.; Higgins, J.A.; Cooke, M.; Mistry, V. Inter-laboratory variation in DNA damage using a standard comet assay protocol. Mutagenesis 2012, 27, 665–672. [Google Scholar] [CrossRef] [Green Version]
- Olive, P.L.; Banáth, J.P. The comet assay: A method to measure DNA damage in individual cells. Nat. Protoc. 2006, 1, 23–29. [Google Scholar] [CrossRef]
- Fairbairn, D.W.; O’Neill, K.L. Necrotic DNA degradation mimics apoptotic nucleosomal fragmentation comet tail length. Vitr. Cell. Dev. Biol. Anim. 1995, 31, 171–173. [Google Scholar] [CrossRef] [PubMed]
- Fabiani, R.; Rosignoli, P.; De Bartolomeo, A.; Fuccelli, R.; Morozzi, G. Genotoxicity of alkene epoxides in human peripheral blood mononuclear cells and hl60 leukaemia cells evaluated with the comet assay. Mutat. Res. 2012, 747, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kitts, D.D.; Wu, C.; Kopec, A.; Nagasawa, T. Chemistry and genotoxicity of caramelized sucrose. Mol. Nutr. Food Res. 2006, 50, 1180–1190. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Serra, L.; Esteller, M. Proteins that bind methylated DNA and human cancer: Reading the wrong words. Br. J. Cancer 2008, 98, 1881–1885. [Google Scholar] [CrossRef] [PubMed]
- Qin, T.; Jelinek, J.; Si, J.; Shu, J.; Issa, J.P.J. Mechanisms of resistance to 5-aza-2′-deoxycytidine in human cancer cell lines. Blood 2009, 113, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Lander, E.S.; Linton, L.M.; Birren, B.; Nusbaum, C.; Zody, M.C.; Baldwin, J.; Devon, K.; Dewar, K.; Doyle, M.; FitzHugh, W. Initial sequencing and analysis of the human genome. Nature 2001, 409, 860–921. [Google Scholar] [Green Version]
- Boissinot, S.; Entezam, A.; Furano, A.V. Selection against deleterious line-1-containing loci in the human lineage. Mol. Biol. Evol. 2001, 18, 926–935. [Google Scholar] [CrossRef]
- Grover, D.; Majumder, P.P.; Rao, C.B.; Brahmachari, S.K.; Mukerji, M. Nonrandom distribution of alu elements in genes of various functional categories: Insight from analysis of human chromosomes 21 and 22. Mol. Biol. Evol. 2003, 20, 1420–1424. [Google Scholar] [CrossRef]
- Waye, J.; Willard, H. Structure, organization, and sequence of alpha satellite DNA from human chromosome 17: Evidence for evolution by unequal crossing-over and an ancestral pentamer repeat shared with the human x chromosome. Mol. Cell. Biol. 1986, 6, 3156–3165. [Google Scholar] [CrossRef] [PubMed]
- Martínez, J.G.; Pérez-Escuredo, J.; Castro-Santos, P.; Marcos, C.Á.; Pendás, J.L.L.; Fraga, M.F.; Hermsen, M.A. Hypomethylation of line-1, and not centromeric sat-α, is associated with centromeric instability in head and neck squamous cell carcinoma. Cell. Oncol. 2012, 35, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Ting, D.T.; Lipson, D.; Paul, S.; Brannigan, B.W.; Akhavanfard, S.; Coffman, E.J.; Contino, G.; Deshpande, V.; Iafrate, A.J.; Letovsky, S. Aberrant overexpression of satellite repeats in pancreatic and other epithelial cancers. Science (New York, NY) 2011, 331, 593–596. [Google Scholar] [CrossRef] [PubMed]
- Wild, L.; Flanagan, J.M. Genome-wide hypomethylation in cancer may be a passive consequence of transformation. BBA Rev. Cancer 2010, 1806, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Lima, P.M.; Orsoli, P.C.; Araújo, T.G.; Brandao, D. Effects of a carbonated soft drink on epithelial tumor incidence in Drosophila melanogaster. J. Pharm. Pharmacol. 2018, 6, 240–2047. [Google Scholar]




| Primer | Forward Primer Sequence 5′ to 3′ (N) | Reverse Primer Sequence 5′ to 3′ (N) |
|---|---|---|
| ALU-C4 | GGTTAGGTATAGTGGTTTATATTTGTAATTTTAGTA (-36) | ATTAACTAAACTAATCTTAAACTCCTAACCTCA (-33) |
| ALU-M1 | ATTATGTTAGTTAGGATGGTTTCGATTTT (-29) | CAATCGACCGAACGCGA (-17) |
| LINE-1-M1 | GGACGTATTTGGAAAATCGGG (-21) | AATCTCGCGATACGCCGTT (-19) |
| SAT-α-M1 | TGATGGAGTATTTTTAAAATATACGTTTTGTAGT (-34) | AATTCTAAAAATATTCCTCTTCAATTACGTAAA (-33) |
| CAR (mg/mL) | Survival (%) | |
|---|---|---|
| Simple Treatment 1 | Combined Treatment 2 | |
| 0 | 100 | 100 |
| H2O2 | - | 62.64 |
| 0.03 | 61 *,3 | 62 |
| 0.125 | 65.7 * | 54.02 |
| 0.25 | 64.3 * | 53 |
| 1 | 61.32 * | 54.02 |
| 4 | 63 * | 23 *,4 |
| Clones per Wings (Number of Spots) | |||||||
|---|---|---|---|---|---|---|---|
| Compound | Wings Number | Small Single Spots (1–2 Cells) m = 2 | Large Simple Spots (>2 Cells) m = 5 | Twin Spots m = 5 | Total Spots m = 2 | Mann–Whitney Test | IP (%) |
| H2O | 41 | 0.147 (6) | 0.048 (2) | 0 | 0.195 (8) | ||
| H2O2 (0.15 M) | 40 | 0.375 (15) | 0.05 (2) | 0 | 0.425 (17) + | ||
| SIMPLE TREATMENT | |||||||
| CAR (mg/mL) [0.125] [4] | 40 | 0.25 (10) | 0.125 (5) | 0 | 0.375 (15) i | Λ | |
| 42 | 0.166 (7) | 0.095 (4) | 0.024 (1) | 0.286 (12) i | Λ | ||
| COMBINED TREATMENT | |||||||
| CAR (mg/mL) [0.125] [4] | 42 | 0.166 (7) | 0 | 0 | 0.166 (7) * | 61 | |
| 46 | 0.065 (3) | 0.02 (1) | 0 | 0.087 (4) * | 79.5 | ||
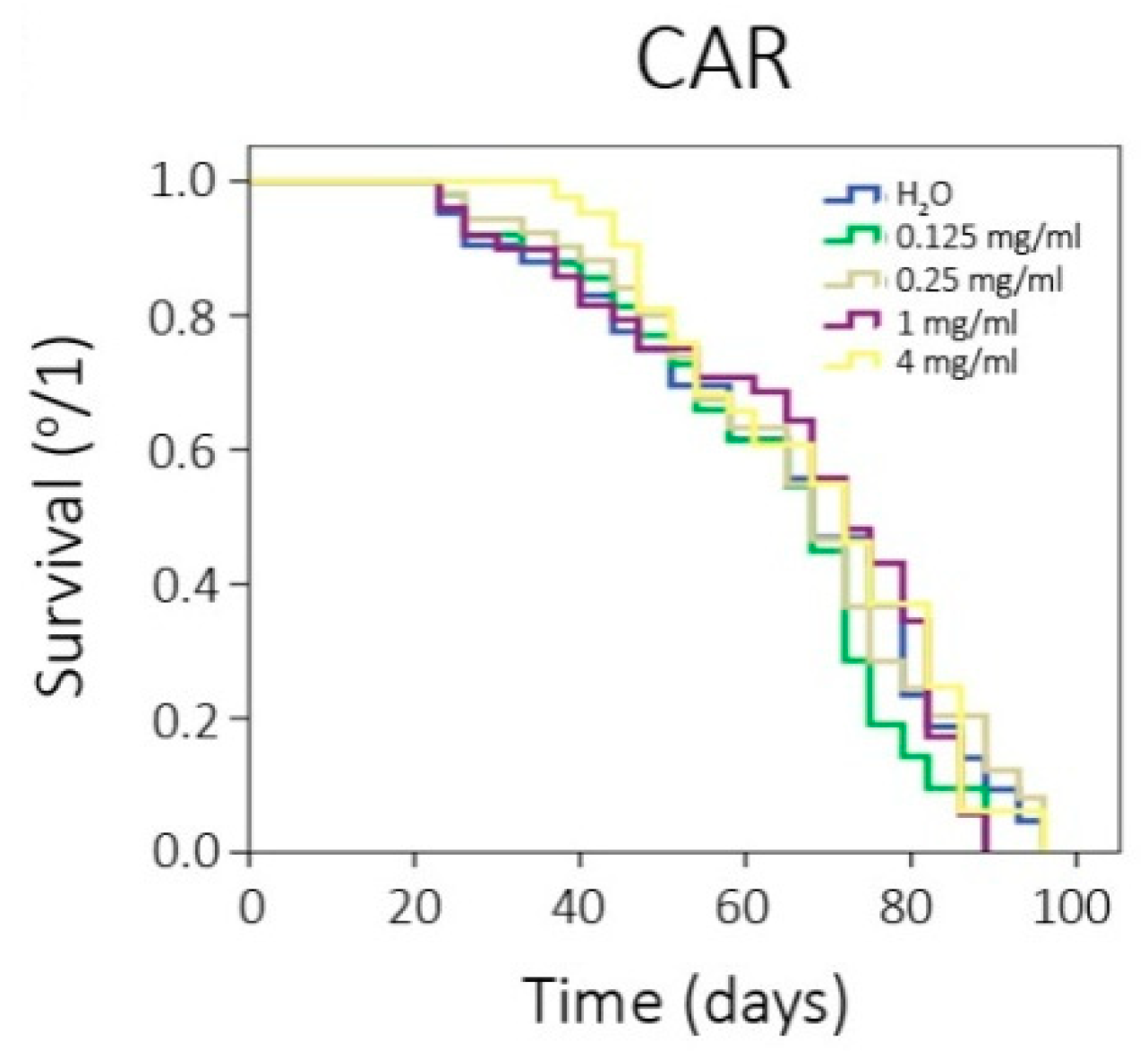
| CAR (mg/mL) | Mean Lifespan (Days) | Mean Lifespan Difference (%) a | Healthspan (80th Percentile) (Days) | Healthspan Difference (%) a |
|---|---|---|---|---|
| Control | 64 ± 3.16 | 0 | 31.21 ± 2.37 | 0 |
| 0.125 | 59.65 ± 2.4 | −6.8 | 31.03 ± 2.12 | −0.5 |
| 0.25 | 60.83 ± 2.73 | −4.9 | 33.68 ± 2.44 | 7.6 |
| 1 | 62.8 ± 2.78 | −1.9 | 30.88 ± 2.1 | −1.1 |
| 4 | 59 ± 3.35 | −7.9 | 37.54 ± 4 | 20.28 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mateo-Fernández, M.; Alves-Martínez, P.; Del Río-Celestino, M.; Font, R.; Merinas-Amo, T.; Alonso-Moraga, Á. Food Safety and Nutraceutical Potential of Caramel Colour Class IV Using In Vivo and In Vitro Assays. Foods 2019, 8, 392. https://doi.org/10.3390/foods8090392
Mateo-Fernández M, Alves-Martínez P, Del Río-Celestino M, Font R, Merinas-Amo T, Alonso-Moraga Á. Food Safety and Nutraceutical Potential of Caramel Colour Class IV Using In Vivo and In Vitro Assays. Foods. 2019; 8(9):392. https://doi.org/10.3390/foods8090392
Chicago/Turabian StyleMateo-Fernández, Marcos, Pilar Alves-Martínez, Mercedes Del Río-Celestino, Rafael Font, Tania Merinas-Amo, and Ángeles Alonso-Moraga. 2019. "Food Safety and Nutraceutical Potential of Caramel Colour Class IV Using In Vivo and In Vitro Assays" Foods 8, no. 9: 392. https://doi.org/10.3390/foods8090392
APA StyleMateo-Fernández, M., Alves-Martínez, P., Del Río-Celestino, M., Font, R., Merinas-Amo, T., & Alonso-Moraga, Á. (2019). Food Safety and Nutraceutical Potential of Caramel Colour Class IV Using In Vivo and In Vitro Assays. Foods, 8(9), 392. https://doi.org/10.3390/foods8090392







