Ketoprofen Photodegradation Kinetics Promoted by TiO2
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Apparatus and Procedure
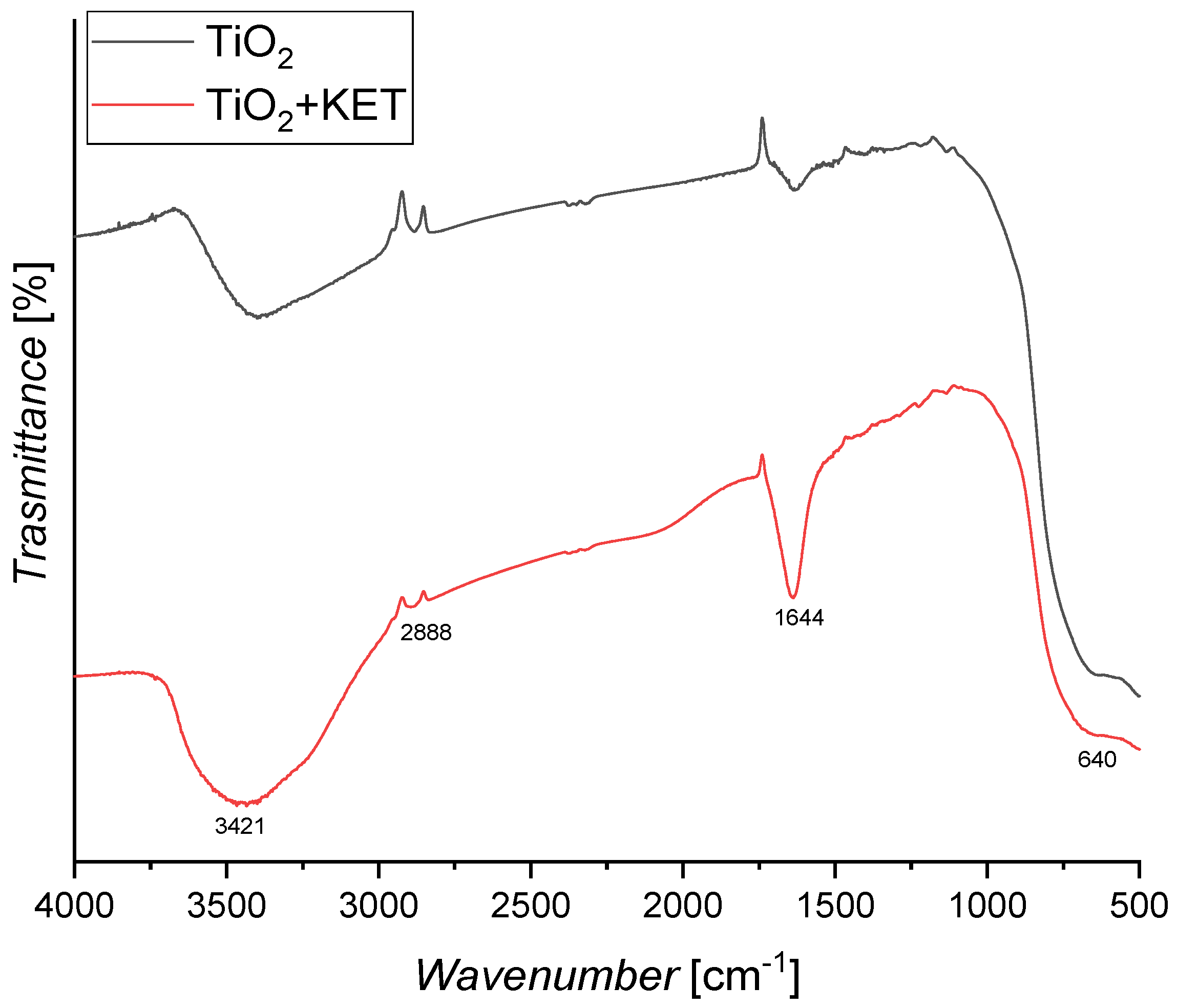
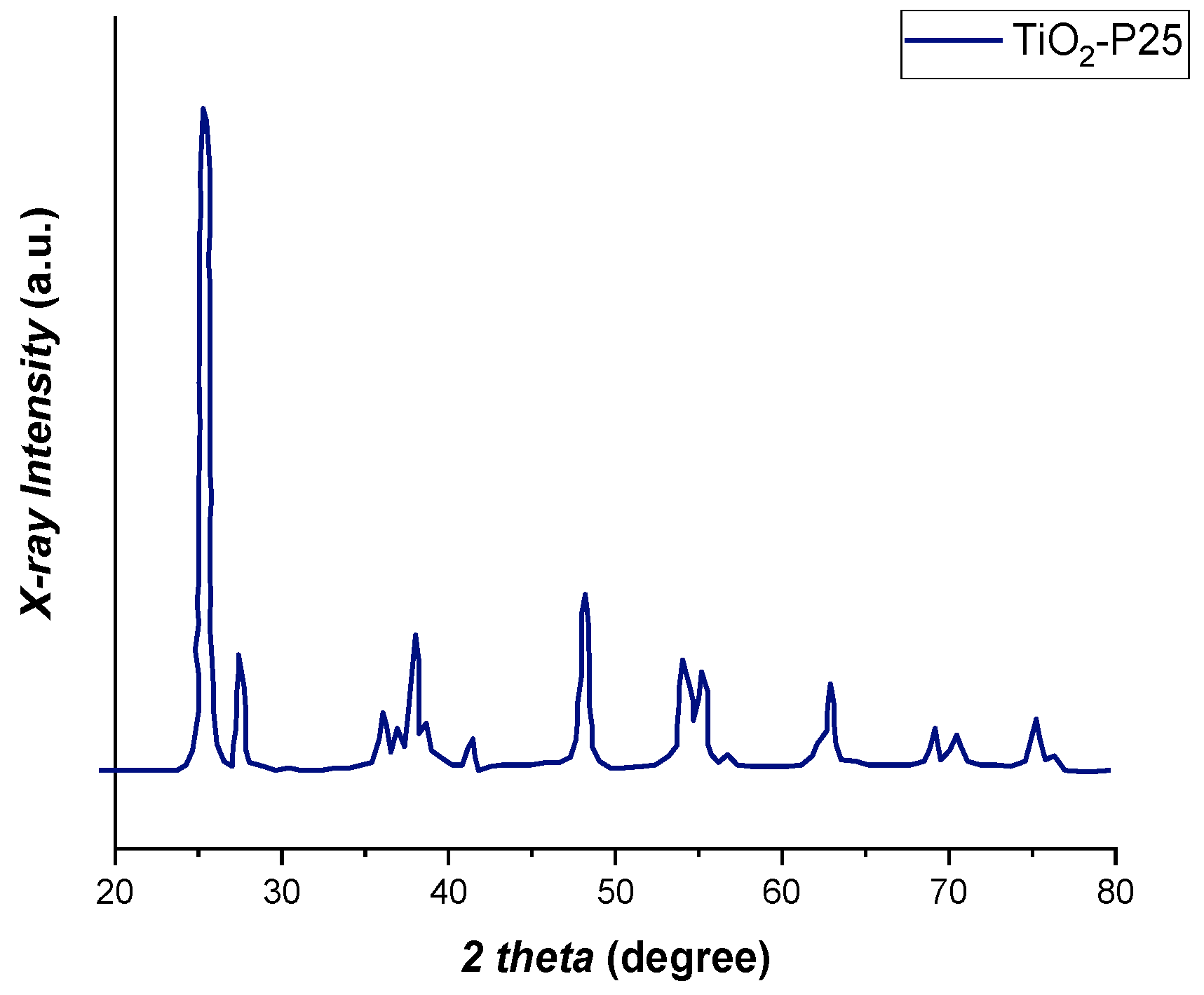
2.3. Catalyst Characterization
2.4. Mass Balances and Numerical Methods
3. Results and Discussion
3.1. Results of the Kinetic Investigation
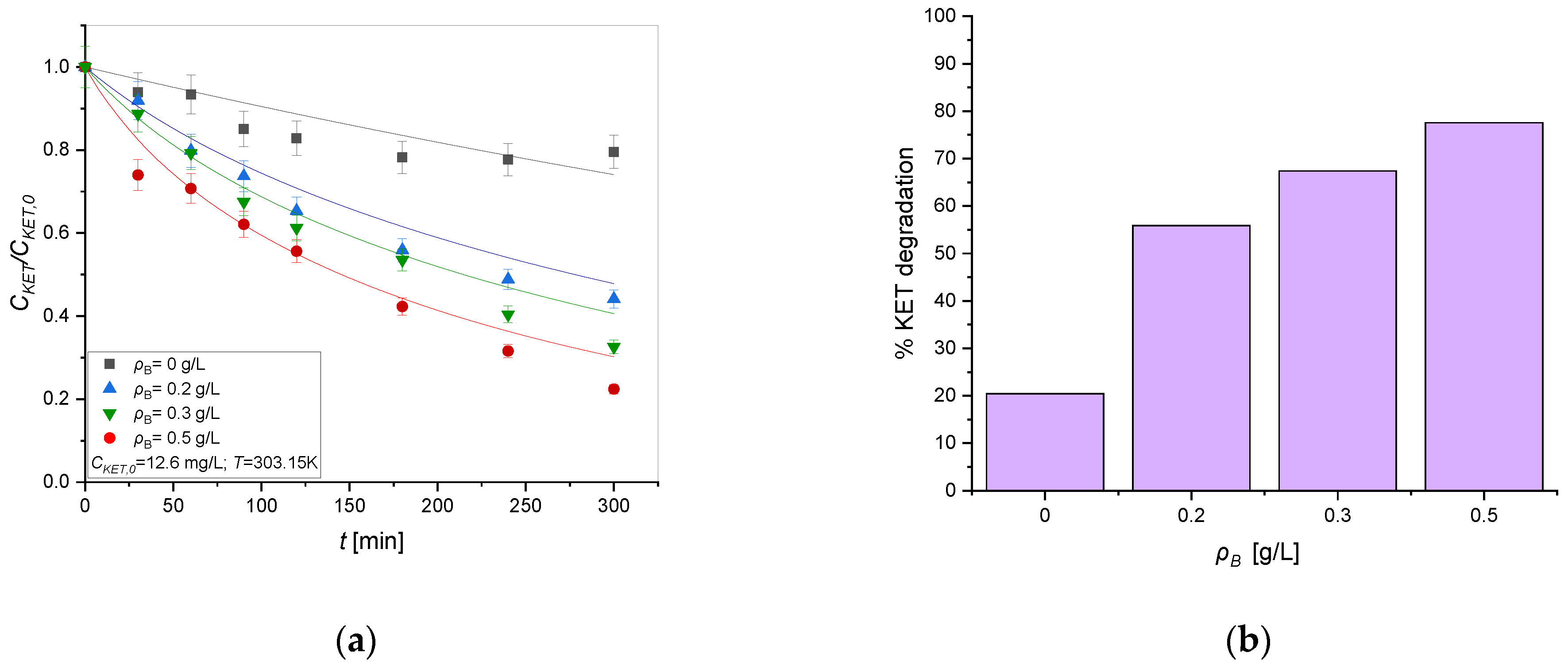
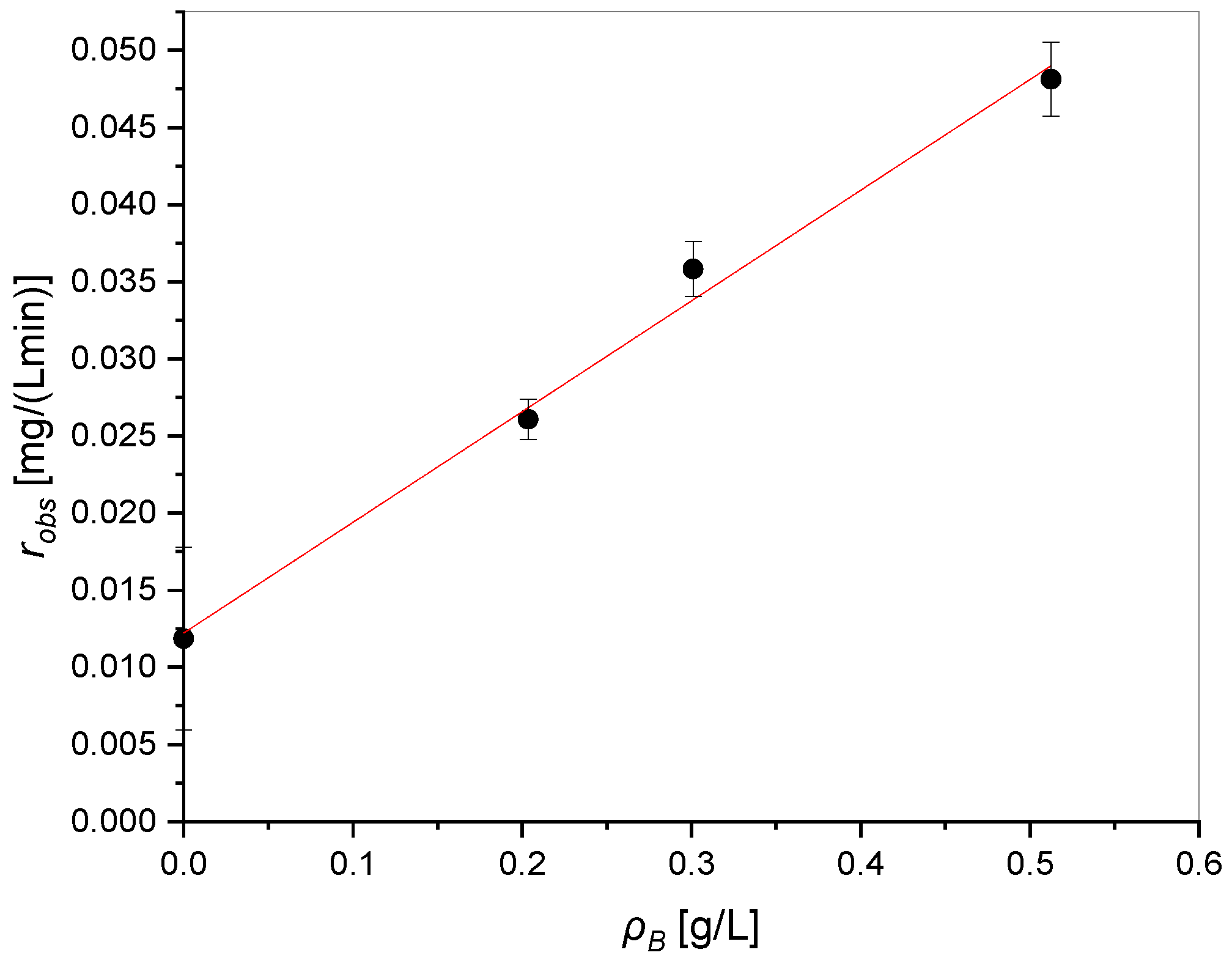
3.1.1. Light Source and Catalyst Effect on the Photodegradation Process
3.1.2. Photodegradation Using UV Light and TiO2
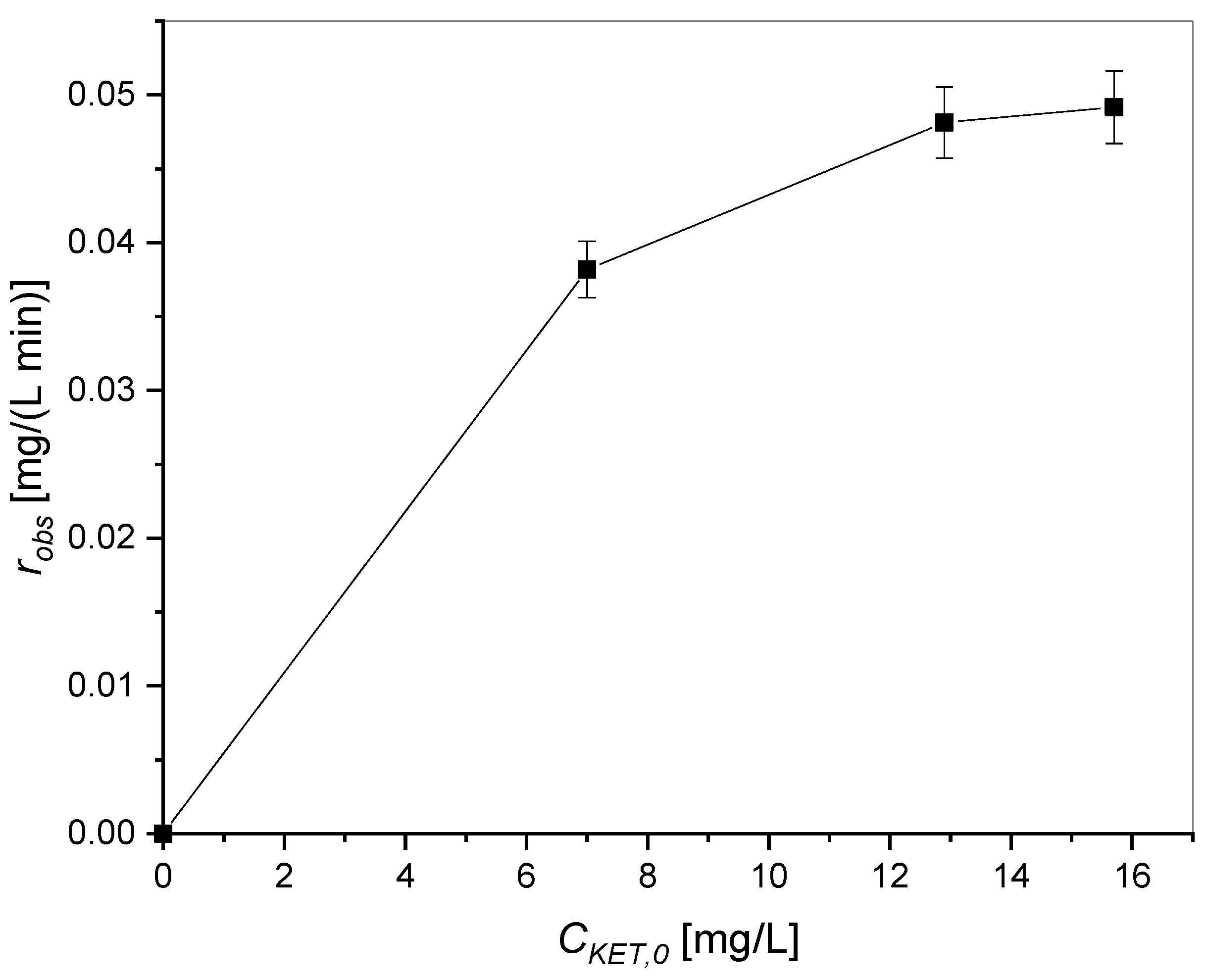
3.1.3. Effect of Initial Concentration of Ketoprofen Using TiO2 and UV Radiation
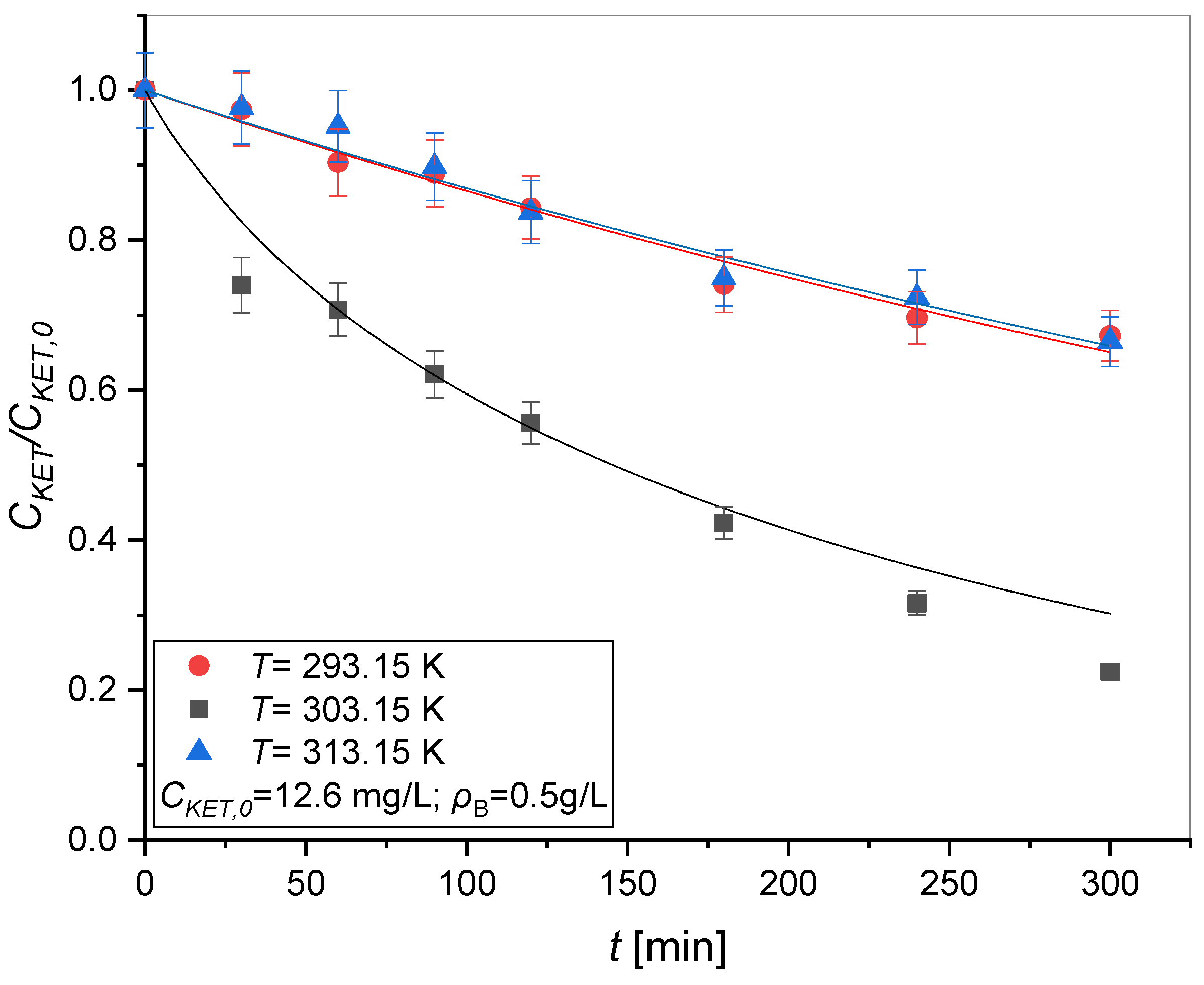
3.1.4. Effect of Temperature Using TiO2 and UV Radiation
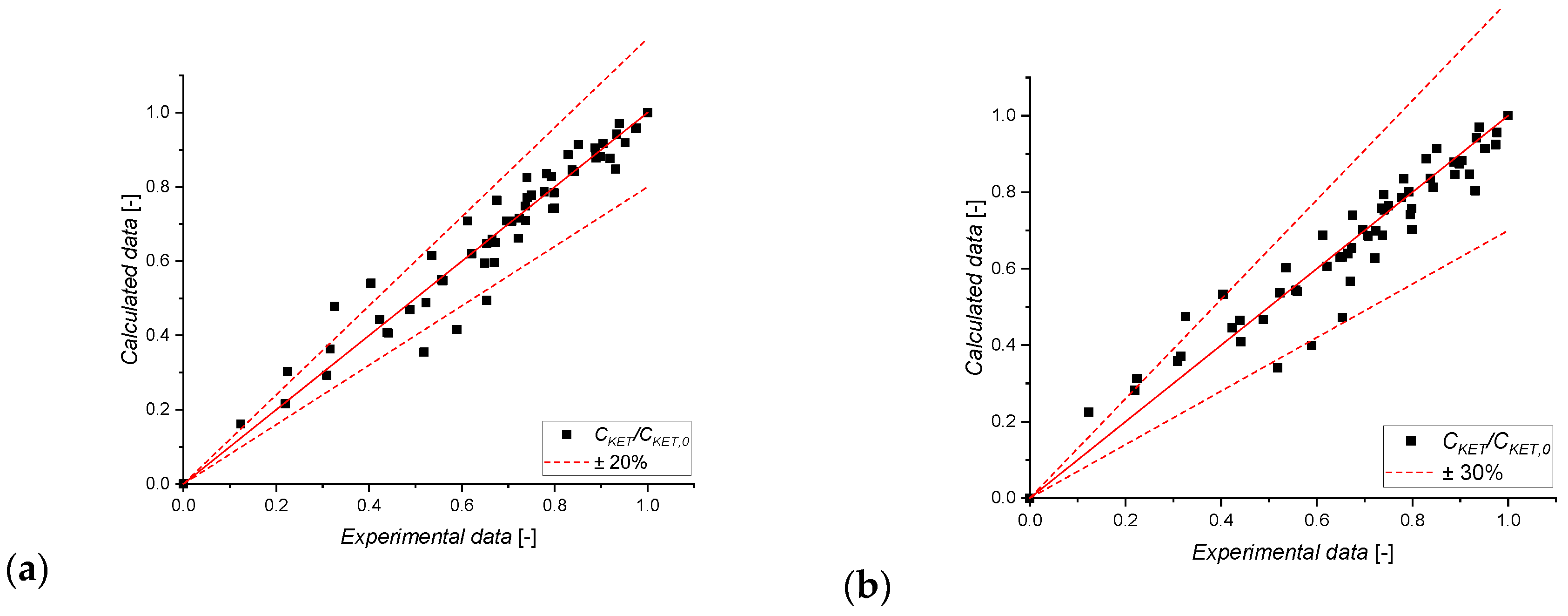
3.2. Kinetic Modeling Results
Preliminary Evaluations
3.3. Kinetic Data Modeling
3.4. Results of Catalyst Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
List of Symbols
| Ci | concentration of component i [mg/L] |
| Ci,0 | initial concentration of component i [mg/L] |
| Ea | activation energy [kJ/mol] |
| Ki | adsorption constant for component i [L/mg] |
| Ki,ref | adsorption constant for component i at the reference temperature [L/mg] |
| k0 | pre-exponential factor [mg/(L min)] |
| k′ | photodegradation kinetic constant [mg/(L min)] |
| k′ref | photodegradation kinetic constant at the reference temperature [mg/(L min)] |
| k″, k‴, kIV | Apparent kinetic constants, units depend on reaction |
| kj | kinetic constant for reaction j, units depend on reaction |
| kj,ref | kinetic constant for reaction j at the reference temperature, units depend on reaction |
| kobs | observed kinetic constant [min−1] |
| kp | kinetic constant of photolysis reaction [min−1] |
| kp,ref | photolysis adsorption constant at reference temperature [min−1] |
| mCAT | mass of the catalyst [g] |
| Q | flow rate [mL/min] |
| R | ideal gas constant [kJ/(K mol)] |
| robs | observed reaction rate [mg/(L min)] |
| r | photodegradation reaction rate [mg/(g min)] |
| rp | photolysis reaction rate [mg/(L min)] |
| T | temperature [K] |
| t | time [min] |
| Tref | reference temperature, 303.15 [K] |
| v | Impeller stirring rate [rpm] |
| VL | liquid volume [L] |
| Greek symbols | |
| α, β | lumped parameters |
| ΔH | adsorption enthalpy change [kJ/mol] |
| θi | fraction of the active sites occupied by the chemical compound i [-] |
| ρB | catalyst bulk density [g/L] |
| vi | stechiometric coefficient of component i [-] |
| Abbreviations | |
| AOP | Advanced oxidation process |
| ECs | Emerging contaminants |
| E-R | Eley–Rideal |
| KET | Ketoprofen |
| L-H | Langmuir–Hinshelwood |
| P | Generic photodegradation by-product |
| PhACs | Pharmaceutically active compounds |
| WWTPs | Wastewater treatment plants |
References
- Papageorgiou, M.; Zioris, I.; Danis, T.; Bikiaris, D.; Lambropoulou, D. Comprehensive Investigation of a Wide Range of Pharmaceuticals and Personal Care Products in Urban and Hospital Wastewaters in Greece. Sci. Total Environ. 2019, 694, 133565. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, I.; Thurman, E.M. Chapter 4—Analysis of Pharmaceuticals in Drinking Water, Groundwater, Surface Water, and Wastewater. In Comprehensive Analytical Chemistry; Petrovic, M., Barcelo, D., Pérez, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 62, pp. 91–128. [Google Scholar] [CrossRef]
- Eniola, J.O.; Kumar, R.; Barakat, M.A.; Rashid, J. A Review on Conventional and Advanced Hybrid Technologies for Pharmaceutical Wastewater Treatment. J. Clean. Prod. 2022, 356, 131826. [Google Scholar] [CrossRef]
- Madi-Azegagh, K.; Yahiaoui, I.; Boudrahem, F.; Aissani-Benissad, F.; Vial, C.; Audonnet, F.; Favier, L. Applied of Central Composite Design for the Optimization of Removal Yield of the Ketoprofen (KTP) Using Electrocoagulation Process. Sep. Sci. Technol. 2019, 54, 3115–3127. [Google Scholar] [CrossRef]
- Yurdakal, S.; Loddo, V.; Augugliaro, V.; Berber, H.; Palmisano, G.; Palmisano, L. Photodegradation of Pharmaceutical Drugs in Aqueous TiO2 Suspensions: Mechanism and Kinetics. Catal. Today 2007, 129, 9–15. [Google Scholar] [CrossRef]
- Hunge, Y.M.; Yadav, A.A.; Kang, S.-W.; Mohite, B.M. Role of Nanotechnology in Photocatalysis Application. Recent Pat. Nanotechnol. 2023, 17, 5–7. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.A.; Hunge, Y.M.; Mathe, V.L.; Kulkarni, S.B. Photocatalytic Degradation of Salicylic Acid Using BaTiO3 Photocatalyst under Ultraviolet Light Illumination. J. Mater. Sci. Mater. Electron. 2018, 29, 15069–15073. [Google Scholar] [CrossRef]
- Yu, Y.; Yang, Q.; Wang, Z.; Ding, Q.; Li, M.; Fang, Y.; He, Q.; Zhu, Y.Z. The Anti-Inflammation and Anti-Nociception Effect of Ketoprofen in Rats Could Be Strengthened through Co-Delivery of a H2S Donor, S-Propargyl-Cysteine. J. Inflamm. Res. 2021, 14, 5863–5875. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, S.; Zhang, M.; He, B. Targeted Eco-Pharmacovigilance for Ketoprofen in the Environment: Need, Strategy and Challenge. Chemosphere 2018, 194, 450–462. [Google Scholar] [CrossRef]
- Feng, L.; van Hullebusch, E.D.; Rodrigo, M.A.; Esposito, G.; Oturan, M.A. Removal of Residual Anti-Inflammatory and Analgesic Pharmaceuticals from Aqueous Systems by Electrochemical Advanced Oxidation Processes. A review. Chem. Eng. J. 2013, 228, 944–964. [Google Scholar] [CrossRef]
- Onchoke, K.K.; Lopez, G.; Broom, A.M. Detection of Carbamazepine, Diclofenac, and Ketoprofen via a SPE-HPLC-PDA Method in a Municipal Wastewater Treatment Plant in East Texas, USA. Anal. Chem. Lett. 2023, 13, 127–140. [Google Scholar] [CrossRef]
- Jelic, A.; Gros, M.; Ginebreda, A.; Cespedes-Sánchez, R.; Ventura, F.; Petrovic, M.; Barcelo, D. Occurrence, Partition and Removal of Pharmaceuticals in Sewage Water and Sludge during Wastewater Treatment. Water Res. 2011, 45, 1165–1176. [Google Scholar] [CrossRef] [PubMed]
- Spongberg, A.L.; Witter, J.D.; Acuña, J.; Vargas, J.; Murillo, M.; Umaña, G.; Gómez, E.; Perez, G. Reconnaissance of Selected PPCP Compounds in Costa Rican Surface. Water Res. 2011, 45, 6709–6717. [Google Scholar] [CrossRef]
- Bendz, D.; Paxéus, N.A.; Ginn, T.R.; Loge, F.J. Occurrence and Fate of Pharmaceutically Active Compounds in the Environment, a Case Study: Höje River in Sweden. J. Hazard. Mater. 2005, 122, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Mantas, A.; Labbe, V.; Loryan, I.; Mihranyan, A. Amorphisation of Free Acid Ibuprofen and Other Profens in Mixtures with Nanocellulose: Dry Powder Formulation Strategy for Enhanced Solubility. Pharmaceutics 2019, 11, 68. [Google Scholar] [CrossRef]
- Albornoz Marin, S.L.; de Oliveira, S.C.; Peralta-Zamora, P. Photocatalytic Degradation of Phenol by Core–Shell Cu@TiO2 Nanostructures under Visible Radiation. J. Photochem. Photobiol. Chem. 2022, 433, 114129. [Google Scholar] [CrossRef]
- Hao, X.; Li, H.; Zhang, Z.; Fan, C.; Liu, S.; Sun, Y. Modeling and Experimentation of a Novel Labyrinth Bubble Photoreactor for Degradation of Organic Pollutant. Chem. Eng. Res. Des. 2009, 87, 1604–1611. [Google Scholar] [CrossRef]
- Mofokeng, L.E.; Hlekelele, L.; Tetana, Z.N.; Moma, J.; Chauke, V.P. CuO-doped TiO2 Supported on Graphitic Carbon Nitride for the Photodegradation of Ketoprofen in Drinking and Groundwater: Process Optimization and Energy Consumption Evaluation. ChemistrySelect 2022, 7, e202101847. [Google Scholar] [CrossRef]
- Djouadi, L.; Khalaf, H.; Boukhatem, H.; Boutoumi, H.; Kezzime, A.; Santaballa, J.A.; Canle, M. Degradation of Aqueous Ketoprofen by Heterogeneous Photocatalysis Using Bi2S3/TiO2–Montmorillonite Nanocomposites under Simulated Solar Irradiation. Appl. Clay Sci. 2018, 166, 27–37. [Google Scholar] [CrossRef]
- Mofokeng, L.E.; Hlekelele, L.; Moma, J.; Tetana, Z.N.; Chauke, V.P. Energy-Efficient CuO/TiO2@GCN Cellulose Acetate-Based Membrane for Concurrent Filtration and Photodegradation of Ketoprofen in Drinking and Groundwater. Appl. Sci. 2022, 12, 1649. [Google Scholar] [CrossRef]
- Suárez-Escobar, A.; Rodríguez-González, V.; Gallardo-Vega, C.; Sarria, E.; Clavijo, L. Ketoprofen Degradation by Surface Reactive Species on TiO2, AgxOy/MoxOy/Catalysts. Mater. Lett. 2021, 294, 129687. [Google Scholar] [CrossRef]
- Sun, J.; Feng, S.; Feng, S. Hydrothermally Synthesis of MWCNT/N-TiO2/UiO-66-NH2 Ternary Composite with Enhanced Photocatalytic Performance for Ketoprofen. Inorg. Chem. Commun. 2020, 111, 107669. [Google Scholar] [CrossRef]
- Piątkowska, A.; Szymański, K.; Mozia, S. Effect of Sulfur on the Solar Light Photoactivity of TiO2-Based Photocatalysts. Chem. Eng. Res. Des. 2023, 195, 721–731. [Google Scholar] [CrossRef]
- Piątkowska, A.; Moszyński, D.; Mozia, S. Enhanced Solar Light Photocatalytic Activity of TiO2 Modified with Ammonium Carbamate for the Removal of Ketoprofen from Water. J. Water Process Eng. 2023, 56, 104534. [Google Scholar] [CrossRef]
- Sacco, O.; Murcia, J.J.; Lara, A.E.; Hernández-Laverde, M.; Rojas, H.; Navío, J.A.; Hidalgo, M.C.; Vaiano, V. Pt–TiO2–Nb2O5 Heterojunction as Effective Photocatalyst for the Degradation of Diclofenac and Ketoprofen. Mater. Sci. Semicond. Process. 2020, 107, 104839. [Google Scholar] [CrossRef]
- Yetti, R.D.; Safni; Rivai, H. Photo Degradation of Ketoprofen Using Titanium Dioxide as Catalyst. IJRDO—J. Biol. Sci. 2017, 3, 30–45. [Google Scholar]
- Martínez, C.; Vilariño, S.; Fernández, M.I.; Faria, J.L.M.C.; Santaballa, J.A. Mechanism of Degradation of Ketoprofen by Heterogeneous Photocatalysis in Aqueous Solution. Appl. Catal. B Environ. 2013, 142–143, 633–646. [Google Scholar] [CrossRef]
- Qin, X.; Jing, L.; Tian, G.; Qu, Y.; Feng, Y. Enhanced Photocatalytic Activity for Degrading Rhodamine B Solution of Commercial Degussa P25 TiO2 and Its Mechanisms. J. Hazard. Mater. 2009, 172, 1168–1174. [Google Scholar] [CrossRef]
- Vijayabalan, A.; Selvam, K.; Velmurugan, R.; Swaminathan, M. Photocatalytic Activity of Surface Fluorinated TiO2-P25 in the Degradation of Reactive Orange 4. J. Hazard. Mater. 2009, 172, 914–921. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, B.; Grzechulska, J.; Grzmil, B.; Morawski, A.W. Photocatalytic Degradation of Reactive Black 5: A Comparison between TiO2-Tytanpol A11 and TiO2-Degussa P25 Photocatalysts. Appl. Catal. B Environ. 2001, 35, L1–L7. [Google Scholar] [CrossRef]
- Kanan, S.; Moyet, M.A.; Arthur, R.B.; Patterson, H.H. Recent Advances on TiO2-Based Photocatalysts toward the Degradation of Pesticides and Major Organic Pollutants from Water Bodies. Catal. Rev. 2020, 62, 1–65. [Google Scholar] [CrossRef]
- Romeiro, A.; Azenha, M.E.; Canle, M.; Rodrigues, V.H.N.; Da Silva, J.P.; Burrows, H.D. Titanium Dioxide Nanoparticle Photocatalysed Degradation of Ibuprofen and Naproxen in Water: Competing Hydroxyl Radical Attack and Oxidative Decarboxylation by Semiconductor Holes. ChemistrySelect 2018, 3, 10915–10924. [Google Scholar] [CrossRef]
- Melcher, J.; Feroz, S.; Bahnemann, D. Comparing Photocatalytic Activities of Commercially Available Iron-Doped and Iron-Undoped Aeroxide TiO2 P25 Powders. J. Mater. Sci. 2017, 52, 6341–6348. [Google Scholar] [CrossRef]
- Szabó, R.K.; Megyeri, C.; Illés, E.; Gajda-Schrantz, K.; Mazellier, P.; Dombi, A. Phototransformation of Ibuprofen and Ketoprofen in Aqueous Solutions. Chemosphere 2011, 84, 1658–1663. [Google Scholar] [CrossRef] [PubMed]
- Fogler, H.S. Elements of Chemical Reaction Engineering; Prentice Hall PTR: Hoboken, NJ, USA, 2006; ISBN 978-0-13-047394-3. [Google Scholar]
- Akpotu, S.O.; Oseghe, E.O.; Ayanda, O.S.; Skelton, A.A.; Msagati, T.A.M.; Ofomaja, A.E. Photocatalysis and Biodegradation of Pharmaceuticals in Wastewater: Effect of Abiotic and Biotic Factors. Clean Technol. Environ. Policy 2019, 21, 1701–1721. [Google Scholar] [CrossRef]
- Nabil, S.; Hammad, A.S.; El-Bery, H.M.; Shalaby, E.A.; El-Shazly, A.H. The CO2 Photoconversion over Reduced Graphene Oxide Based on Ag/TiO2 Photocatalyst in an Advanced Meso-Scale Continuous-Flow Photochemical Reactor. Environ. Sci. Pollut. Res. 2021, 28, 36157–36173. [Google Scholar] [CrossRef] [PubMed]





| Compound | IUPAC Name | Molecular Formula | Molecular Weight | Solubility in Water | pKa | Structure |
|---|---|---|---|---|---|---|
| KET | 2-(3-benzoylphenyl)-propanoic acid | C13H18O2 | 206.28 g/mol | 51 mg/L (at 22 °C) | 4.75 |  |
| Catalyst | Experimental Conditions | Light Source and Photodegradation Degree | System | Reference |
|---|---|---|---|---|
| TiO2-P25 | CKET = 7.0–15.7 mg/L Vsol = 1 L ρB = 0.20–0.50 g/L t = 300 min | UV-A = 78% Visible light = 21% | Batch reactor | [present work] |
| TiO2 | t = 120 min Vsol = 10 mL CKET = 10 mg/L ρB = 0.50 g/L | UV-A = 22.75% UV-C = 61.12% | Petri dish | [26] |
| 0.9-S-TiO2 | CKET = 10 mg/L ρB = 1 g/L t = 5 and 24 h | Solar light = 23–29% (5 h) and 65–73% (24 h) | Batch reactor | [23] |
| TiO2:ACM (wt.%) = 9:1–5:5 | CKET = 10 mg/L ρB = 1 g/L t = 5 and 24 h | Solar light = 29–72% (24 h) Visible light = 6–10% (24 h) UV radiation = 100% (5 h) | Batch reactor | [24] |
| 1%CuOx/TiO2 TiO2@GCN(1:9) | CKET = 10 mg/L t = 120 min mcat = 75 mg | Visible light = 94.7% | Solar simulator | [18] |
| Bi2S3/TiO2(%): (25/75)- Mt | CKET = 15 mg/L ρB = 0.50 g/L t = 120 min Vsol = 200 mL | UV–Vis irradiation (<366 nm) = 100% | Batch reactor | [19] |
| 1%CuO/TiO2@GCN (9:1) | Catalyst/wt% = 0.50 CKET = 10 mg/L t = 300 min | Visible light = 83.7% | Membrane reactor | [20] |
| MoO3/AgO TiO2 | CKET = 200 mg/L t = 180 min Catalyst/wt% = 0.50% | UV light = 63–67% | 350 mL quartz reactor | [21] |
| MWCNT/N-TiO2/UiO-66-NH2 (3:7) | CKET = 50 mg/L t = 120 min Vsol = 100 mL ρB = 0.25 g/L | Visible light = 96% | Batch reactor | [22] |
| Pt–TiO2–Nb2O5 | Vsol = 100 mL ρB = 0.5 g/L CKET = 12.5 mg/L t = 60 min | UV light = 100% | Batch reactor | [25] |
| Property | Value |
|---|---|
| Composition | 80% anatase, 20% rutile |
| Density (g/cm3) | 3.8 |
| BET surface area (m2g−1) | 55 |
| Mean particle size (nm) | 21 |
| pH in aqueous particle | 3–4 |
| Porosity | Non-porous |
| Test | Q [mL/min] | VL [L] | ρB [g/L] | CKET,0 [mg/L] | pH | v [rpm] | T [K] | Light Source |
|---|---|---|---|---|---|---|---|---|
| 1 | 50 | 1 | - | 12.6 | 4.6 | 750 | 303.15 | VIS |
| 2 | 50 | 1 | - | 12.6 | 4.6 | 750 | 303.15 | UV |
| 3 | 50 | 1 | 0.51 | 12.6 | 4.6 | 750 | 303.15 | - |
| 4 | 50 | 1 | 0.51 | 12.6 | 4.6 | 750 | 303.15 | UV |
| 5 | 50 | 1 | 0.52 | 12.6 | 4.6 | 750 | 303.15 | VIS |
| 6 | 50 | 1 | 0.30 | 12.6 | 4.6 | 750 | 303.15 | UV |
| 7 | 50 | 1 | 0.20 | 12.6 | 4.6 | 750 | 303.15 | UV |
| 8 | 50 | 1 | 0.52 | 15.7 | 4.3 | 750 | 303.15 | UV |
| 9 | 50 | 1 | 0.52 | 7.0 | 7.0 | 750 | 303.15 | UV |
| 10 | 50 | 1 | 0.50 | 12.6 | 4.6 | 750 | 293.15 | UV |
| 11 | 50 | 1 | 0.51 | 12.6 | 4.6 | 750 | 313.15 | UV |
| Model | kp,ref [min−1] | k′ref [mg/(L min)] | KKET,ref [L/mg] | KP,ref [L/mg] | KO2CO2 [-] | Ea [kJ/mol] | ΔH [kJ/mol] |
|---|---|---|---|---|---|---|---|
| L-H | (1.0 ± 0.1)·10−3 | 118.5 ± 0.3 | 11 ± 1 | 40.2 ± 0.2 | 177 ± 2 | 100 ± 5 | −410 ± 3 |
| E-R | (1.0 ± 0.1)·10−3 | 80 ± 10 | 11 ± 8 | (2 ± 7)·104 | (3 ± 6)·104 | 240 ± 100 | −500 ± 80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paparo, R.; Viscovo, A.; Trifuoggi, M.; Di Serio, M.; Russo, V. Ketoprofen Photodegradation Kinetics Promoted by TiO2. ChemEngineering 2024, 8, 90. https://doi.org/10.3390/chemengineering8050090
Paparo R, Viscovo A, Trifuoggi M, Di Serio M, Russo V. Ketoprofen Photodegradation Kinetics Promoted by TiO2. ChemEngineering. 2024; 8(5):90. https://doi.org/10.3390/chemengineering8050090
Chicago/Turabian StylePaparo, Rosanna, Alessia Viscovo, Marco Trifuoggi, Martino Di Serio, and Vincenzo Russo. 2024. "Ketoprofen Photodegradation Kinetics Promoted by TiO2" ChemEngineering 8, no. 5: 90. https://doi.org/10.3390/chemengineering8050090










