Determination of Bisphenol Compounds and the Bioaccumulation after Co-Exposure with Polyethylene Microplastics in Zebrafish
Abstract
:1. Introduction
2. Materials and Method
2.1. Chemicals
2.2. Exposure Experiment and Sample Collection
2.3. Sample Preparation
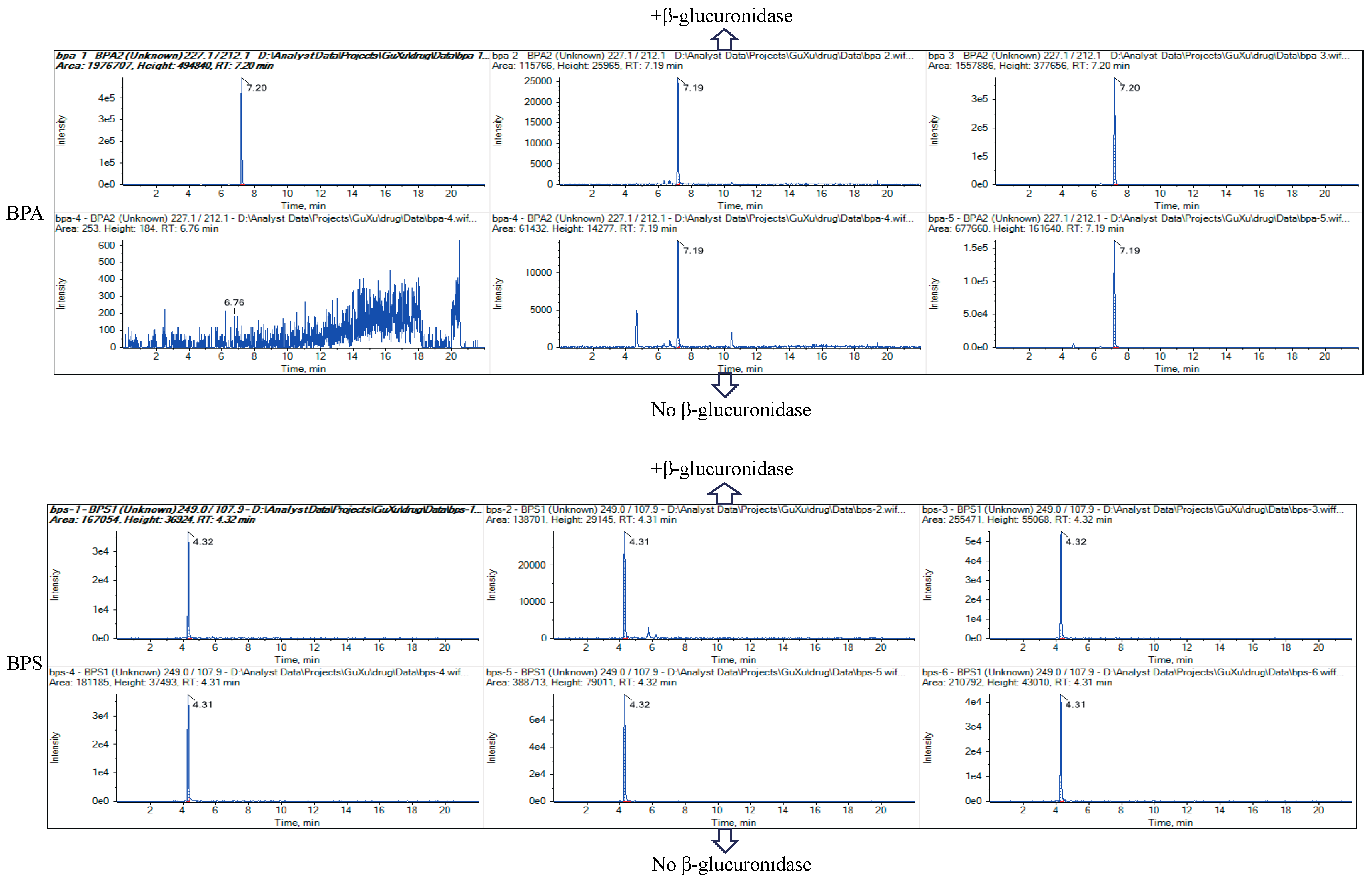
2.4. Analytical Conditions
2.5. Data Analysis
3. Results
3.1. UPLC-MS/MS Conditions
3.2. Sample Preparation
3.3. Method Validation
3.4. Tissue Accumulation of Bisphenol Compounds in Zebrafish
4. Discussion
4.1. Optimization of Sample Pretreatment
4.2. Tissue Accumulation of Bisphenol Compounds in Zebrafish
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, N.C.; Seebacher, F. Effect of the plastic pollutant bisphenol A on the biology of aquatic organisms: A meta-analysis. Glob. Chang. Biol. 2020, 26, 3821–3833. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Villanueva, E.; Jaumot, J.; Martinez, R.; Navarro-Martin, L.; Pina, B.; Taulet, R. Assessment of endocrine disruptors effects on zebrafish (Danio rerio) embryos by untargeted LC-HRMS metabolomic analysis. Sci. Total Environ. 2018, 635, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Pan, S.; Cheng, H.; Sweetman, A.J.; Zhang, H.; Jones, K.C. Diffusive gradients in thin-films (DGT) for in situ sampling of selected endocrine disrupting chemicals (EDCs) in waters. Water Res. 2018, 137, 211–219. [Google Scholar] [CrossRef]
- MacKay, H.; Abizaid, A. A plurality of molecular targets: The receptor ecosystem for bisphenol-A (BPA). Horm. Behav. 2018, 101, 59–67. [Google Scholar] [CrossRef]
- Miglioli, A.; Balbi, T.; Besnardeau, L.; Rumollard, R.; Canesi, L. Bisphenol A interferes with first shell formation and development of the serotoninergic system in early larval stages of Mytilus galloprovincialis. Sci. Total Environ. 2021, 758, 144003. [Google Scholar] [CrossRef]
- Qiu, W.; Chen, J.; Li, Y.; Chen, Z.; Jiang, L.; Yang, M.; Wu, M. Oxidative stress and immune disturbance after long-term exposure to bisphenol A in juvenile common carp (Cyprinus carpio). Ecotoxicol. Environ. Saf. 2016, 130, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Rochester, J.R.; Bolden, A.L. Bisphenol S and F: A systematic review and comparison of the hormonal activity of bisphenol A substitutes. Environ. Health Perspect. 2015, 123, 643–650. [Google Scholar] [CrossRef]
- Almeida, S.; Raposo, A.; Almeida-González, M.; Carrascosa, C. Bisphenol A: Food exposure and impact on human health. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1503–1517. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.H.; Zhou, E.X.; Yang, Z.L. Waterborne exposure to BPS causes thyroid endocrine disruption in zebrafish larvae. PLoS ONE 2017, 12, e0176927. [Google Scholar] [CrossRef]
- Tang, Y.; Han, Y.; Zhang, W.; Yu, Y.; Huang, L.; Zhou, W.; Shi, W.; Tian, D.; Liu, G. Bisphenol A and microplastics weaken the antimicrobial ability of blood clams by disrupting humoral immune responses and suppressing hemocyte chemotactic activity. Environ. Pollut. 2022, 307, 119497. [Google Scholar] [CrossRef]
- Galloway, T.S.; Lewis, C.N. Marine microplastics spell big problems for future generations. Proc. Natl. Acad. Sci. USA 2016, 113, 2331–2333. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zhou, W.; Sun, S.; Du, X.; Han, Y.; Shi, W.; Liu, G. Immunotoxicity and neurotoxicity of bisphenol A and microplastics alone or in combination to a bivalve species, Tegillarca granosa. Environ. Pollut. 2020, 265, 115115. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Han, Y.; Tang, Y.; Shi, W.; Du, X.; Sun, S.; Liu, G. Microplastics aggravate the bioaccumulation of two waterborne veterinary antibiotics in an edible bivalve species: Potential mechanisms and implications for human health. Environ. Sci. Technol. 2020, 54, 8115–8122. [Google Scholar] [CrossRef]
- Lionetto, F.; Esposito Corcione, C. An overview of the sorption studies of contaminants on poly (Ethylene Terephthalate) microplastics in the marine environment. J. Mar. Sci. Eng. 2021, 9, 445. [Google Scholar] [CrossRef]
- Wu, P.; Cai, Z.; Jin, H.; Tang, Y. Adsorption mechanisms of five bisphenol analogues on PVC microplastics. Sci. Total Environ. 2019, 650, 671–678. [Google Scholar] [CrossRef]
- Han, Y.; Shi, W.; Tang, Y.; Zhou, W.; Sun, H.; Zhang, J.; Yan, M.; Hu, L.; Liu, G. Microplastics and bisphenol A hamper gonadal development of whiteleg shrimp (Litopenaeus vannamei) by interfering with metabolism and disrupting hormone regulation. Sci. Total Environ. 2022, 810, 152354. [Google Scholar] [CrossRef]
- Wang, T.; Hu, M.; Xu, G.; Shi, H.; Leung, Y.S.; Wang, Y. Microplastic accumulation via trophic transfer: Can a predatory crab counter the adverse effects of microplastics by body defence? Sci. Total Environ. 2021, 754, 142099. [Google Scholar] [CrossRef]
- Azzouz, A.; Rascón, A.J.; Ballesteros, E. Simultaneous determination of parabens, alkylphenols, phenylphenols, bisphenol A and triclosan in human urine, blood and breast milk by continuous solid-phase extraction and gas chromatography–mass spectrometry. J. Pharm. Biomed. Anal. 2016, 119, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Liu, F.; Alomirah, H.; Loi, V.; Mohd, M.; Moon, H.; Nakata, H.; Kannan, K. Bisphenol S in urine from the United States and seven Asian countries: Occurrence and human exposures. Environ. Sci. Technol. 2012, 46, 6860–6866. [Google Scholar] [CrossRef]
- Zhou, X.; Kramer, J.P.; Calafat, A.M.; Ye, X. Automated on-line column-switching high performance liquid chromatography isotope dilution tandem mass spectrometry method for the quantification of bisphenol A, bisphenol F, bisphenol S, and 11 other phenols in urine. J. Chromatogr. B 2014, 944, 152–156. [Google Scholar] [CrossRef]
- Grumetto, L.; Gennari, O.; Montesano, D.; Ferracane, R.; Ritieni, A.; Albrizio, S.; Barbato, F. Determination of five bisphenols in commercial milk samples by liquid chromatography coupled to fluorescence detection. J. Food Prot. 2013, 76, 1590–1596. [Google Scholar] [CrossRef] [PubMed]
- Cunha, S.C.; Inácio, T.; Almada, M.; Ferreira, R.; Fernandes, J.O. Gas chromatography–mass spectrometry analysis of nine bisphenols in canned meat products and human risk estimation. Food Res. Int. 2020, 135, 109293. [Google Scholar] [CrossRef] [PubMed]
- Alnaimat, A.S.; Barciela-Alonso, M.C.; Bermejo-Barrera, P. Determination of bisphenol A in tea samples by solid phase extraction and liquid chromatography coupled to mass spectrometry. Microchem. J. 2019, 147, 598–604. [Google Scholar] [CrossRef]
- Sun, X.; Wang, J.; Li, Y.; Jing, J.; Zhang, B.; Shah, S.M.; Wang, X.; Chen, J. Highly selective dummy molecularly imprinted polymer as a solid-phase extraction sorbent for five bisphenols in tap and river water. J. Chromatogr. A 2014, 1343, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, K.K.; Shanmugam, G.; Sampath, S.; Larsson, D.G.; Ramaswamy, B.R. GC–MS determination of bisphenol A and alkylphenol ethoxylates in river water from India and their ecotoxicological risk assessment. Ecotoxicol. Environ. Saf. 2014, 99, 13–20. [Google Scholar] [CrossRef]
- Kazemi, S.; Bahramifar, N.; Moghadamnia, A.A.; Jorsarae, S.G. Detection of bisphenol A and nonylphenol in rat’s blood serum, tissue and impact on reproductive system. Electron. Physician 2016, 8, 2772. [Google Scholar] [CrossRef]
- Shi, X.; Liu, C.; Wu, G.; Zhou, B. Waterborne exposure to PFOS causes disruption of the hypothalamus-pituitary-thyroid axis in zebrafish larvae. Chemosphere 2009, 77, 1010–1018. [Google Scholar] [CrossRef]
- Wei, J.; Liu, J.; Liang, S.; Sun, M.; Duan, J. Low-dose exposure of silica nanoparticles induces neurotoxicity via neuroactive ligand–receptor interaction signaling pathway in zebrafish embryos. Int. J. Nanomed. 2020, 15, 4407–4415. [Google Scholar] [CrossRef] [PubMed]
- New, L.S.; Chan, E.C. Evaluation of BEH C18, BEH HILIC, and HSS T3 (C18) column chemistries for the UPLC-MS-MS analysis of glutathione, glutathione disulfide, and ophthalmic acid in mouse liver and human plasma. J. Chromatogr. Sci. 2008, 46, 209–214. [Google Scholar] [CrossRef]
- Chen, G.; Tang, C.; Tan, J.; Zhu, Z.; Guo, S.; Zhou, J.; Peng, X. Multi-residue determination of bisphenol analogues in organism tissues by ultra-high performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2022, 1682, 463489. [Google Scholar] [CrossRef]
- Xiao, Z.; Wang, R.; Suo, D.; Li, T.; Su, X. Trace analysis of bisphenol A and its analogues in eggs by ultra-performance liquid chromatography-tandem mass spectrometry. Food Chem. 2020, 327, 126882. [Google Scholar] [CrossRef] [PubMed]
- Konermann, L. Addressing a common misconception: Ammonium acetate as neutral pH “buffer” for native electrospray mass spectrometry. J. Am. Soc. Mass Spectrom. 2017, 28, 1827–1835. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yin, J.; Yang, Y.; Zhou, N.; Zhang, J.; Shao, B.; Wu, Y. Determination of bisphenol AF (BPAF) in tissues, serum, urine and feces of orally dosed rats by ultra-high-pressure liquid chromatography–electrospray tandem mass spectrometry. J. Chromatogr. B 2012, 901, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Owczarek, K.; Kubica, P.; Kudłak, B.; Rutkowska, A.; Konieczna, A.; Rachon, D.; Namiesnik, J.; Wasik, A. Determination of trace levels of eleven bisphenol A analogues in human blood serum by high performance liquid chromatography–tandem mass spectrometry. Sci. Total Environ. 2018, 628, 1362–1368. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Zhang, S.; Razanajatovo, R.; Zou, H.; Zhu, W. Accumulation, tissue distribution, and biochemical effects of polystyrene microplastics in the freshwater fish red tilapia (Oreochromis niloticus). Environ. Pollut. 2018, 238, 1–9. [Google Scholar] [CrossRef]
- Petrarca, M.H.; Fernandes, J.O.; Marmelo, I.; Marques, A.; Cunha, S. Multi-analyte gas chromatography-mass spectrometry method to monitor bisphenols, musk fragrances, ultraviolet filters, and pesticide residues in seafood. J. Chromatogr. A 2022, 1663, 462755. [Google Scholar] [CrossRef]
- Niu, Y.; Wang, B.; Zhao, Y.; Zhang, J.; Shao, B. Highly sensitive and high-throughput method for the analysis of bisphenol analogues and their halogenated derivatives in breast milk. J. Agric. Food Chem. 2017, 65, 10452–10463. [Google Scholar] [CrossRef]
- Wang, Q.; Zhu, L.; Chen, M.; Ma, X.; Wang, X.; Xia, J. Simultaneously determination of bisphenol A and its alternatives in sediment by ultrasound-assisted and solid phase extractions followed by derivatization using GC-MS. Chemosphere 2017, 169, 709–715. [Google Scholar] [CrossRef]
- Li, Y.; Yang, L.; Zhen, H.; Chen, X.; Sheng, M.; Li, K.; Xue, W.; Zhao, H.; Meng, S.; Cao, G. Determination of estrogens and estrogen mimics by solid-phase extraction with liquid chromatography-tandem mass spectrometry. J. Chromatogr. B 2021, 1168, 122559. [Google Scholar] [CrossRef]
- Guo, F.; Liu, Q.; Qu, G.; Song, S.; Sun, J.; Shi, J.; Jiang, G. Simultaneous determination of five estrogens and four androgens in water samples by online solid-phase extraction coupled with high-performance liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2013, 1281, 9–18. [Google Scholar] [CrossRef]
- Cui, J.; Wei, Y.; Jiang, J.; Xiao, S.; Liu, X.; Zhou, Z.; Liu, D.; Wang, P. Bioaccumulation, metabolism and toxicological effects of chiral insecticide malathion and its metabolites in zebrafish (Danio rerio). Chemosphere 2023, 318, 137898. [Google Scholar] [CrossRef]
- Shi, J.; Yang, Y.; Zhang, J.; Feng, Y.; Shao, B. Uptake, depuration and bioconcentration of bisphenol AF (BPAF) in whole-body and tissues of zebrafish (Danio rerio). Ecotoxicol. Environ. Saf. 2016, 132, 339–344. [Google Scholar] [CrossRef]
- Chen, Q.; Yin, D.; Jia, Y.; Schiwy, S.; Legradi, J.; Yang, S.; Hollert, H. Enhanced uptake of BPA in the presence of nanoplastics can lead to neurotoxic effects in adult zebrafish. Sci. Total Environ. 2017, 609, 1312–1321. [Google Scholar] [CrossRef]
- Kelly, B.C.; Gobas, F.A.P.C.; McLachlan, M.S. Intestinal absorption and biomagnification of organic contaminants in fish, wildlife, and humans. Environ. Toxicol. Chem. 2004, 23, 2324–2336. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, Y.; Deng, Y.; Jiang, W.; Zhao, Y.; Geng, J.; Ding, L.; Ren, H. Uptake and accumulation of polystyrene microplastics in zebrafish (Danio rerio) and toxic effects in liver. Environ. Sci. Technol. 2016, 50, 4054–4060. [Google Scholar] [CrossRef]
- Sundaram, E.; Manna, A.; Servarayan, K.L.; Vasantha, V.S. Colorimetric detection and bio-magnification of bisphenol A in fish organs and water sources using 3′, 6′-bis (diethylamino)-2-((3, 4, 5trimethyl benzylidene) amino) spiro [isoindoline-1, 9′-xanthen]-3-one (BTSIXO)-Fe3+ ion conjugate. Food Chem. 2021, 345, 128627. [Google Scholar] [CrossRef]
- Abdulhameed, A.S.A.R.; Lim, V.; Bahari, H.; Khoo, B.Y.; Abdullah, M.N.H.; Tan, J.; Yong, Y.K. Adverse effects of bisphenol A on the liver and its underlying mechanisms: Evidence from in vivo and in vitro studies. BioMed Res. Int. 2022, 2022, 8227314. [Google Scholar] [CrossRef]
- Mukherjee, U.; Samanta, A.; Biswas, S.; Das, S.; Ghosh, S.; Mandal, D.K.; Maitra, S. Bisphenol A-induced oxidative stress, hepatotoxicity and altered estrogen receptor expression in Labeo bata: Impact on metabolic homeostasis and inflammatory response. Ecotoxicol. Environ. Saf. 2020, 202, 110944. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, J.; Wang, D.; Wan, M.; Li, X.; Zhang, L.; Yang, D.; Liu, F.; Liu, J.; Li, K.; et al. BPA induces hepatotoxicity in zebrafish through oxidative stress and apoptosis pathways. Fish Physiol. Biochem. 2024, 50, 403–412. [Google Scholar] [CrossRef]
- Huang, W.; Shi, X.; Chen, Y.; Zhang, Q.; Peng, J.; Zheng, S.; Wu, K. Comparative pharyngeal cartilage developmental toxicity of bisphenol A, bisphenol S and bisphenol AF to zebrafish (Danio rerio) larvae: A combination of morphometry and global transcriptome analyses. Sci. Total Environ. 2023, 868, 161702. [Google Scholar] [CrossRef]
- Bao, Y.; Zhu, M.; Su, G. Tissue-specific accumulation, bioaccumulation, and depuration of liquid crystal monomers (LCMs) in adult zebrafish (Danio rerio). Sci. Total Environ. 2023, 859, 160267. [Google Scholar] [CrossRef]
- Negri-Cesi, P. Bisphenol A interaction with brain development and functions. Dose-Response 2015, 13, 1559325815590394. [Google Scholar] [CrossRef]
- Gu, J.; Zhang, J.; Chen, Y.; Wang, H.; Guo, M.; Wang, L.; Wang, Z.; Wu, S.; Shi, L.; Gu, A.; et al. Neurobehavioral effects of bisphenol S exposure in early life stages of zebrafish larvae (Danio rerio). Chemosphere 2019, 217, 629–635. [Google Scholar] [CrossRef]
- Mornagui, B.; Rezg, R.; Repond, C.; Pellerin, L. Effects of bisphenol S, a major substitute of bisphenol A, on neurobehavioral responses and cerebral monocarboxylate transporters expression in mice. Food Chem. Toxicol. 2019, 132, 110670. [Google Scholar] [CrossRef]
- Mukherjee, U.; Das, S.; Ghosh, S.; Maitra, S. Reproductive toxicity of bisphenol A, at environmentally relevant concentrations, on ovarian redox balance, maturational response, and intra-oocyte signalling events in Labeo bata. Sci. Total Environ. 2024, 906, 167415. [Google Scholar] [CrossRef]
- Qiu, W.; Liu, S.; Chen, H.; Luo, S.; Xiong, Y.; Wang, X.; Xu, B.; Zheng, C.; Wang, K. The comparative toxicities of BPA, BPB, BPS, BPF, and BPAF on the reproductive neuroendocrine system of zebrafish embryos and its mechanisms. J. Hazard. Mater. 2021, 406, 124303. [Google Scholar] [CrossRef]
- Mu, X.; Qi, S.; Liu, J.; Yuan, L.; Huang, Y.; Xue, J.; Qian, L.; Wang, C.; Li, Y. Toxicity and behavioral response of zebrafish exposed to combined microplastic and bisphenol analogues. Environ. Chem. Lett. 2022, 20, 41–48. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, L.; Lu, G.; Jiang, R.; Yan, Z.; Li, Y. Occurrence, toxicity and ecological risk of Bisphenol A analogues in aquatic environment–A review. Ecotoxicol. Environ. Saf. 2021, 208, 111481. [Google Scholar] [CrossRef]
- Qiu, W.; Zhan, H.; Hu, J.; Zhang, T.; Xu, H.; Wong, M.; Xu, B.; Zheng, C. The occurrence, potential toxicity, and toxicity mechanism of bisphenol S, a substitute of bisphenol A: A critical review of recent progress. Ecotoxicol. Environ. Saf. 2019, 173, 192–202. [Google Scholar] [CrossRef]
- Boucher, J.G.; Gagné, R.; Rowan-Carroll, A.; Boudreau, A.; Yauk, C.L.; Atlas, E. Bisphenol A and bisphenol S induce distinct transcriptional profiles in differentiating human primary preadipocytes. PLoS ONE 2016, 11, e0163318. [Google Scholar] [CrossRef]
- Moreman, J.; Lee, O.; Trznadel, M.; David, A.; Kudoh, T.; Tyler, C.R. Acute toxicity, teratogenic, and estrogenic effects of bisphenol A and its alternative replacements bisphenol S, bisphenol F, and bisphenol AF in zebrafish embryo-larvae. Environ. Sci. Technol. 2017, 51, 12796–12805. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Tang, Y.; Du, X.; Han, Y.; Shi, W.; Sun, S.; Zhang, W.; Zheng, H.; Liu, G. Fine polystyrene microplastics render immune responses more vulnerable to two veterinary antibiotics in a bivalve species. Mar. Pollut. Bull. 2021, 164, 111995. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Zhou, W.; Tang, Y.; Shi, W.; Shao, Y.; Ren, P.; Zhang, J.; Xiao, G.; Sun, H.; Liu, G. Microplastics aggravate the bioaccumulation of three veterinary antibiotics in the thick shell mussel Mytilus coruscus and induce synergistic immunotoxic effects. Sci. Total Environ. 2021, 770, 145273. [Google Scholar] [CrossRef]
- Tang, Y.; Rong, J.; Guan, X.; Zha, S.; Shi, W.; Han, Y.; Du, X.; Wu, F.; Huang, W.; Liu, G. Immunotoxicity of microplastics and two persistent organic pollutants alone or in combination to a bivalve species. Environ. Pollut. 2020, 258, 113845. [Google Scholar] [CrossRef]


| BPA | BPS | |
|---|---|---|
| Extract Solvents | Recoveries (%) | Recoveries (%) |
| Acetonitrile | 72.6 ± 4.2 | 62.2 ± 15.6 |
| Acidified acetonitrile | 84.0 ± 1.9 | 66.0 ± 2.7 |
| 75% acetonitrile | 68.3 ± 6.2 | 67.9 ± 5.8 |
| 75% acidified acetonitrile | 69.7 ± 1.4 | 62.3 ± 2.9 |
| Ammonia acetonitrile | 75.7 ± 8.5 | 54.4 ± 16.6 |
| Acetonitrile: EDTA–Mcllvaine = 9:1 | 79.5 ± 6.8 | 62.7 ± 11.1 |
| Acetonitrile: EDTA–Mcllvaine = 8:2 | 75.8 ± 2.4 | 70.3 ± 3.2 |
| Acetonitrile: EDTA–Mcllvaine = 7:3 | 45.4 ± 3.1 | 39.2 ± 9.2 |
| Acetonitrile: EDTA–Mcllvaine = 6:4 | 34.5 ± 2.3 | 37.6 ± 3.0 |
| Acetonitrile: EDTA–Mcllvaine = 5:5 | 50.9 ± 10.1 | 54.2 ± 1.1 |
| Ammonium acetate buffer solution | 98.5 ± 4.5 | 92.7 ± 0.8 |
| Tissues | Spiked (μg/L) | BPA | BPS | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Intra-Day | Inter-Day | Intra-Day | Inter-Day | ||||||
| Recovery (%) | RSD | Recovery (%) | RSD | Recovery (%) | RSD | Recovery (%) | RSD | ||
| Brain | 5 | 78.8 | 8.2 | 89.2 | 8.7 | 81.6 | 3.6 | 72.9 | 11.1 |
| 10 | 83.7 | 4.3 | 90.3 | 5.8 | 84.3 | 1.8 | 86.8 | 4.85 | |
| 20 | 83.9 | 5.5 | 91.2 | 3.9 | 79.6 | 4.5 | 89.7 | 8.0 | |
| Gill | 20 | 92.6 | 5.2 | 92.4 | 7.1 | 96.5 | 2.9 | 95.6 | 1.9 |
| 50 | 89.2 | 3.8 | 91.2 | 6.7 | 96.2 | 3.2 | 96.7 | 3.4 | |
| 100 | 93.8 | 3.6 | 84.6 | 3.3 | 94.8 | 2.9 | 89.0 | 6.2 | |
| Muscle | 20 | 96.8 | 4.1 | 95.8 | 2.9 | 99.1 | 3.6 | 105.2 | 8.4 |
| 50 | 94.1 | 5.3 | 95.4 | 4.2 | 86.4 | 1.1 | 83.9 | 1.8 | |
| 100 | 90.5 | 1.8 | 86.8 | 5.7 | 113.2 | 7.8 | 83.2 | 7.5 | |
| Gonad | 20 | 91.4 | 5.9 | 93.2 | 9.1 | 98.2 | 7.9 | 84.1 | 6.1 |
| 50 | 86.9 | 7.2 | 84.1 | 7.5 | 95.6 | 7.4 | 92.5 | 6.2 | |
| 100 | 87.6 | 1.4 | 84.6 | 5.2 | 92.9 | 6.3 | 93.8 | 4.8 | |
| Liver | 20 | 96.5 | 9.2 | 94.9 | 6.8 | 94.8 | 4.2 | 84.6 | 9.7 |
| 50 | 99.1 | 6.7 | 96.5 | 5.5 | 97.3 | 6.5 | 101.4 | 5.6 | |
| 100 | 101.3 | 11.1 | 93.6 | 3.8 | 97.1 | 9.8 | 95.6 | 11.9 | |
| Intestine | 20 | 98.6 | 3.0 | 101.9 | 8.0 | 99.3 | 8.8 | 104.6 | 5.8 |
| 50 | 89.7 | 6.6 | 97.1 | 9.8 | 95.8 | 10.1 | 95.6 | 9.0 | |
| 100 | 109.5 | 5.6 | 86.9 | 6.3 | 90.0 | 5.3 | 92.1 | 3.2 | |
| BPA | |||||
|---|---|---|---|---|---|
| Tissues | Linearity Range (μg/L) | Correlation Coefficient (r) | Limit of Detection (μg/L) | Limit of Quantitation (μg/L) | Matrix Effect (%) |
| Brain | 5.0–50.0 | 0.9984 | 0.3 | 1.0 | 5.6 ± 2.1 |
| Gill | 5.0–500.0 | 0.9994 | 0.3 | 1.0 | 8.8 ± 2.4 |
| Muscle | 5.0–500.0 | 0.9999 | 0.2 | 0.6 | 5.2 ± 1.6 |
| Gonad | 5.0–2000.0 | 0.9997 | 0.2 | 0.6 | −9.4 ± 3.8 |
| Liver | 5.0–2500.0 | 0.9989 | 0.3 | 1.0 | 12.0 ± 2.7 |
| Intestine | 5.0–10,000.0 | 0.9992 | 1.0 | 3.0 | −11.2 ± 3.5 |
| BPS | |||||
| Tissues | Linearity Range (μg/L) | Correlation Coefficient (r) | Limit of Detection (μg/L) | Limit of Quantitation (μg/L) | Matrix Effect (%) |
| Brain | 5.0–50.0 | 0.9990 | 0.3 | 1.0 | 13.9 ± 4.2 |
| Gill | 5.0–500.0 | 0.9992 | 0.3 | 1.0 | 5.7 ± 1.7 |
| Muscle | 5.0–500.0 | 0.9999 | 0.3 | 1.0 | 8.3 ± 1.1 |
| Gonad | 5.0–1000.0 | 0.9989 | 0.26 | 0.8 | −4.8 ± 3.0 |
| Liver | 5.0–2000.0 | 0.9998 | 0.3 | 1.0 | −15.6 ± 6.9 |
| Intestine | 5.0–10,000.0 | 0.9993 | 1.0 | 3.0 | −13.8 ± 5.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, M.; Jia, M.; Qin, Y.; Li, J.; Yao, T.; Francis, F.; Gu, X. Determination of Bisphenol Compounds and the Bioaccumulation after Co-Exposure with Polyethylene Microplastics in Zebrafish. Toxics 2024, 12, 702. https://doi.org/10.3390/toxics12100702
Xue M, Jia M, Qin Y, Li J, Yao T, Francis F, Gu X. Determination of Bisphenol Compounds and the Bioaccumulation after Co-Exposure with Polyethylene Microplastics in Zebrafish. Toxics. 2024; 12(10):702. https://doi.org/10.3390/toxics12100702
Chicago/Turabian StyleXue, Moyong, Ming Jia, Yuchang Qin, Jing Li, Ting Yao, Frédéric Francis, and Xu Gu. 2024. "Determination of Bisphenol Compounds and the Bioaccumulation after Co-Exposure with Polyethylene Microplastics in Zebrafish" Toxics 12, no. 10: 702. https://doi.org/10.3390/toxics12100702







