Integrated Approach for Testing and Assessment for Developmental Neurotoxicity (DNT) to Prioritize Aromatic Organophosphorus Flame Retardants
Abstract
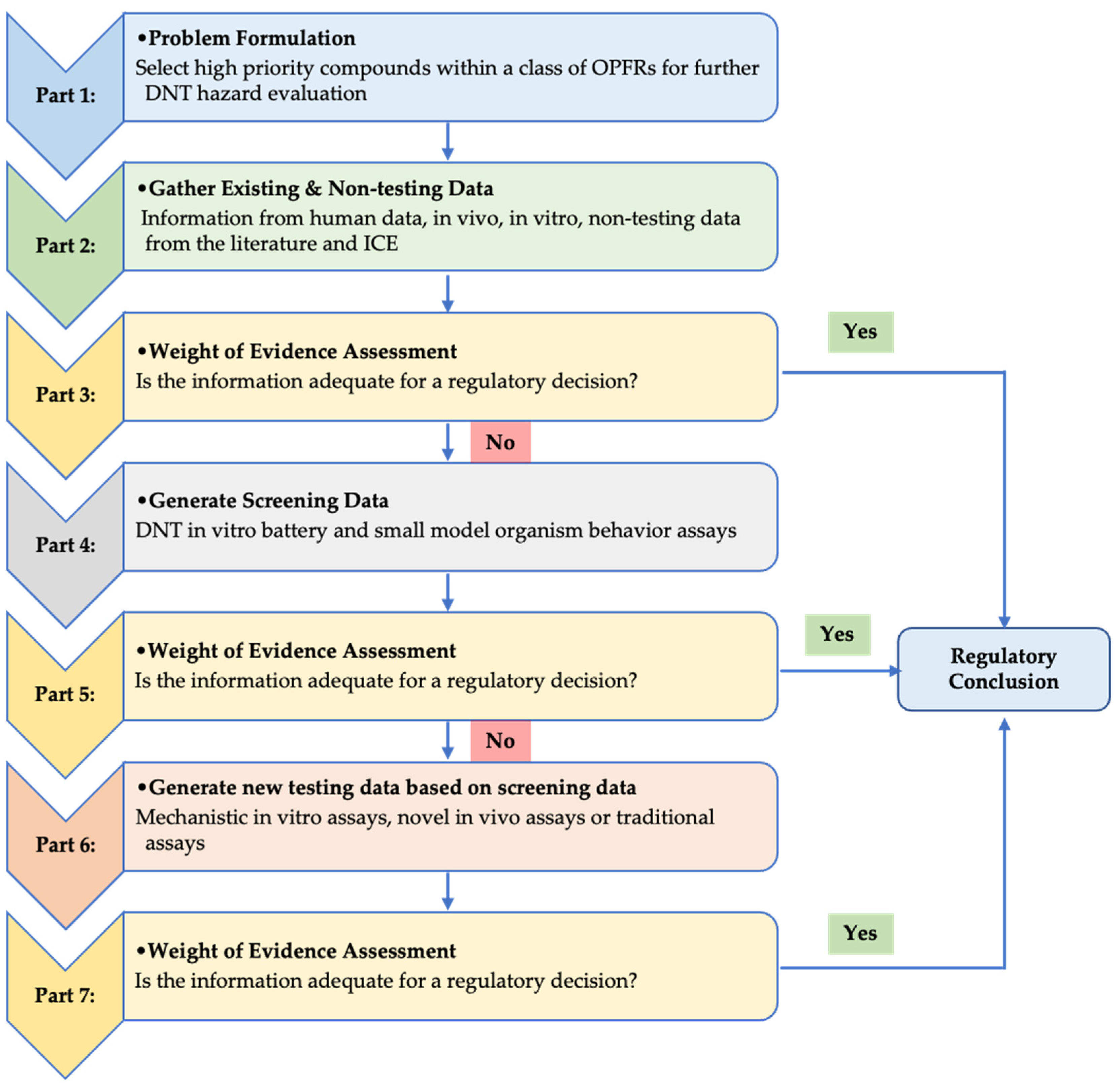
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Assays in the DNT Battery
2.2.1. Proliferation
- i.
- Proliferation@IUF
- ii.
- Proliferation@USEPA
2.2.2. Migration
- i.
- NCC migration@UKonstanz
- ii.
- Radial glia migration@IUF
- iii.
- Neuronal migration@IUF
- iv.
- Oligo migration@IUF
2.2.3. Differentiation
- i.
- Neuronal differentiation@IUF
- ii.
- Oligodendrocyte differentiation@IUF
2.2.4. Neurite Outgrowth
- i.
- Neurite outgrowth@USEPA (rat)
- ii.
- Neurite outgrowth@MolDevices
- iii.
- Neurite outgrowth@USEPA (human)
- iv.
- CNS neurite outgrowth@UKonstanz
- v.
- PNS neurite outgrowth@UKonstanz
- vi.
- Neurite outgrowth@IUF
- vii./iix.
- CNS and PNS neurite outgrowth@NCATS
2.2.5. Network Formation and Function
- i.
- Acute neuronal firing@USEPA
- ii.
- Network formation@USEPA
2.2.6. Behavior
- i.
- Behavior@Biobide
- ii.
- Behavior@OregonStateU
- iii.
- Behavior@UCDavis
- iv.
- Behavior@UCSanDiego
| Assay | Model | References |
|---|---|---|
| Proliferation | ||
| Proliferation@IUF | Human 3D neurosphere | [21] |
| Proliferation@USEPA | Human hNP1 | [23] |
| Migration | ||
| NCC migration@UKonstanz | Human crest cells | [25] |
| Radial glia migration@IUF 1 | Human 3D neurosphere | [21] |
| Neuronal migration@IUF 1 | Human 3D neurosphere | [21] |
| Oligo migration@IUF 1 | Human 3D neurosphere | [21] |
| Differentiation | ||
| Neuron differentiation@IUF 1 | Human 3D neurosphere | [21] |
| Oligodendrocyte differentiation@IUF 1 | Human 3D neurosphere | [21] |
| Neurite outgrowth | ||
| Neurite outgrowth@USEPA | Rat primary cortical | [23] |
| Neurite outgrowth@MolDevices | Human iPSC-derived | [29] |
| Neurite outgrowth@USEPA | Human hN2 | [23] |
| CNS neurite outgrowth@UKonstanz | Human LUHMES | [30] |
| PNS neurite outgrowth@UKonstanz | Human ESC-derived | [30] |
| Neurite outgrowth@IUF 1 | Human 3D neurosphere | [21] |
| CNS neurite outgrowth@NCATS | Human GFP-labeled cortical iPSCs | [34] |
| PNS neurite outgrowth@NCATS | Human GFP-labeled spinal iPSCs | [34] |
| Firing/Network formation | ||
| Acute neuronal firing@USEPA | Rat primary cortical | [23] |
| Network formation@USEPA | Rat primary cortical | [35] |
| Behavior | ||
| Behavior@Biobide | Zebrafish | [41] |
| Behavior@OregoneStateU | Zebrafish | [42] |
| Behavior@UCSanDiego | Planarian | [45] |
| Behavior@UCDavis | Zebrafish | [45] |
2.3. Data Analysis
2.3.1. Benchmark Concentration (BMC) Approach
2.3.2. Human Exposure Evaluation
2.4. Gathering of Additional Data
2.4.1. Integrated Chemical Environment (ICE)
2.4.2. Literature Review
3. Results
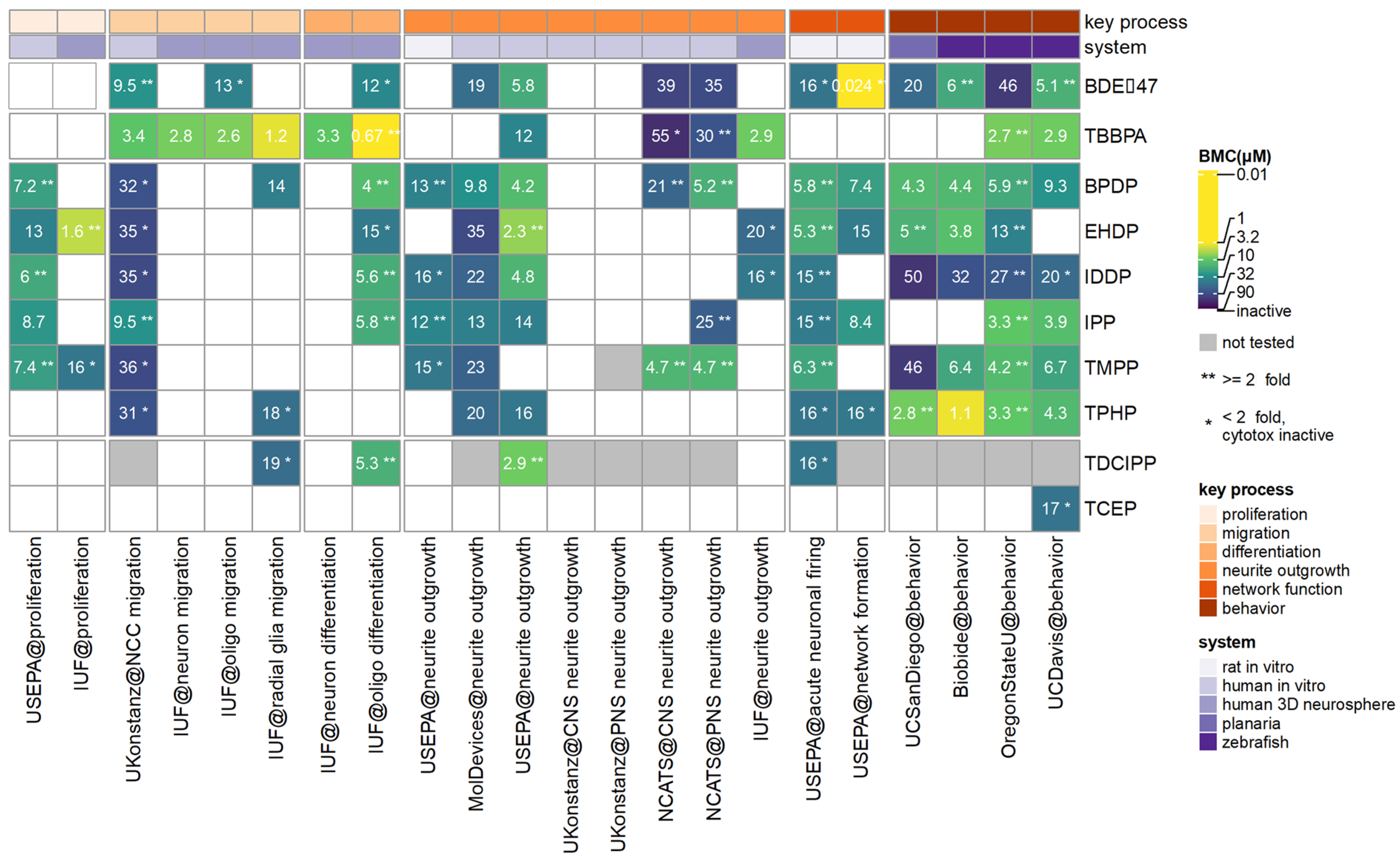
3.1. Most Sensitive Endpoint in Each Assay of the DNT Battery
3.2. Data Integration of All Endpints in the DNT Battery
3.3. Data Integration from ICE and Literature Review
3.4. Relevance to Human Exposure
4. Discussion
4.1. Comparison of Potency across Phased-Out BFRs, Halogenated FRs, and OPFRs
4.1.1. Brominated FRs
4.1.2. Halogenated FRs
4.1.3. Aromatic FRs
4.2. Comparison of In Vitro and Small Model Organisms Data to Human Exposures and In Vivo Studies
4.3. Chemical Considerations
4.4. Prioritization for Further Testing
4.5. Uncertatinites and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Watanabe, I.; Sakai, S. Environmental release and behavior of brominated flame retardants. Environ. Int. 2003, 29, 665–682. [Google Scholar] [CrossRef] [PubMed]
- Blum, A.; Behl, M.; Birnbaum, L.; Diamond, M.L.; Phillips, A.; Singla, V.; Sipes, N.S.; Stapleton, H.M.; Venier, M. Organophosphate Ester Flame Retardants: Are They a Regrettable Substitution for Polybrominated Diphenyl Ethers? Environ. Sci. Technol. Lett. 2019, 6, 638–649. [Google Scholar] [CrossRef]
- Plichta, V.; Steinwider, J.; Vogel, N.; Weber, T.; Kolossa-Gehring, M.; Murinova, L.P.; Wimmerova, S.; Tratnik, J.S.; Horvat, M.; Koppen, G.; et al. Risk Assessment of Dietary Exposure to Organophosphorus Flame Retardants in Children by Using HBM-Data. Toxics 2022, 10, 234. [Google Scholar] [CrossRef] [PubMed]
- National Academies of Sciences, Division on Earth, Life Studies, Board on Environmental Studies and Committee to Develop a Scoping Plan to Assess the Hazards of Organohalogen Flame Retardants. A Class Approach to Hazard Assessment of Organohalogen Flame Retardants; The National Academies Press: Washington, DC, USA, 2019; p. 102. [Google Scholar]
- Thomas, R.S.; Bahadori, T.; Buckley, T.J.; Cowden, J.; Deisenroth, C.; Dionisio, K.L.; Frithsen, J.B.; Grulke, C.M.; Gwinn, M.R.; Harrill, J.A.; et al. The Next Generation Blueprint of Computational Toxicology at the U.S. Environmental Protection Agency. Toxicol. Sci. 2019, 169, 317–332. [Google Scholar] [CrossRef]
- Judson, R.; Richard, A.; Dix, D.J.; Houck, K.; Martin, M.; Kavlock, R.; Dellarco, V.; Henry, T.; Holderman, T.; Sayre, P.; et al. The toxicity data landscape for environmental chemicals. Environ. Health Perspect. 2009, 117, 685–695. [Google Scholar] [CrossRef] [PubMed]
- Casida, J.E.; Durkin, K.A. Pesticide Chemical Research in Toxicology: Lessons from Nature. Chem. Res. Toxicol. 2017, 30, 94–104. [Google Scholar] [CrossRef]
- Mie, A.; Ruden, C. Non-disclosure of developmental neurotoxicity studies obstructs the safety assessment of pesticides in the European Union. Environ. Health 2023, 22, 44. [Google Scholar] [CrossRef]
- Grandjean, P.; Landrigan, P.J. Neurobehavioural effects of developmental toxicity. Lancet Neurol. 2014, 13, 330–338. [Google Scholar] [CrossRef]
- Stapleton, H.M.; Kelly, S.M.; Allen, J.G.; McClean, M.D.; Webster, T.F. Measurement of polybrominated diphenyl ethers on hand wipes: Estimating exposure from hand-to-mouth contact. Environ. Sci. Technol. 2008, 42, 3329–3334. [Google Scholar] [CrossRef]
- Stapleton, H.M.; Misenheimer, J.; Hoffman, K.; Webster, T.F. Flame retardant associations between children’s handwipes and house dust. Chemosphere 2014, 116, 54–60. [Google Scholar] [CrossRef]
- Bal-Price, A.; Hogberg, H.T.; Crofton, K.M.; Daneshian, M.; FitzGerald, R.E.; Fritsche, E.; Heinonen, T.; Hougaard Bennekou, S.; Klima, S.; Piersma, A.H.; et al. Recommendation on test readiness criteria for new approach methods in toxicology: Exemplified for developmental neurotoxicity. ALTEX 2018, 35, 306–352. [Google Scholar] [CrossRef] [PubMed]
- Bal-Price, A.K.; Hogberg, H.T.; Buzanska, L.; Coecke, S. Relevance of in vitro neurotoxicity testing for regulatory requirements: Challenges to be considered. Neurotoxicol. Teratol. 2010, 32, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Masjosthusmann, S.; Blum, J.; Bartmann, K.; Dolde, X.; Holzer, A.-K.; Stürzl, L.-C.; Keßel, E.H.; Förster, N.; Dönmez, A.; Klose, J.; et al. Establishment of An a Priori Protocol for the Implementation and Interpretation of an In-Vitro Testing Battery for the Assessment of Developmental Neurotoxicity; EFSA Supporting Publications (European Food Safety Authority): Parma, Italy, 2020; Volume 17, p. 1938E. [Google Scholar] [CrossRef]
- OECD. Initial Recommendations on Evaluation of Data from the Developmental Neurotoxicity (DNT) In-Vitro Testing Battery; OECD: Paris, France, 2023. [Google Scholar]
- OECD. Case Study on the Use of Integrated Approaches for Testing and Assessment for DNT to Prioritize a Class of Organophosphorus Flame Retardants; OECD: Paris, France, 2022. [Google Scholar]
- OECD. Guidance Document on the Reporting of Defined Approaches to be Used Within Integrated Approaches to Testing and Assessment; OECD: Paris, France, 2017. [Google Scholar]
- Bell, S.; Abedini, J.; Ceger, P.; Chang, X.; Cook, B.; Karmaus, A.L.; Lea, I.; Mansouri, K.; Phillips, J.; McAfee, E.; et al. An integrated chemical environment with tools for chemical safety testing. Toxicol. In Vitro 2020, 67, 104916. [Google Scholar] [CrossRef] [PubMed]
- Cugola, F.R.; Fernandes, I.R.; Russo, F.B.; Freitas, B.C.; Dias, J.L.; Guimaraes, K.P.; Benazzato, C.; Almeida, N.; Pignatari, G.C.; Romero, S.; et al. The Brazilian Zika virus strain causes birth defects in experimental models. Nature 2016, 534, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Ming, G.L.; Tang, H.; Song, H. Advances in Zika Virus Research: Stem Cell Models, Challenges, and Opportunities. Cell Stem Cell 2016, 19, 690–702. [Google Scholar] [CrossRef] [PubMed]
- Klose, J.; Pahl, M.; Bartmann, K.; Bendt, F.; Blum, J.; Dolde, X.; Forster, N.; Holzer, A.K.; Hubenthal, U.; Kessel, H.E.; et al. Neurodevelopmental toxicity assessment of flame retardants using a human DNT in vitro testing battery. Cell Biol. Toxicol. 2022, 38, 781–807. [Google Scholar] [CrossRef] [PubMed]
- Nimtz, L.; Klose, J.; Masjosthusmann, S.; Barenys, M.; Fritsche, E. The neurosphere assay as an in vitro method for developmental neurotoxicity (DNT) evaluation. In Neuromethods; Aschner, M., Costa, L., Eds.; Humana Press Inc.: New York, NY, USA, 2019; Volume 145, pp. 141–168. [Google Scholar]
- Behl, M.; Hsieh, J.H.; Shafer, T.J.; Mundy, W.R.; Rice, J.R.; Boyd, W.A.; Freedman, J.H.; Hunter, E.S., 3rd; Jarema, K.A.; Padilla, S.; et al. Use of alternative assays to identify and prioritize organophosphorus flame retardants for potential developmental and neurotoxicity. Neurotoxicol. Teratol. 2015, 52, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Rice, D.; Barone, S., Jr. Critical periods of vulnerability for the developing nervous system: Evidence from humans and animal models. Environ. Health Perspect. 2000, 108 (Suppl. S3), 511–533. [Google Scholar] [CrossRef] [PubMed]
- Nyffeler, J.; Dolde, X.; Krebs, A.; Pinto-Gil, K.; Pastor, M.; Behl, M.; Waldmann, T.; Leist, M. Combination of multiple neural crest migration assays to identify environmental toxicants from a proof-of-concept chemical library. Arch. Toxicol. 2017, 91, 3613–3632. [Google Scholar] [CrossRef]
- Schmuck, M.R.; Temme, T.; Dach, K.; de Boer, D.; Barenys, M.; Bendt, F.; Mosig, A.; Fritsche, E. Omnisphero: A high-content image analysis (HCA) approach for phenotypic developmental neurotoxicity (DNT) screenings of organoid neurosphere cultures in vitro. Arch. Toxicol. 2017, 91, 2017–2028. [Google Scholar] [CrossRef]
- Wiggins, R.C. Myelination: A critical stage in development. Neurotoxicology 1986, 7, 103–120. [Google Scholar] [PubMed]
- Audesirk, G.; Audesirk, T. Neurocytoskeleton and Neuritic Development. In Handbook of Developmental Neurotoxicology, 1st ed.; Slikker, W.J., Chang, L.W., Eds.; Academic Press: San Diego, CA, USA, 1998; pp. 61–85. [Google Scholar]
- Ryan, K.R.; Sirenko, O.; Parham, F.; Hsieh, J.H.; Cromwell, E.F.; Tice, R.R.; Behl, M. Neurite outgrowth in human induced pluripotent stem cell-derived neurons as a high-throughput screen for developmental neurotoxicity or neurotoxicity. Neurotoxicology 2016, 53, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Delp, J.; Gutbier, S.; Klima, S.; Hoelting, L.; Pinto-Gil, K.; Hsieh, J.H.; Aichem, M.; Klein, K.; Schreiber, F.; Tice, R.R.; et al. A high-throughput approach to identify specific neurotoxicants/ developmental toxicants in human neuronal cell function assays. ALTEX 2018, 35, 235–253. [Google Scholar] [CrossRef] [PubMed]
- Qian, K.; Huang, C.T.; Chen, H.; Blackbourn, L.W.t.; Chen, Y.; Cao, J.; Yao, L.; Sauvey, C.; Du, Z.; Zhang, S.C. A simple and efficient system for regulating gene expression in human pluripotent stem cells and derivatives. Stem Cells 2014, 32, 1230–1238. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, H.; Sauvey, C.; Yao, L.; Zarnowska, E.D.; Zhang, S.C. Directed differentiation of forebrain GABA interneurons from human pluripotent stem cells. Nat. Protoc. 2013, 8, 1670–1679. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.W.; Chen, H.; Liu, H.; Lu, J.; Qian, K.; Huang, C.L.; Zhong, X.; Fan, F.; Zhang, S.C. Generation and expansion of highly pure motor neuron progenitors from human pluripotent stem cells. Nat. Commun. 2015, 6, 6626. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, L.; Huang, R.; Xu, T.; Parham, F.; Behl, M.; Xia, M. Evaluation of chemical compounds that inhibit neurite outgrowth using GFP-labeled iPSC-derived human neurons. Neurotoxicology 2021, 83, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Frank, C.L.; Brown, J.P.; Wallace, K.; Mundy, W.R.; Shafer, T.J. From the Cover: Developmental Neurotoxicants Disrupt Activity in Cortical Networks on Microelectrode Arrays: Results of Screening 86 Compounds During Neural Network Formation. Toxicol. Sci. 2017, 160, 121–135. [Google Scholar] [CrossRef]
- Brown, J.P.; Hall, D.; Frank, C.L.; Wallace, K.; Mundy, W.R.; Shafer, T.J. Editor’s Highlight: Evaluation of a Microelectrode Array-Based Assay for Neural Network Ontogeny Using Training Set Chemicals. Toxicol. Sci. 2016, 154, 126–139. [Google Scholar] [CrossRef]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef]
- Khan, K.M.; Collier, A.D.; Meshalkina, D.A.; Kysil, E.V.; Khatsko, S.L.; Kolesnikova, T.; Morzherin, Y.Y.; Warnick, J.E.; Kalueff, A.V.; Echevarria, D.J. Zebrafish models in neuropsychopharmacology and CNS drug discovery. Br. J. Pharmacol. 2017, 174, 1925–1944. [Google Scholar] [CrossRef]
- Nishimura, Y.; Murakami, S.; Ashikawa, Y.; Sasagawa, S.; Umemoto, N.; Shimada, Y.; Tanaka, T. Zebrafish as a systems toxicology model for developmental neurotoxicity testing. Congenit. Anom. 2015, 55, 1–16. [Google Scholar] [CrossRef]
- Padilla, S.; Hunter, D.L.; Padnos, B.; Frady, S.; MacPhail, R.C. Assessing locomotor activity in larval zebrafish: Influence of extrinsic and intrinsic variables. Neurotoxicol. Teratol. 2011, 33, 624–630. [Google Scholar] [CrossRef]
- Quevedo, C.; Behl, M.; Ryan, K.; Paules, R.S.; Alday, A.; Muriana, A.; Alzualde, A. Detection and Prioritization of Developmentally Neurotoxic and/or Neurotoxic Compounds Using Zebrafish. Toxicol. Sci. 2019, 168, 225–240. [Google Scholar] [CrossRef]
- Hagstrom, D.; Truong, L.; Zhang, S.; Tanguay, R.; Collins, E.S. Comparative Analysis of Zebrafish and Planarian Model Systems for Developmental Neurotoxicity Screens Using an 87-Compound Library. Toxicol. Sci. 2019, 167, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Roell, K.R.; Truong, L.; Tanguay, R.L.; Reif, D.M. A data-driven weighting scheme for multivariate phenotypic endpoints recapitulates zebrafish developmental cascades. Toxicol. Appl. Pharmacol. 2017, 314, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Truong, L.; Reif, D.M.; St Mary, L.; Geier, M.C.; Truong, H.D.; Tanguay, R.L. Multidimensional in vivo hazard assessment using zebrafish. Toxicol. Sci. 2014, 137, 212–233. [Google Scholar] [CrossRef]
- Dach, K.; Yaghoobi, B.; Schmuck, M.R.; Carty, D.R.; Morales, K.M.; Lein, P.J. Teratological and Behavioral Screening of the National Toxicology Program 91-Compound Library in Zebrafish (Danio rerio). Toxicol. Sci. 2019, 167, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Pearce, R.G.; Setzer, R.W.; Strope, C.L.; Wambaugh, J.F.; Sipes, N.S. httk: R Package for High-Throughput Toxicokinetics. J. Stat. Softw. 2017, 79, 1–26. [Google Scholar] [CrossRef]
- Lactation, Institute of Medicine (US) Committee on Nutritional Status During Pregnancy and Lactation. Milk Volume. In Nutrition During Lactation; National Academies Press: Washington DC, USA, 1991. [Google Scholar]
- Hines, D.E.; Bell, S.; Chang, X.; Mansouri, K.; Allen, D.; Kleinstreuer, N. Application of an Accessible Interface for Pharmacokinetic Modeling and In Vitro to In Vivo Extrapolation. Front. Pharmacol. 2022, 13, 864742. [Google Scholar] [CrossRef]
- Cariou, R.; Antignac, J.P.; Zalko, D.; Berrebi, A.; Cravedi, J.P.; Maume, D.; Marchand, P.; Monteau, F.; Riu, A.; Andre, F.; et al. Exposure assessment of French women and their newborns to tetrabromobisphenol-A: Occurrence measurements in maternal adipose tissue, serum, breast milk and cord serum. Chemosphere 2008, 73, 1036–1041. [Google Scholar] [CrossRef] [PubMed]
- Pac-Kozuchowska, E.; Rakus-Kwiatosz, A.; Krawiec, P. Cord blood lipid profile in healthy newborns: A prospective single-center study. Adv. Clin. Exp. Med. 2018, 27, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Abedini, J.; Cook, B.; Bell, S.; Chang, X.; Choksi, N.; Daniel, A.B.; Hines, D.; Karmaus, A.L.; Mansouri, K.; McAfee, E.; et al. Application of new approach methodologies: ICE tools to support chemical evaluations. Comput. Toxicol. 2021, 20, 100184. [Google Scholar] [CrossRef]
- Daniel, A.B.; Choksi, N.; Abedini, J.; Bell, S.; Ceger, P.; Cook, B.; Karmaus, A.L.; Rooney, J.; To, K.T.; Allen, D.; et al. Data curation to support toxicity assessments using the Integrated Chemical Environment. Front. Toxicol. 2022, 4, 987848. [Google Scholar] [CrossRef] [PubMed]
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological profile for Phosphate Ester Flame Retardants; CDC: Atlanta, GA, USA, 2012. [Google Scholar]
- Patisaul, H.B.; Behl, M.; Birnbaum, L.S.; Blum, A.; Diamond, M.L.; Rojello Fernandez, S.; Hogberg, H.T.; Kwiatkowski, C.F.; Page, J.D.; Soehl, A.; et al. Beyond Cholinesterase Inhibition: Developmental Neurotoxicity of Organophosphate Ester Flame Retardants and Plasticizers. Environ. Health Perspect. 2021, 129, 105001. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.; Wiersielis, K.; Yasrebi, A.; Conde, K.; Armstrong, L.; Guo, G.L.; Roepke, T.A. Sex- and age-dependent effects of maternal organophosphate flame-retardant exposure on neonatal hypothalamic and hepatic gene expression. Reprod. Toxicol. 2020, 94, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Wiersielis, K.R.; Adams, S.; Yasrebi, A.; Conde, K.; Roepke, T.A. Maternal exposure to organophosphate flame retardants alters locomotor and anxiety-like behavior in male and female adult offspring. Horm. Behav. 2020, 122, 104759. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhao, X.; Wang, Y.; Hong, J.; Shi, M.; Pfaff, D.; Guo, L.; Tang, H. Triphenyl phosphate permeates the blood brain barrier and induces neurotoxicity in mouse brain. Chemosphere 2020, 252, 126470. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Zhao, F.; Fang, Y.; Li, L.; Li, C.; Ta, N. (1)H-nuclear magnetic resonance metabolomics revealing the intrinsic relationships between neurochemical alterations and neurobehavioral and neuropathological abnormalities in rats exposed to tris(2-chloroethyl)phosphate. Chemosphere 2018, 200, 649–659. [Google Scholar] [CrossRef]
- Bal-Price, A.; Pistollato, F.; Sachana, M.; Bopp, S.K.; Munn, S.; Worth, A. Strategies to improve the regulatory assessment of developmental neurotoxicity (DNT) using in vitro methods. Toxicol. Appl. Pharmacol. 2018, 354, 7–18. [Google Scholar] [CrossRef]
- OECD. Case Study for the Integration of In Vitro Data in the Developmental Neurotoxicity Hazard Identification and Characterisation Using Deltamethrin as a Prototype Chemical; OECD: Paris, France, 2022. [Google Scholar]
- Dobreniecki, S.; Mendez, E.; Lowit, A.; Freudenrich, T.M.; Wallace, K.; Carpenter, A.; Wetmore, B.A.; Kreutz, A.; Korol-Bexell, E.; Friedman, K.P.; et al. Integration of toxicodynamic and toxicokinetic new approach methods into a weight-of-evidence analysis for pesticide developmental neurotoxicity assessment: A case-study with DL- and L-glufosinate. Regul. Toxicol. Pharmacol. 2022, 131, 105167. [Google Scholar] [CrossRef]
- Zheng, K.; Zeng, Z.; Lin, Y.; Wang, Q.; Tian, Q.; Huo, X. Current status of indoor dust PBDE pollution and its physical burden and health effects on children. Environ. Sci. Pollut. Res. Int. 2023, 30, 19642–19661. [Google Scholar] [CrossRef] [PubMed]
- Lam, J.; Lanphear, B.P.; Bellinger, D.; Axelrad, D.A.; McPartland, J.; Sutton, P.; Davidson, L.; Daniels, N.; Sen, S.; Woodruff, T.J. Developmental PBDE Exposure and IQ/ADHD in Childhood: A Systematic Review and Meta-analysis. Environ. Health Perspect. 2017, 125, 086001. [Google Scholar] [CrossRef] [PubMed]
- Gomes, J.; Begum, M.; Kumarathasan, P. Polybrominated diphenyl ether (PBDE) exposure and adverse maternal and infant health outcomes: Systematic review. Chemosphere 2024, 347, 140367. [Google Scholar] [CrossRef]
- Villapol, S. Roles of Peroxisome Proliferator-Activated Receptor Gamma on Brain and Peripheral Inflammation. Cell Mol. Neurobiol. 2018, 38, 121–132. [Google Scholar] [CrossRef]
- Eriksson, P.S.; Perfilieva, E.; Bjork-Eriksson, T.; Alborn, A.M.; Nordborg, C.; Peterson, D.A.; Gage, F.H. Neurogenesis in the adult human hippocampus. Nat. Med. 1998, 4, 1313–1317. [Google Scholar] [CrossRef] [PubMed]
- Roth, A.D.; Leisewitz, A.V.; Jung, J.E.; Cassina, P.; Barbeito, L.; Inestrosa, N.C.; Bronfman, M. PPAR gamma activators induce growth arrest and process extension in B12 oligodendrocyte-like cells and terminal differentiation of cultured oligodendrocytes. J. Neurosci. Res. 2003, 72, 425–435. [Google Scholar] [CrossRef]
- Lupu, D.; Andersson, P.; Bornehag, C.G.; Demeneix, B.; Fritsche, E.; Gennings, C.; Lichtensteiger, W.; Leist, M.; Leonards, P.E.G.; Ponsonby, A.L.; et al. The ENDpoiNTs Project: Novel Testing Strategies for Endocrine Disruptors Linked to Developmental Neurotoxicity. Int. J. Mol. Sci. 2020, 21, 3978. [Google Scholar] [CrossRef]
- Klose, J.; Tigges, J.; Masjosthusmann, S.; Schmuck, K.; Bendt, F.; Hubenthal, U.; Petzsch, P.; Kohrer, K.; Koch, K.; Fritsche, E. TBBPA targets converging key events of human oligodendrocyte development resulting in two novel AOPs. ALTEX 2021, 38, 215–234. [Google Scholar] [CrossRef]
- Baker, A.D.; Malur, A.; Barna, B.P.; Kavuru, M.S.; Malur, A.G.; Thomassen, M.J. PPARgamma regulates the expression of cholesterol metabolism genes in alveolar macrophages. Biochem. Biophys. Res. Commun. 2010, 393, 682–687. [Google Scholar] [CrossRef]
- Hirte, S.; Burk, O.; Tahir, A.; Schwab, M.; Windshugel, B.; Kirchmair, J. Development and Experimental Validation of Regularized Machine Learning Models Detecting New, Structurally Distinct Activators of PXR. Cells 2022, 11, 1253. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Xu, W.; Peng, T.; Chen, H.; Ren, L.; Tan, H.; Xiao, D.; Qian, H.; Fu, Z. Developmental exposure of zebrafish larvae to organophosphate flame retardants causes neurotoxicity. Neurotoxicol. Teratol. 2016, 55, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Baas, D.; Bourbeau, D.; Sarlieve, L.L.; Ittel, M.E.; Dussault, J.H.; Puymirat, J. Oligodendrocyte maturation and progenitor cell proliferation are independently regulated by thyroid hormone. Glia 1997, 19, 324–332. [Google Scholar] [CrossRef]
- Balazs, R.; Brooksbank, B.W.; Davison, A.N.; Eayrs, J.T.; Wilson, D.A. The effect of neonatal thyroidectomy on myelination in the rat brain. Brain Res. 1969, 15, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Oliveri, A.N.; Bailey, J.M.; Levin, E.D. Developmental exposure to organophosphate flame retardants causes behavioral effects in larval and adult zebrafish. Neurotoxicol. Teratol. 2015, 52, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Glazer, L.; Hawkey, A.B.; Wells, C.N.; Drastal, M.; Odamah, K.A.; Behl, M.; Levin, E.D. Developmental Exposure to Low Concentrations of Organophosphate Flame Retardants Causes Life-Long Behavioral Alterations in Zebrafish. Toxicol. Sci. 2018, 165, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Rone, M.B.; Fan, J.; Papadopoulos, V. Cholesterol transport in steroid biosynthesis: Role of protein-protein interactions and implications in disease states. Biochim. Biophys. Acta 2009, 1791, 646–658. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, V.; Lecanu, L.; Brown, R.C.; Han, Z.; Yao, Z.X. Peripheral-type benzodiazepine receptor in neurosteroid biosynthesis, neuropathology and neurological disorders. Neuroscience 2006, 138, 749–756. [Google Scholar] [CrossRef]
- Liu, X.; Ji, K.; Choi, K. Endocrine disruption potentials of organophosphate flame retardants and related mechanisms in H295R and MVLN cell lines and in zebrafish. Aquat. Toxicol. 2012, 114–115, 173–181. [Google Scholar] [CrossRef]
- Chen, C.; Kuo, J.; Wong, A.; Micevych, P. Estradiol modulates translocator protein (TSPO) and steroid acute regulatory protein (StAR) via protein kinase A (PKA) signaling in hypothalamic astrocytes. Endocrinology 2014, 155, 2976–2985. [Google Scholar] [CrossRef]
- Beattie, R.; Hippenmeyer, S. Mechanisms of radial glia progenitor cell lineage progression. FEBS Lett. 2017, 591, 3993–4008. [Google Scholar] [CrossRef] [PubMed]
- Bayraktar, O.A.; Fuentealba, L.C.; Alvarez-Buylla, A.; Rowitch, D.H. Astrocyte development and heterogeneity. Cold Spring Harb. Perspect. Biol. 2014, 7, a020362. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Adhikari, N.; Banerjee, S.; Amin, S.A.; Jha, T. Matrix metalloproteinase-9 (MMP-9) and its inhibitors in cancer: A minireview. Eur. J. Med. Chem. 2020, 194, 112260. [Google Scholar] [CrossRef] [PubMed]
- Vafadari, B.; Salamian, A.; Kaczmarek, L. MMP-9 in translation: From molecule to brain physiology, pathology, and therapy. J. Neurochem. 2016, 139 (Suppl. S2), 91–114. [Google Scholar] [CrossRef] [PubMed]
- Vandooren, J.; Van Damme, J.; Opdenakker, G. On the structure and functions of gelatinase B/matrix metalloproteinase-9 in neuroinflammation. Prog. Brain Res. 2014, 214, 193–206. [Google Scholar] [CrossRef] [PubMed]
- Vorkamp, K.; Balmer, J.; Hung, H.; Letcher, R.J.; Rigét, F.F.; de Wit, C.A. Current-use halogenated and organophosphorous flame retardants: A review of their presence in Arctic ecosystems. Emerg. Contam. 2019, 5, 179–200. [Google Scholar] [CrossRef]
- Gibson, E.A.; Stapleton, H.M.; Calero, L.; Holmes, D.; Burke, K.; Martinez, R.; Cortes, B.; Nematollahi, A.; Evans, D.; Anderson, K.A.; et al. Differential exposure to organophosphate flame retardants in mother-child pairs. Chemosphere 2019, 219, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Li, M.; Pan, L.; Duan, Y.; Duan, X.; Li, Y.; Sun, H. Exposure to organophosphate ester flame retardants and plasticizers during pregnancy: Thyroid endocrine disruption and mediation role of oxidative stress. Environ. Int. 2021, 146, 106215. [Google Scholar] [CrossRef] [PubMed]
- Kincaid, B.; Piechota, P.; Golden, E.; Maertens, M.; Hartung, T.; Maertens, A. Using in silico tools to predict flame retardant metabolites for more informative exposomics-based approaches. Front. Toxicol. 2023, 5, 1216802. [Google Scholar] [CrossRef]
- Commission, E.; Centre, J.R.; Dura, A.; Zuang, V. EURL ECVAM Status Report on the Development, Validation and Regulatory Acceptance of Alternative Methods and Approaches (2019); JRC Publications Repository: Geneva, Switzerland, 2020. [Google Scholar]
- Doherty, B.T.; Hammel, S.C.; Daniels, J.L.; Stapleton, H.M.; Hoffman, K. Organophosphate Esters: Are These Flame Retardants and Plasticizers Affecting Children’s Health? Curr. Environ. Health Rep. 2019, 6, 201–213. [Google Scholar] [CrossRef]
- Doherty, B.T.; Hoffman, K.; Keil, A.P.; Engel, S.M.; Stapleton, H.M.; Goldman, B.D.; Olshan, A.F.; Daniels, J.L. Prenatal exposure to organophosphate esters and behavioral development in young children in the Pregnancy, Infection, and Nutrition Study. Neurotoxicology 2019, 73, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Castorina, R.; Bradman, A.; Stapleton, H.M.; Butt, C.; Avery, D.; Harley, K.G.; Gunier, R.B.; Holland, N.; Eskenazi, B. Current-use flame retardants: Maternal exposure and neurodevelopment in children of the CHAMACOS cohort. Chemosphere 2017, 189, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Sugeng, E.J.; Leonards, P.E.G.; van de Bor, M. Brominated and organophosphorus flame retardants in body wipes and house dust, and an estimation of house dust hand-loadings in Dutch toddlers. Environ. Res. 2017, 158, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Lipscomb, S.T.; McClelland, M.M.; MacDonald, M.; Cardenas, A.; Anderson, K.A.; Kile, M.L. Cross-sectional study of social behaviors in preschool children and exposure to flame retardants. Environ. Health 2017, 16, 23. [Google Scholar] [CrossRef] [PubMed]
- Luo, K.; Liu, J.; Wang, Y.; Aimuzi, R.; Luo, F.; Ao, J.; Zhang, J. Associations between organophosphate esters and sex hormones among 6–19-year old children and adolescents in NHANES 2013-2014. Environ. Int. 2020, 136, 105461. [Google Scholar] [CrossRef] [PubMed]
- Blum, J.; Masjosthusmann, S.; Bartmann, K.; Bendt, F.; Dolde, X.; Donmez, A.; Forster, N.; Holzer, A.K.; Hubenthal, U.; Kessel, H.E.; et al. Establishment of a human cell-based in vitro battery to assess developmental neurotoxicity hazard of chemicals. Chemosphere 2023, 311, 137035. [Google Scholar] [CrossRef] [PubMed]
- Dishaw, L.V.; Powers, C.M.; Ryde, I.T.; Roberts, S.C.; Seidler, F.J.; Slotkin, T.A.; Stapleton, H.M. Is the PentaBDE replacement, tris (1,3-dichloro-2-propyl) phosphate (TDCPP), a developmental neurotoxicant? Studies in PC12 cells. Toxicol. Appl. Pharmacol. 2011, 256, 281–289. [Google Scholar] [CrossRef]
- Dishaw, L.V.; Hunter, D.L.; Padnos, B.; Padilla, S.; Stapleton, H.M. Developmental exposure to organophosphate flame retardants elicits overt toxicity and alters behavior in early life stage zebrafish (Danio rerio). Toxicol. Sci. 2014, 142, 445–454. [Google Scholar] [CrossRef]
- Fang, M.; Webster, T.F.; Stapleton, H.M. Activation of Human Peroxisome Proliferator-Activated Nuclear Receptors (PPARgamma1) by Semi-Volatile Compounds (SVOCs) and Chemical Mixtures in Indoor Dust. Environ. Sci. Technol. 2015, 49, 10057–10064. [Google Scholar] [CrossRef]
- Hausherr, V.; van Thriel, C.; Krug, A.; Leist, M.; Schobel, N. Impairment of glutamate signaling in mouse central nervous system neurons in vitro by tri-ortho-cresyl phosphate at noncytotoxic concentrations. Toxicol. Sci. 2014, 142, 274–284. [Google Scholar] [CrossRef]
- Hogberg, H.T.; de Cassia da Silveira, E.S.R.; Kleensang, A.; Bouhifd, M.; Cemiloglu Ulker, O.; Smirnova, L.; Behl, M.; Maertens, A.; Zhao, L.; Hartung, T. Organophosphorus flame retardants are developmental neurotoxicants in a rat primary brainsphere in vitro model. Arch. Toxicol. 2021, 95, 207–228. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Jung, J.; Lee, I.; Jung, D.; Youn, H.; Choi, K. Thyroid disruption by triphenyl phosphate, an organophosphate flame retardant, in zebrafish (Danio rerio) embryos/larvae, and in GH3 and FRTL-5 cell lines. Aquat. Toxicol. 2015, 160, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Kojima, H.; Takeuchi, S.; Itoh, T.; Iida, M.; Kobayashi, S.; Yoshida, T. In vitro endocrine disruption potential of organophosphate flame retardants via human nuclear receptors. Toxicology 2013, 314, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Guo, W.; Lei, L.; Zhang, L.; Liu, Y.; Han, J.; Chen, L.; Zhou, B. Early-life exposure to the organophosphorus flame-retardant tris (1,3-dichloro-2-propyl) phosphate induces delayed neurotoxicity associated with DNA methylation in adult zebrafish. Environ. Int. 2020, 134, 105293. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.; Peng, J.; Behl, M.; Sipes, N.S.; Shockley, K.R.; Rao, M.S.; Tice, R.R.; Zeng, X. Comparative neurotoxicity screening in human iPSC-derived neural stem cells, neurons and astrocytes. Brain Res. 2016, 1638, 57–73. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Wang, M.; Shi, F.; Yang, L.; Guo, Y.; Feng, C.; Liu, J.; Zhou, B. Developmental neurotoxicity of triphenyl phosphate in zebrafish larvae. Aquat. Toxicol. 2018, 203, 80–87. [Google Scholar] [CrossRef]
- Slotkin, T.A.; Skavicus, S.; Stapleton, H.M.; Seidler, F.J. Brominated and organophosphate flame retardants target different neurodevelopmental stages, characterized with embryonic neural stem cells and neuronotypic PC12 cells. Toxicology 2017, 390, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Tan, H.; Peng, T.; Wang, S.; Xu, W.; Qian, H.; Jin, Y.; Fu, Z. Developmental neurotoxicity of organophosphate flame retardants in early life stages of Japanese medaka (Oryzias latipes). Environ. Toxicol. Chem. 2016, 35, 2931–2940. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Ji, G.; Liu, J.; Zhang, S.; Gong, Y.; Shi, L. TBBPA induces developmental toxicity, oxidative stress, and apoptosis in embryos and zebrafish larvae (Danio rerio). Environ. Toxicol. 2016, 31, 1241–1249. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, X.; Shi, Z. Organophosphorus flame retardants in breast milk from Beijing, China: Occurrence, nursing infant’s exposure and risk assessment. Sci. Total Environ. 2021, 771, 145404. [Google Scholar] [CrossRef]
- Hammel, S.C.; Phillips, A.L.; Hoffman, K.; Stapleton, H.M. Evaluating the Use of Silicone Wristbands To Measure Personal Exposure to Brominated Flame Retardants. Environ. Sci. Technol. 2018, 52, 11875–11885. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Isobe, T.; Muto, M.; Tue, N.M.; Katsura, K.; Malarvannan, G.; Sudaryanto, A.; Chang, K.H.; Prudente, M.; Viet, P.H.; et al. Organophosphorus flame retardants (PFRs) in human breast milk from several Asian countries. Chemosphere 2014, 116, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.K.; Kang, H.; Lee, S.; Kim, S.; Choi, K.; Moon, H.B. Human exposure to legacy and emerging flame retardants in indoor dust: A multiple-exposure assessment of PBDEs. Sci. Total Environ. 2020, 719, 137386. [Google Scholar] [CrossRef] [PubMed]
- Phillips, A.L.; Hammel, S.C.; Hoffman, K.; Lorenzo, A.M.; Chen, A.; Webster, T.F.; Stapleton, H.M. Children’s residential exposure to organophosphate ester flame retardants and plasticizers: Investigating exposure pathways in the TESIE study. Environ. Int. 2018, 116, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, H.M.; Eagle, S.; Sjodin, A.; Webster, T.F. Serum PBDEs in a North Carolina toddler cohort: Associations with handwipes, house dust, and socioeconomic variables. Environ. Health Perspect. 2012, 120, 1049–1054. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Harris, S.A.; Jantunen, L.M.; Siddique, S.; Kubwabo, C.; Tsirlin, D.; Latifovic, L.; Fraser, B.; St-Jean, M.; De La Campa, R.; et al. Are cell phones an indicator of personal exposure to organophosphate flame retardants and plasticizers? Environ. Int. 2019, 122, 104–116. [Google Scholar] [CrossRef]
- Yang, W.; Braun, J.M.; Vuong, A.M.; Percy, Z.; Xu, Y.; Xie, C.; Deka, R.; Calafat, A.M.; Ospina, M.; Burris, H.H.; et al. Associations of gestational exposure to organophosphate esters with gestational age and neonatal anthropometric measures: The HOME study. Environ. Pollut. 2023, 316, 120516. [Google Scholar] [CrossRef]


| CAS | Chemical Name | Chemical ID |
|---|---|---|
| 5436-43-1 | 2,2′4,4′-Tetrabromodiphenyl ether | BDE-47 1 |
| 79-94-7 | 3,3′,5,5′-Tetrabromobisphenol A | TBBPA |
| 115-86-6 | Triphenyl phosphate | TPHP |
| 68937-41-7 | Phenol, isopropylated, phosphate (3:1) | IPP 2,3 |
| 1241-94-7 | 2-Ethylhexyl diphenyl phosphate | EHDP 2 |
| 1330-78-5 | Tricresyl phosphate | TMPP 2 |
| 29761-21-5 | Isodecyl diphenyl phosphate | IDDP 3 |
| 56803-37-3 | tert-Butylphenyl diphenyl phosphate | BPDP 2 |
| 13674-87-8 | Tris(1,3-dichloro-2-propyl) phosphate | TDCIPP |
| 115-96-8 | Tris(2-chloroethyl) phosphate | TCEP 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kreutz, A.; Oyetade, O.B.; Chang, X.; Hsieh, J.-H.; Behl, M.; Allen, D.G.; Kleinstreuer, N.C.; Hogberg, H.T. Integrated Approach for Testing and Assessment for Developmental Neurotoxicity (DNT) to Prioritize Aromatic Organophosphorus Flame Retardants. Toxics 2024, 12, 437. https://doi.org/10.3390/toxics12060437
Kreutz A, Oyetade OB, Chang X, Hsieh J-H, Behl M, Allen DG, Kleinstreuer NC, Hogberg HT. Integrated Approach for Testing and Assessment for Developmental Neurotoxicity (DNT) to Prioritize Aromatic Organophosphorus Flame Retardants. Toxics. 2024; 12(6):437. https://doi.org/10.3390/toxics12060437
Chicago/Turabian StyleKreutz, Anna, Oluwakemi B. Oyetade, Xiaoqing Chang, Jui-Hua Hsieh, Mamta Behl, David G. Allen, Nicole C. Kleinstreuer, and Helena T. Hogberg. 2024. "Integrated Approach for Testing and Assessment for Developmental Neurotoxicity (DNT) to Prioritize Aromatic Organophosphorus Flame Retardants" Toxics 12, no. 6: 437. https://doi.org/10.3390/toxics12060437






