Combined Dietary Spirulina platensis and Citrus limon Essential Oil Enhances the Growth, Immunity, Antioxidant Capacity and Intestinal Health of Nile Tilapia
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Committee Approval
2.2. Fish Management
2.3. Feeding and Experimental Design
2.4. Growth Performance
2.5. Sampling
2.6. Hematological and Immunological Parameter Analysis
2.7. Serum Biochemical Measurements
2.8. Serum Antioxidant Enzyme Activity and MDA Concentration Assessment
2.9. Histomorphological Features of Intestine, Liver, and Spleen
2.10. Total RNA Extraction and cDNA Synthesis
2.11. Relative Gene Expression Using qPCR
2.12. Statistical Analysis
3. Results
3.1. Dietary Supplementation of LEO, SP, and Their Mixture Significantly Modified the Growth Performance of Nile Tilapia
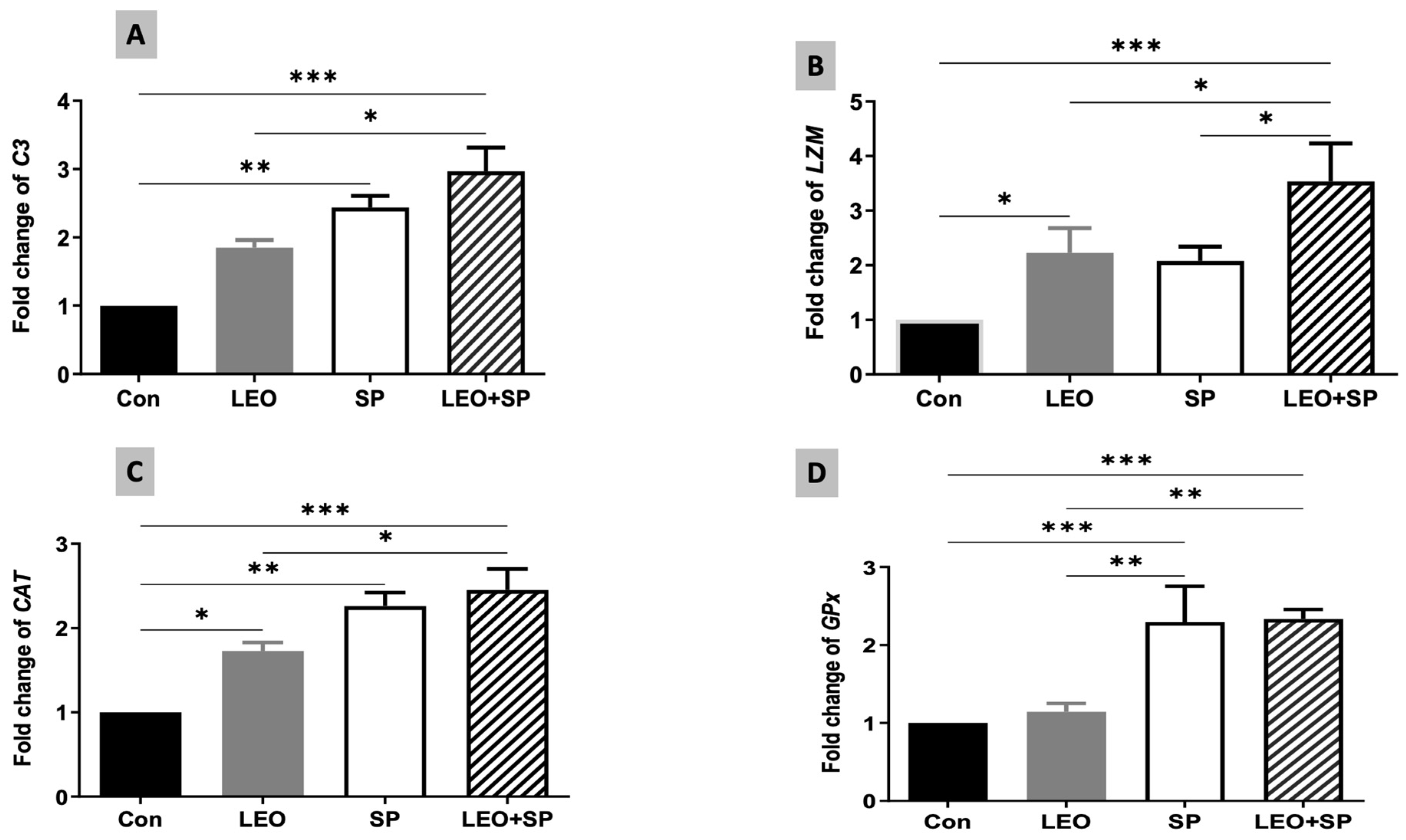
3.2. LEO, SP, and Their Combination Improved the Antioxidant and Non-Specific Immune Responses
3.3. The Effects of LEO, SP, and Their Combination on Nile Tilapia Hematological Response and the Serum Biochemical Constituents
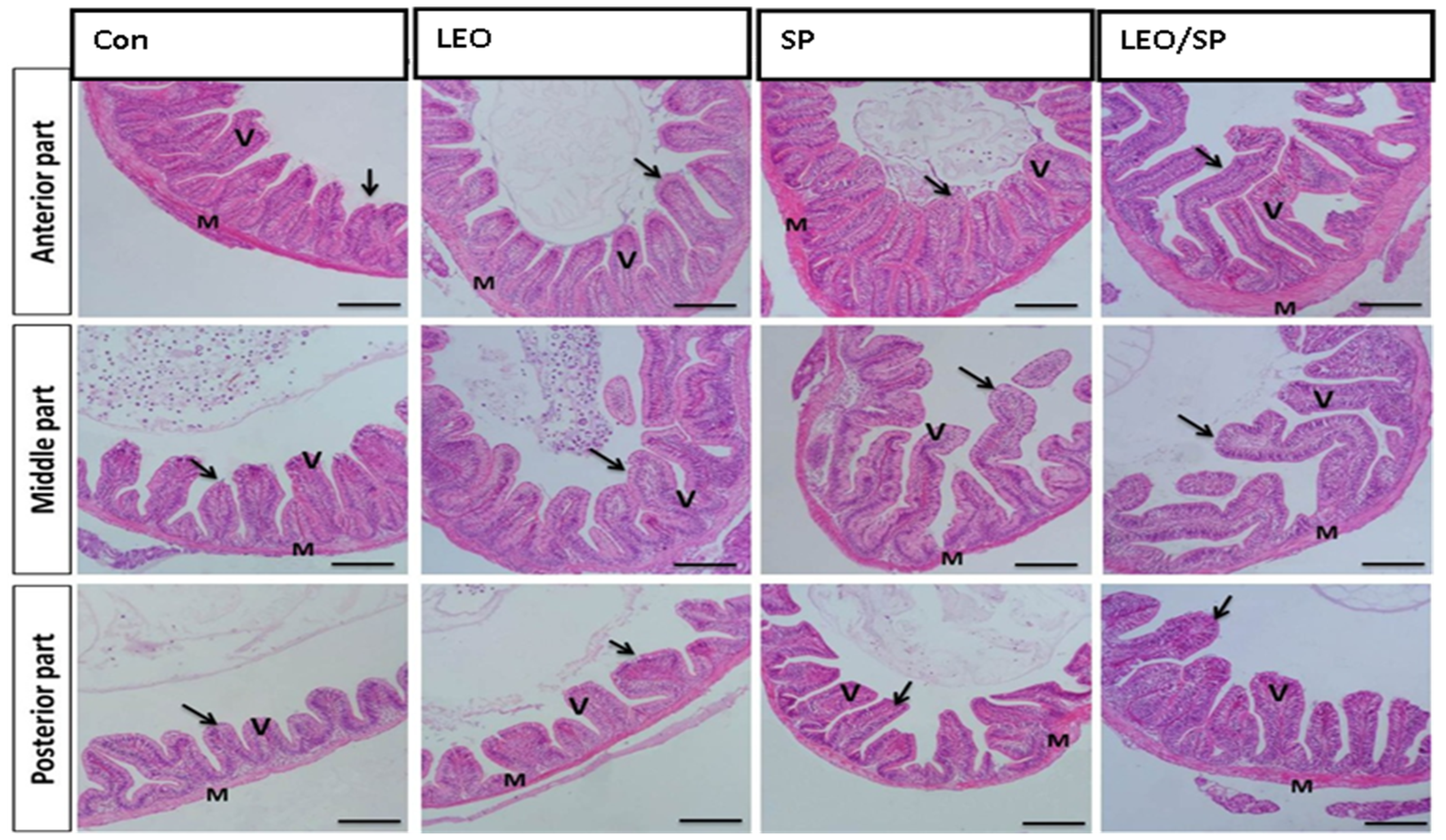
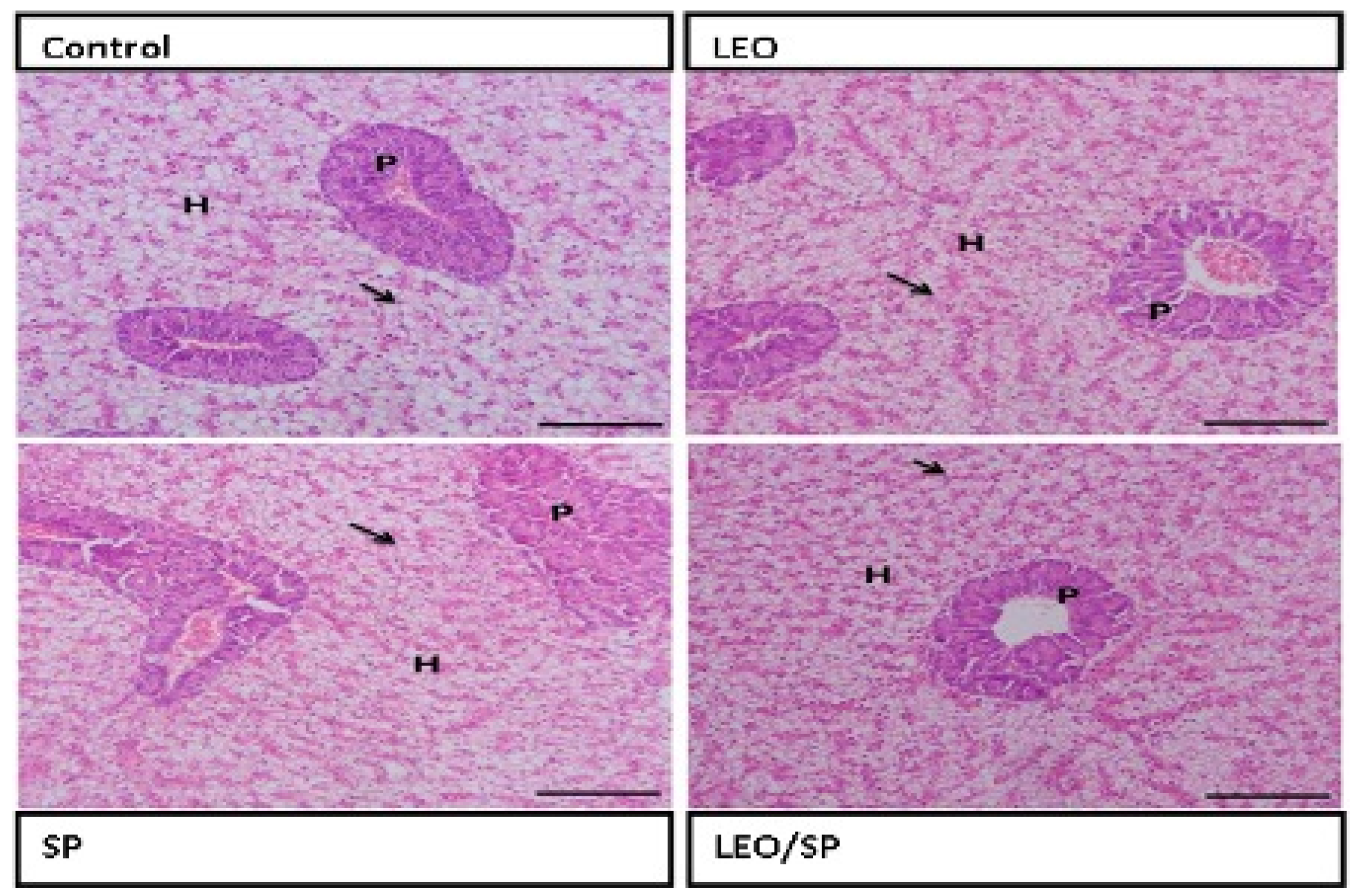
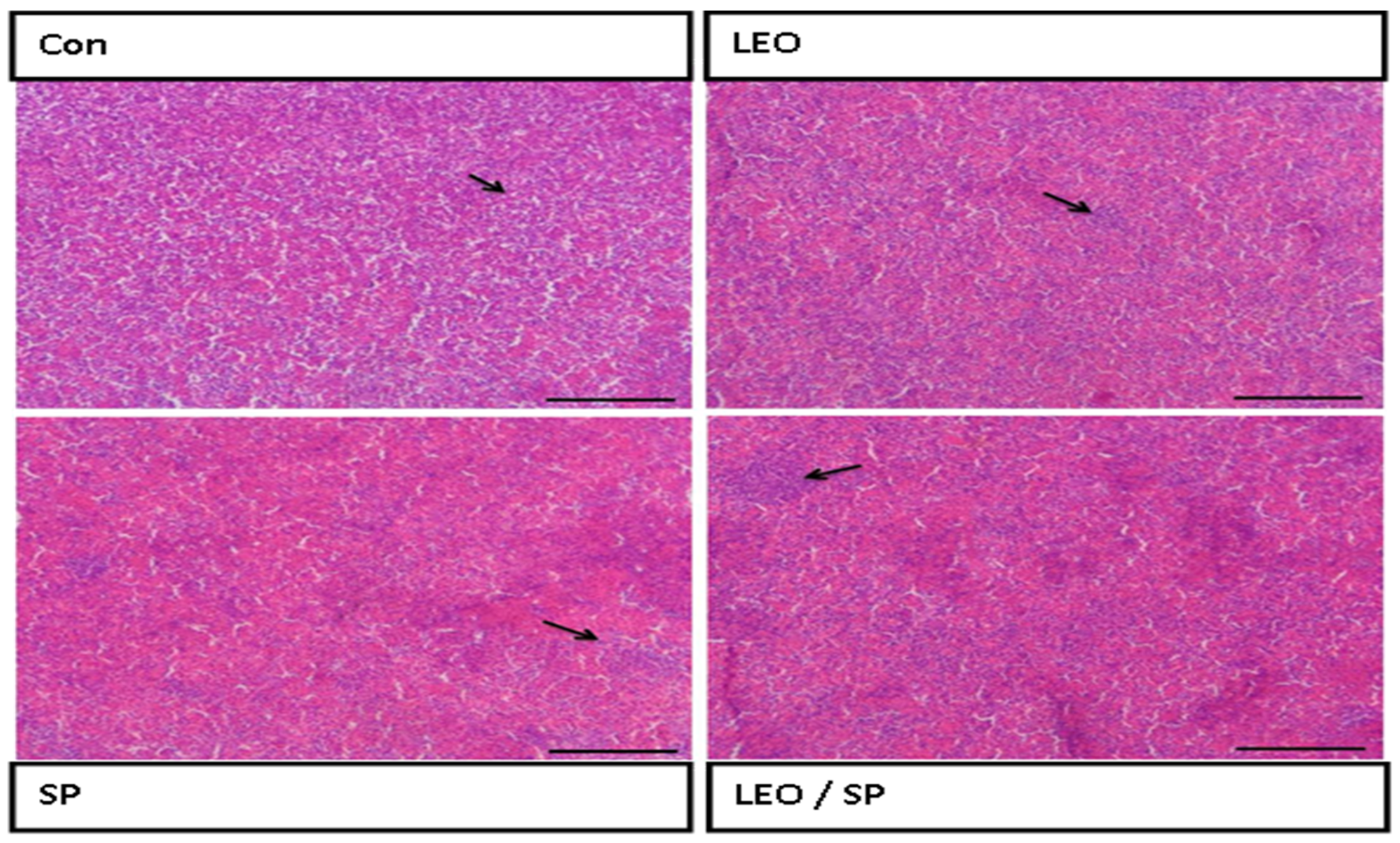
3.4. Histological Features of Intestine, Spleen, and Hepatopancreas of Nile Tilapia Supplemented with LEO, SP, and Their Combination
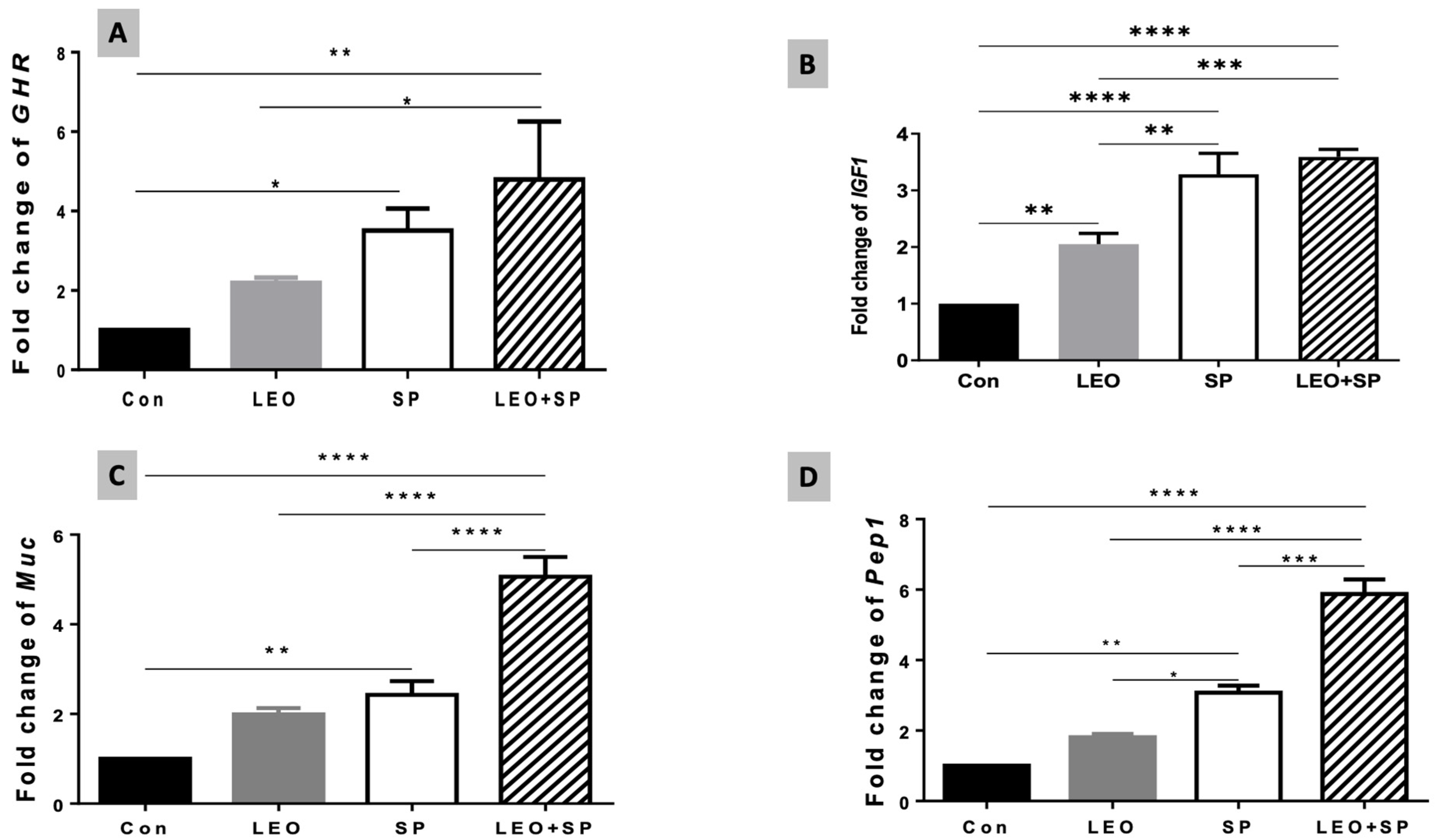
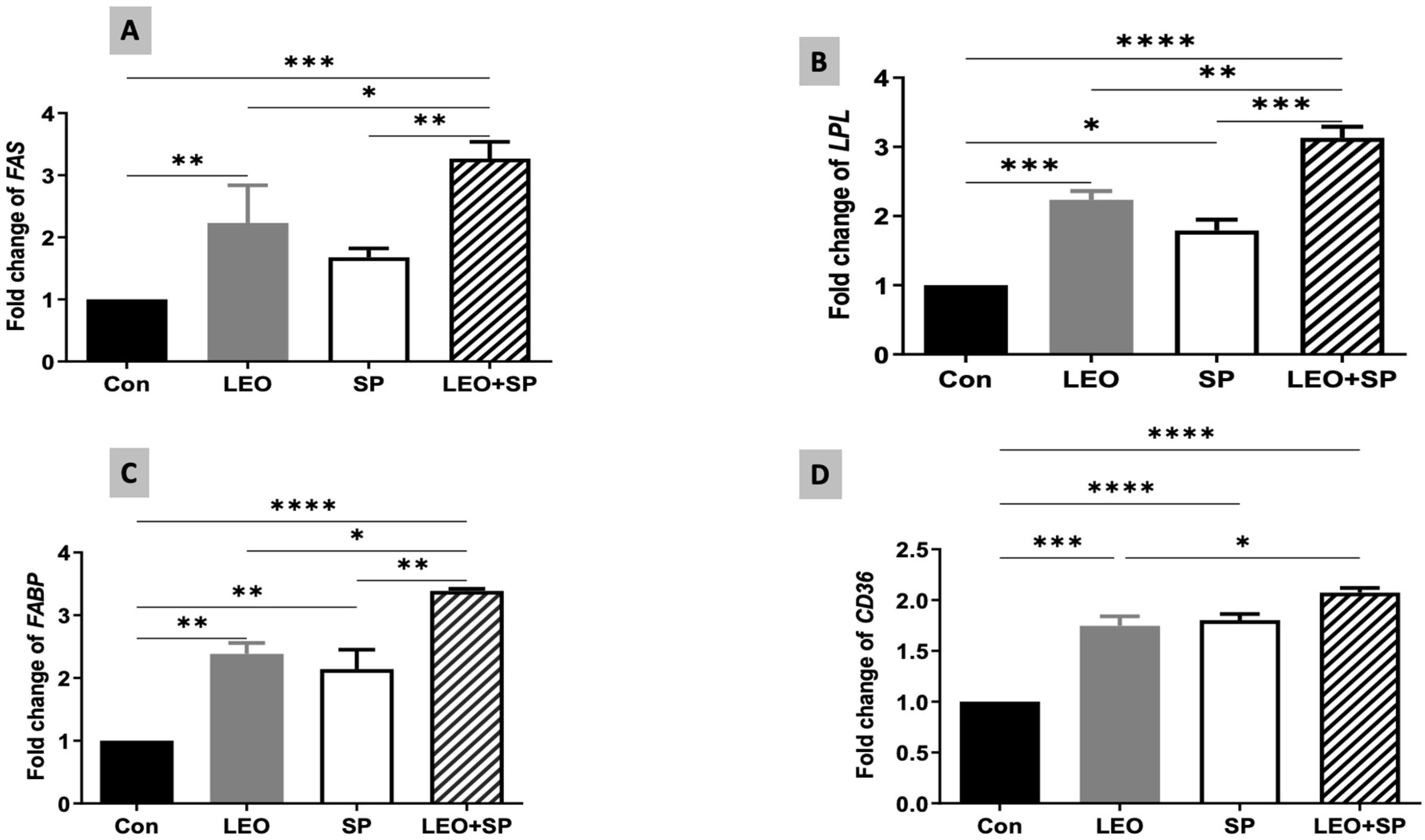
3.5. The Effects of Dietary Supplementation of LEO, SP, and Their Combination on the Expression Levels of Growth, Antioxidant, and Fat Metabolism-Regulating Genes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Subramaniam, B.; Antony, C.; Cbt, R.; Arumugam, U.; Ahilan, B.; Aanand, S. Functional feed additives used in fish feeds. Int. J. Fish. Aquat. Stud. 2019, 7, 44–52. [Google Scholar]
- Beltrán, J.M.G.; Esteban, M.Á. Nature-identical compounds as feed additives in aquaculture. Fish Shellfish Immunol. 2022, 123, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Reverter, M.; Bontemps, N.; Lecchini, D.; Banaigs, B.; Sasal, P. Use of plant extracts in fish aquaculture as an alternative to chemotherapy: Current status and future perspectives. Aquaculture 2014, 433, 50–61. [Google Scholar] [CrossRef]
- Abdo, S.E.; El-Nahas, A.F.; Abdelmenam, S.; Elmadawy, M.A.; Mohamed, R.; Helal, M.A.; El-Kassas, S. The synergetic effect of Bacillus species and Yucca shidigera extract on water quality, histopathology, antioxidant, and innate immunity in response to acute ammonia exposure in Nile tilapia. Fish Shellfish Immunol. 2022, 128, 123–135. [Google Scholar] [CrossRef]
- El-Kassas, S.; Abdo, S.E.; Abosheashaa, W.; Mohamed, R.; Moustafa, E.M.; Helal, M.A.; El-Naggar, K. Growth performance, serum lipid profile, intestinal morphometry, and growth and lipid indicator gene expression analysis of mono-sex Nile tilapia fed Moringa oleifera leaf powder. Aquac. Rep. 2020, 18, 100422. [Google Scholar] [CrossRef]
- El-Kassas, S.; Aljahdali, N.; Abdo, S.E.; Alaryani, F.S.; Moustafa, E.M.; Mohamed, R.; Abosheashaa, W.; Abdulraouf, E.; Helal, M.A.; Shafi, M.E.; et al. Moringa oleifera Leaf Powder Dietary Inclusion Differentially Modulates the Antioxidant, Inflammatory, and Histopathological Responses of Normal and Aeromonas hydrophila-Infected Mono-Sex Nile Tilapia (Oreochromis niloticus). Front. Vet. Sci. 2022, 9, 918933. [Google Scholar] [CrossRef]
- Elangovan, P.; Felix, S.; Nathan, F.; Ahilan, B. An overview on significance of fish nutrition in aquaculture industry. Int. J. Fish. Aquat. Stud. 2017, 5, 349–355. [Google Scholar]
- Zeng, Z.; Zhang, S.; Wang, H.; Piao, X. Essential oil and aromatic plants as feed additives in non-ruminant nutrition: A review. J. Anim. Sci. Biotechnol. 2015, 6, 7. [Google Scholar] [CrossRef]
- Bozkurt, T.; Gulnaz, O.; Aka Kaçar, Y. Chemical composition of the essential oils from some citrus species and evaluation of the antimicrobial activity. IOSR J. Environ. Sci. Toxicol. Food Technol. 2017, 11, 29–33. [Google Scholar] [CrossRef]
- Sun, J. D-Limonene: Safety and clinical applications. Altern. Med. Rev. A J. Clin. Ther. 2007, 12, 259–264. [Google Scholar]
- Kazyoba, P.; Viljoen, A. Limonene—A Review: Biosynthetic, Ecological and Pharmacological Relevance. Nat. Prod. Commun. 2008, 3, 1193–1202. [Google Scholar] [CrossRef]
- Aanyu, M.; Betancor, M.; Monroig, Ó. Effects of dietary limonene and thymol on the growth and nutritional physiology of Nile tilapia (Oreochromis niloticus). Aquaculture 2018, 488, 217–226. [Google Scholar] [CrossRef]
- Mohamed, R.; Yousef, M.; El-Tras, W.; Khalafallaa, M. Dietary essential oil extract from sweet orange (Citrus sinensis) and bitter lemon (Citrus limon) peels improved Nile tilapia performance and health status. Aquac. Res. 2020, 52, 1463–1479. [Google Scholar] [CrossRef]
- Gültepe, N. Protective effect of d-limonene derived from orange peel essential oil against Yersinia ruckeri in rainbow trout. Aquac. Rep. 2020, 18, 100417. [Google Scholar] [CrossRef]
- Keinan, E.; Alt, A.; Amir, G.; Bentur, L.; Bibi, H.; Shoseyov, D. Natural ozone scavenger prevents asthma in sensitized rats. Bioorg. Med. Chem. 2005, 13, 557–562. [Google Scholar] [CrossRef]
- Magara, G.; Prearo, M.; Vercelli, C.; Barbero, R.; Micera, M.; Botto, A.; Caimi, C.; Caldaroni, B.; Bertea, C.M.; Mannino, G.; et al. Modulation of Antioxidant Defense in Farmed Rainbow Trout (Oncorhynchus mykiss) Fed with a Diet Supplemented by the Waste Derived from the Supercritical Fluid Extraction of Basil (Ocimum basilicum). Antioxidants 2022, 11, 415. [Google Scholar] [CrossRef]
- Fisher, K.; Phillips, C.A. The effect of lemon, orange and bergamot essential oils and their components on the survival of Campylobacter jejuni, Escherichia coli O157, Listeria monocytogenes, Bacillus cereus and Staphylococcus aureus in vitro and in food systems. J. Appl. Microbiol. 2006, 101, 1232–1240. [Google Scholar] [CrossRef]
- Fisher, K.; Phillips, C. Potential antimicrobial uses of essential oils in food: Is citrus the answer? Trends Food Sci. Technol. 2008, 19, 156–164. [Google Scholar] [CrossRef]
- Ngugi, C.; Oyoo-Okoth, E.; Muchiri, M. Effects of dietary levels of essential oil (EO) extract from bitter lemon (Citrus limon) fruit peels on growth, biochemical, hemato-immunological parameters and disease resistance in Juvenile Labeo victorianus fingerlings challenged with Aeromonas hydrophila. Aquac. Res. 2016, 47, 2253–2265. [Google Scholar] [CrossRef]
- Christaki, E.; Florou-Paneri, P.; Bonos, E. Microalgae: A novel ingredient in nutrition. Int. J. Food Sci. Nutr. 2011, 62, 794–799. [Google Scholar] [CrossRef]
- Zhang, F.; Man, Y.B.; Mo, W.Y.; Wong, M.H. Application of Spirulina in aquaculture: A review on wastewater treatment and fish growth. Rev. Aquac. 2020, 12, 582–599. [Google Scholar] [CrossRef]
- Velasquez, S.F.; Chan, M.A.; Abisado, R.G.; Traifalgar, R.F.M.; Tayamen, M.M.; Maliwat, G.C.F.; Ragaza, J.A. Dietary Spirulina (Arthrospira platensis) replacement enhances performance of juvenile Nile tilapia (Oreochromis niloticus). J. Appl. Phycol. 2016, 28, 1023–1030. [Google Scholar] [CrossRef]
- Alagawany, M.; Taha, A.E.; Noreldin, A.; El-Tarabily, K.A.; Abd El-Hack, M.E. Nutritional applications of species of Spirulina and Chlorella in farmed fish: A review. Aquaculture 2021, 542, 736841. [Google Scholar] [CrossRef]
- El-Shall, N.A.; Jiang, S.; Farag, M.R.; Azzam, M.; Al-Abdullatif, A.A.; Alhotan, R.; Dhama, K.; Hassan, F.-u.; Alagawany, M. Potential of Spirulina platensis as a feed supplement for poultry to enhance growth performance and immune modulation. Front. Immunol. 2023, 14, 1072787. [Google Scholar] [CrossRef]
- Teimouri, M.; Yeganeh, S.; Keramat, A. The effects of Spirulina platensis meal on proximate composition, fatty acid profile and lipid peroxidation of rainbow trout (Oncorhynchus mykiss) muscle. Aquac. Nutr. 2015, 22, 559–566. [Google Scholar] [CrossRef]
- Lu, J.; Takeuchi, T.; Ogawa, H. Flesh quality of tilapia Oreochromis niloticus fed solely on raw Spirulina. Fish. Sci. 2003, 69, 529–534. [Google Scholar] [CrossRef]
- Liu, C.; Liu, H.; Han, D.; Xie, S.; Jin, J.; Yang, Y.; Zhu, X. Effects of dietary Arthrospira platensis supplementation on the growth performance, antioxidation and immune related-gene expression in yellow catfish (Pelteobagrus fulvidraco). Aquac. Rep. 2020, 17, 100297. [Google Scholar] [CrossRef]
- Yu, W.; Wen, G.; Lin, H.; Yang, Y.; Huang, X.; Zhou, C.; Zhang, Z.; Duan, Y.; Huang, Z.; Li, T. Effects of dietary Spirulina platensis on growth performance, hematological and serum biochemical parameters, hepatic antioxidant status, immune responses and disease resistance of Coral trout Plectropomus leopardus (Lacepede, 1802). Fish Shellfish Immunol. 2018, 74, 649–655. [Google Scholar] [CrossRef]
- Al-Deriny, S.; Dawood, M.; Abouzaid, A.; El-Tras, W.; Paray, B.; Doan, H.; Mohamed, R. The synergistic effects of Spirulina platensis and Bacillus amyloliquefaciens on the growth performance, intestinal histomorphology, and immune response of Nile tilapia (Oreochromis niloticus). Aquac. Rep. 2020, 17, 100390. [Google Scholar] [CrossRef]
- Amer, S. Effect of Spirulina platensis as feed supplement on growth performance, immune response and antioxidant status of mono-sex Nile Tilapia (Oreochromis niloticus). BVMJ 2016, 30, 1–10. [Google Scholar] [CrossRef]
- Shalata, H.A.; Bahattab, O.; Zayed, M.M.; Farrag, F.; Salah, A.S.; Al-Awthan, Y.S.; Ebied, N.A.; Mohamed, R.A. Synergistic effects of dietary sodium butyrate and Spirulina platensis on growth performance, carcass composition, blood health, and intestinal histomorphology of Nile tilapia (Oreochromis niloticus). Aquac. Rep. 2021, 19, 100637. [Google Scholar] [CrossRef]
- Awed, E.M.; Sadek, K.M.; Soliman, M.K.; Khalil, R.H.; Younis, E.M.; Abdel-Warith, A.A.; Van Doan, H.; Dawood, M.A.O.; Abdel-Latif, H.M.R. Spirulina platensis Alleviated the Oxidative Damage in the Gills, Liver, and Kidney Organs of Nile Tilapia Intoxicated with Sodium Sulphate. Animals 2020, 10, 2423. [Google Scholar] [CrossRef] [PubMed]
- El-Araby, D.A.; Amer, S.A.; Attia, G.A.; Osman, A.; Fahmy, E.M.; Altohamy, D.E.; Alkafafy, M.; Elakkad, H.A.; Tolba, S.A. Dietary Spirulina platensis phycocyanin improves growth, tissue histoarchitecture, and immune responses, with modulating immunoexpression of CD3 and CD20 in Nile tilapia, Oreochromis niloticus. Aquaculture 2022, 546, 737413. [Google Scholar] [CrossRef]
- Liu, C.; Liu, H.; Zhu, X.; Han, D.; Jin, J.; Yang, Y.; Xie, S. The Effects of Dietary Arthrospira platensis on Oxidative Stress Response and Pigmentation in Yellow Catfish Pelteobagrus fulvidraco. Antioxidants 2022, 11, 1100. [Google Scholar] [CrossRef] [PubMed]
- Faheem, M.; Jamal, R.; Nazeer, N.; Khaliq, S.; Hoseinifar, S.H.; Van Doan, H.; Paolucci, M. Improving Growth, Digestive and Antioxidant Enzymes and Immune Response of Juvenile Grass Carp (Ctenopharyngodon idella) by Using Dietary Spirulina platensis. Fishes 2022, 7, 237. [Google Scholar] [CrossRef]
- Abdel-Latif, H.M.R.; El-Ashram, S.; Sayed, A.E.-D.H.; Alagawany, M.; Shukry, M.; Dawood, M.A.O.; Kucharczyk, D. Elucidating the ameliorative effects of the cyanobacterium Spirulina (Arthrospira platensis) and several microalgal species against the negative impacts of contaminants in freshwater fish: A review. Aquaculture 2022, 554, 738155. [Google Scholar] [CrossRef]
- Thrall, M.A.; Weiser, G.; Allison, R.W.; Campbell, T.W. Veterinary Hematology and Clinical Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Kawahara, E.; Ueda, T.; Nomura, S. In Vitro Phagocytic Activity of White-Spotted Char Blood Cells after Injection with Aeromonas salmonicida Extracellular Products. Fish. Pathol. 1991, 26, 213–214. [Google Scholar] [CrossRef]
- Abo-Al-Ela, H.G.; El-Nahas, A.F.; Mahmoud, S.; Ibrahim, E.M. Vitamin C Modulates the Immunotoxic Effect of 17α-Methyltestosterone in Nile Tilapia. Biochemistry 2017, 56, 2042–2050. [Google Scholar] [CrossRef]
- Doumas, B.T.; Bayse, D.D.; Carter, R.J.; Peters, T., Jr.; Schaffer, R. A candidate reference method for determination of total protein in serum. I. Development and validation. Clin. Chem. 1981, 27, 1642–1650. [Google Scholar] [CrossRef]
- Reitman, S.; Frankel, S. A Colorimetric Method for the Determination of Serum Glutamic Oxalacetic and Glutamic Pyruvic Transaminases. Am. J. Clin. Pathol. 1957, 28, 56–63. [Google Scholar] [CrossRef]
- Ozdemir, C.; Yeni, F.; Odaci, D.; Timur, S. Electrochemical glucose biosensing by pyranose oxidase immobilized in gold nanoparticle-polyaniline/AgCl/gelatin nanocomposite matrix. Food Chem. 2010, 119, 380–385. [Google Scholar] [CrossRef]
- Houston, A. Blood and circulation. In Methods for Fish Biology; American Fisheries Society: Bethesda, MA, USA, 1990; pp. 415–488. [Google Scholar]
- Nishikimi, M.; Appaji Rao, N.; Yagi, K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Commun. 1972, 46, 849–854. [Google Scholar] [CrossRef] [PubMed]
- Satoh, K. Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clin. Chim. Acta 1978, 90, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Suvarna, K.S.; Layton, C.; Bancroft, J.D. Bancroft’s Theory and Practice of Histological Techniques E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Dwivedi, S.; Purohit, P.; Misra, R.; Pareek, P.; Vishnoi, J.R.; Misra, S.; Sharma, P. Methods for Isolation of High Quality and Quantity of miRNA and Single Cell Suspension for Flow-Cytometry from Breast Cancer Tissue: A Comparative Analysis. Indian. J. Clin. Biochem. 2019, 34, 39–44. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Con, P.; Nitzan, T.; Slosman, T.; Harpaz, S.; Cnaani, A. Peptide Transporters in the Primary Gastrointestinal Tract of Pre-Feeding Mozambique Tilapia Larva. Front. Physiol. 2019, 10. [Google Scholar] [CrossRef]
- El-Naggar, K.; Mohamed, R.; El-katcha, M.I.; Abdo, S.E.; Soltan, M.A. Plant Ingredient diet supplemented with lecithin as fish meal and fish oil alternative affects growth performance, serum biochemical, lipid metabolism and growth-related gene expression in Nile tilapia. Aquac. Res. 2021, 52, 6308–6321. [Google Scholar] [CrossRef]
- Zhang, X.; Zhong, H.; Han, Z.; Tang, J.; Xiao, Z.; Guo, F.; Wang, Y.; Luo; Zhou, Y. Effects of waterborne exposure to 17β-estradiol on hepatic lipid metabolism genes in tilapia (Oreochromis niloticus). Aquac Rep. 2020b, 17, 100382. [Google Scholar] [CrossRef]
- Esam, F.; Khalafalla, M.M.; Gewaily, M.S.; Abdo, S.; Hassan, A.M.; Dawood, M.A.O. Acute ammonia exposure combined with heat stress impaired the histological features of gills and liver tissues and the expression responses of immune and antioxidative related genes in Nile tilapia. Ecotoxicol. Environ. Saf. 2022, 231, 113187. [Google Scholar] [CrossRef]
- Aanyu, M.; Betancor, M.B.; Monroig, Ó. The effects of combined phytogenics on growth and nutritional physiology of Nile tilapia Oreochromis niloticus. Aquaculture 2020, 519, 734867. [Google Scholar] [CrossRef]
- Lourenço, S.C.; Moldão-Martins, M.; Alves, V.D. Antioxidants of Natural Plant Origins: From Sources to Food Industry Applications. Molecules 2019, 24, 4132. [Google Scholar] [CrossRef] [PubMed]
- Abdelkhalek, N.K.M.; Eissa, I.A.M.; Ahmed, E.; Kilany, O.E.; El-Adl, M.; Dawood, M.A.O.; Hassan, A.M.; Abdel-Daim, M.M. Protective role of dietary Spirulina platensis against diazinon-induced Oxidative damage in Nile tilapia; Oreochromis niloticus. Environ. Toxicol. Phar. 2017, 54, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Rombenso, A.; Araújo, B.; Li, E.-C. Recent Advances in Fish Nutrition: Insights on the Nutritional Implications of Modern Formulations. Animals 2022, 12, 1705. [Google Scholar] [CrossRef] [PubMed]
- Mugwanya, M.; Dawood, M.A.O.; Kimera, F.; Sewilam, H. Updating the Role of Probiotics, Prebiotics, and Synbiotics for Tilapia Aquaculture as Leading Candidates for Food Sustainability: A Review. Probiotics Antimicrob. Proteins 2022, 14, 130–157. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Abo-Al-Ela, H.G.; Hasan, M.T. Modulation of transcriptomic profile in aquatic animals: Probiotics, prebiotics and synbiotics scenarios. Fish Shellfish Immunol. 2020, 97, 268–282. [Google Scholar] [CrossRef]
- Gewaily, M.S.; Abdo, S.E.; Moustafa, E.M.; AbdEl-kader, M.F.; Abd El-Razek, I.M.; El-Sharnouby, M.; Alkafafy, M.; Raza, S.H.; El Basuini, M.F.; Van Doan, H.; et al. Dietary Synbiotics Can Help Relieve the Impacts of Deltamethrin Toxicity of Nile Tilapia Reared at Low Temperatures. Animals 2021, 11, 1790. [Google Scholar] [CrossRef]
- Bortolini, D.; Maciel, G.M.; Fernandes, I.; Pedro, A.; Rubio, F.; Brancod, I.; Haminiuk, C. Functional properties of bioactive compounds from Spirulina spp.: Current status and future trends. Food Chem. Mol. Sci. 2022, 5, 100134. [Google Scholar] [CrossRef]
- Rosas, V.T.; Poersch, L.H.; Romano, L.A.; Tesser, M.B. Feasibility of the use of Spirulina in aquaculture diets. Rev. Aquac. 2019, 11, 1367–1378. [Google Scholar] [CrossRef]
- Saleh, H.; Gaber, H.; El-Khayat, H.; Abdel-Motleb, A.; Mohammed, W.; Okasha, H. Influences of Dietary Supplementation of Chlorella vulgaris and Spirulina platensis on Growth-Related Genes Expression and Antioxidant Enzymes in Oreochromis niloticus Fish Exposed to Heavy Metals. Aquac. Studies. 2021, 22, AQUAST793. [Google Scholar] [CrossRef]
- Adel, M.; Yeganeh, S.; Dadar, M.; Sakai, M.; Dawood, M. Effects of dietary Spirulina platensis on growth performance, humoral and mucosal immune responses and disease resistance in juvenile great sturgeon (Huso huso Linnaeus, 1754). Fish Shellfish Immunol. 2016, 56, 436–444. [Google Scholar] [CrossRef]
- Belal, E.; Khalafalla, M.; El-hais, A.M.A. Use of spirulina (Arthrospira fusiformis) for promoting growth of Nile Tilapia fingerlings. Afr. J. Microbiol. Res. 2012, 6, 6423–6431. [Google Scholar] [CrossRef]
- Elsayed, H.A.G.; Salem, M. Effects of dietary orange peel on growth performance of Nile tilapia (Oreochromis niloticus) fingerlings. Aquac. Studies. 2018, 18, 127–134. [Google Scholar] [CrossRef]
- Toutou, M.; Soliman, A.A.; Elokaby, M.; Ahmed, R. Growth performance and biochemical blood parameters of Nile tilapia, Oreochromis niloticus, and thinlip mullet, Liza ramada, fed a diet supplemented with lemon (Citrus aurantifolia) peel in a polyculture system ARTICLE INFO ABSTRACT. Egypt. J. Aquat. Biol. Fish. 2018, 22, 183–192. [Google Scholar] [CrossRef]
- Sutili, F.; Gatlin, D.; Heinzmann, B.; Baldisserotto, B. Plant essential oils as fish diet additives: Benefits on fish health and stability in feed. Rev. Aquac. 2017, 10, 716–726. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; El Basuini, M.F.; Yilmaz, S.; Abdel-Latif, H.M.R.; Alagawany, M.; Kari, Z.A.; Abdul Razab, M.K.A.; Hamid, N.K.A.; Moonmanee, T.; Van Doan, H. Exploring the Roles of Dietary Herbal Essential Oils in Aquaculture: A Review. Animals 2022, 12, 823. [Google Scholar] [CrossRef] [PubMed]
- Acar, Ü.; Kesbiç, O.S.; Yılmaz, S.; Gültepe, N.; Türker, A. Evaluation of the effects of essential oil extracted from sweet orange peel (Citrus sinensis) on growth rate of tilapia (Oreochromis mossambicus) and possible disease resistance against Streptococcus iniae. Aquaculture 2015, 437, 282–286. [Google Scholar] [CrossRef]
- Zhang, W.; Deng, Y.; Yang, Z.; Kong, Q.; Liu, P.; Liao, H.; Tang, H. Effects of partial replacement of fishmeal with Spirulina platensis powder and addition of Spirulina platensis polysaccharide on growth, nutrition, antioxidant capacity and gut microbiota of Micropterus salmoides. Aquaculture 2024, 586, 740802. [Google Scholar] [CrossRef]
- Abbasali, H.S.H.F.L.; Tizkar, Z.B. Influence of Spirulina sp. and citric acid dietary supplements on the growth performance and immune parameters of com-mon carp (Cyprinus carpio). Int. Aquat. Res. 2024, 16, 91–99. [Google Scholar]
- Macedo, J.d.S.; Copatti, C.E.; Costa, E.V.; Da Silva, F.M.A.; Dutra, L.M.; Santos, V.L.d.A.; Almeida, J.R.G.d.S.; Tavares-Dias, M.; Melo, J.F.B. Effects of Citrus limon extract on growth performance and immunity in striped catfish (Pangasius hypophthalmus). Aquac. Int. 2023, 31, 719–738. [Google Scholar] [CrossRef]
- Yousefi, M.; Hoseini, S.M.; Abdel Rahman, A.N.; Vatnikov, Y.A.; Kulikov, E.V.; Kharlitskaya, E.V.; Seleznev, S.B. Effects of Dietary Limonene Supplementation on Growth Performance and Immunological Parameters of Common Carp, Cyprinus carpio, Challenged by Aeromonas hydrophila. Animals 2023, 13, 3197. [Google Scholar] [CrossRef]
- Da Silva, E.G.; Finamor, I.A.; Bressan, C.A.; Schoenau, W.; Vencato, M.D.S.; Pavanato, M.A.; Cargnelutti, J.F.; Da Costa, S.T.; Antoniazzi, A.Q.; Baldisserotto, B. Dietary Supplementation with R-(+)-Limonene Improves Growth, Metabolism, Stress, and Antioxidant Responses of Silver Catfish Uninfected and Infected with Aeromonas hydrophila. Animals 2023, 13, 3307. [Google Scholar] [CrossRef] [PubMed]
- Si, W.; Gong, J.; Tsao, R.; Zhou, T.; Yu, H.; Poppe, C.; Johnson, R.; Du, Z. Antimicrobial activity of essential oils and structurally related synthetic food additives towards selected pathogenic and beneficial gut bacteria. J. Appl. Microbiol. 2006, 100, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Thapa, D.; Losa, R.; Zweifel, B.; Wallace, R.J. Sensitivity of pathogenic and commensal bacteria from the human colon to essential oils. Microbiology (Reading) 2012, 158, 2870–2877. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, Y.; Fan, G.; Ren, J.N.; Zhang, L.L.; Pan, S.Y. Effects of orange essential oil on intestinal microflora in mice. J. Sci. Food Agric. 2019, 99, 4019–4028. [Google Scholar] [CrossRef]
- Albalat, A.; Saera-Vila, A.; Capilla, E.; Gutierrez, J.; Pérez-Sánchez, J.; Navarro, I. Insulin regulation of lipoprotein lipase (LPL) activity and expression in Gilthead Sea bream (Sparus aurata). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2007, 148, 151–159. [Google Scholar] [CrossRef]
- Jensen-Urstad, A.P.; Semenkovich, C.F. Fatty acid synthase and liver triglyceride metabolism: Housekeeper or messenger? Biochim. Biophys. Acta. 2012, 1821, 747–753. [Google Scholar] [CrossRef]
- Storch, J.; Thumser, A.E. The fatty acid transport function of fatty acid-binding proteins. Biochim. Biophys. Acta 2000, 1486, 28–44. [Google Scholar] [CrossRef]
- Nakharuthai, C.; Rodrigues, P.M.; Schrama, D.; Kumkhong, S.; Boonanuntanasarn, S. Effects of Different Dietary Vegetable Lipid Sources on Health Status in Nile Tilapia (Oreochromis niloticus): Haematological Indices, Immune Response Parameters and Plasma Proteome. Animals 2020, 10, 1377. [Google Scholar] [CrossRef]
- Silverstein, R.; Febbraio, M. CD36, a Scavenger Receptor Involved in Immunity, Metabolism, Angiogenesis, and Behavior. Sci. Signal. 2009, 2, re3. [Google Scholar] [CrossRef]
- Zuo, H.; Gao, J.; Yuan, J.; Deng, H.; Yang, L.; Weng, S.; He, J.; Xu, X. Fatty acid synthase plays a positive role in shrimp immune responses against Vibrio parahaemolyticus infection. Fish Shellfish Immunol. 2017, 60, 282–288. [Google Scholar] [CrossRef]
- Yeganeh, S.; Teimouri, M.; Amirkolaie, A.K. Dietary effects of Spirulina platensis on hematological and serum biochemical parameters of rainbow trout (Oncorhynchus mykiss). Res. Vet. Sci. 2015, 101, 84–88. [Google Scholar] [CrossRef]
- Muga, M.A.; Chao, J.C. Effects of fish oil and spirulina on oxidative stress and inflammation in hypercholesterolemic hamsters. BMC Complement. Altern. Med. 2014, 14, 470. [Google Scholar] [CrossRef] [PubMed]
- Serban, M.-C.; Sahebkar, A.; Dragan, S.; Stoichescu-Hogea, G.; Ursoniu, S.; Andrica, F.; Banach, M. A systematic review and meta-analysis of the impact of Spirulina supplementation on plasma lipid concentrations. Clin. Nutr. 2016, 35, 842–851. [Google Scholar] [CrossRef] [PubMed]
- Dawood, M. Nutritional immunity of fish intestines: Important insights for sustainable aquaculture. Rev. Aquac. 2020, 13, 642–663. [Google Scholar] [CrossRef]
- Fu, Y.; Liang, X.; Li, D.; Gao, H.; Wang, Y.; Li, W.; Xu, K.; Hu, F. Effect of Dietary Tryptophan on Growth, Intestinal Microbiota, and Intestinal Gene Expression in an Improved Triploid Crucian Carp. Front. Nutr. 2021, 8, 676035. [Google Scholar] [CrossRef] [PubMed]
- Paola, P.; Patrice, D.C. Mucus barrier, mucins and gut microbiota: The expected slimy partners? Gut 2020, 69, 2232. [Google Scholar] [CrossRef]
- Kim, Y.; Ho, S. Intestinal Goblet Cells and Mucins in Health and Disease: Recent Insights and Progress. Curr. Gastroenterol. Rep. 2010, 12, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Sangani, A.K.; Masoudi, A.A.; Hosseini, S.A. The effects of herbal plants on Mucin 2 gene expression and performance in ascetic broilers. Iran. J. Vet. Med. 2014, 8, 47–52. [Google Scholar]
- Verri, T.; Terova, G.; Dabrowski, K.; Saroglia, M. Peptide transport and animal growth: The fish paradigm. Biol. Lett. 2011, 7, 597–600. [Google Scholar] [CrossRef]
- Jang, I.; Ko, Y.; Kang, S.; Lee, C. Effect of a commercial essential oil on growth performance, digestive enzyme activity and intestinal microflora population in broiler chickens. Anim. Feed Sci. Technol. 2007, 134, 304–315. [Google Scholar] [CrossRef]
- Di Cerbo, A.; Pezzuto, F.; Bondi, M. Preliminary evaluation of Spirulina maxima and Ascophyllum nodosum effect on 3 different bacterial strains. Minerva Biotecnol. 2015, 27, 131–136. [Google Scholar]
- Farag, M.R.; Alagawany, M.; Abd El-Hack, M.E.; Dhama, K. Nutritional and healthical aspects of Spirulina (Arthrospira) for poultry, animals and human. Int. J. Pharmacol. 2016, 12, 36–51. [Google Scholar] [CrossRef]
- Bora, H.; Kamle, M.; Mahato, D.K.; Tiwari, P.; Kumar, P. Citrus essential oils (CEOs) and their applications in food: An overview. Plants 2020, 9, 357. [Google Scholar] [CrossRef] [PubMed]
- Sheikhzadeh, N.; Mousavi, S.; Hamidian, G.; Firouzamandi, M.; Khani Oushani, A.; Mardani, K. Role of dietary Spirulina platensis in improving mucosal immune responses and disease resistance of rainbow trout (Oncorhynchus mykiss). Aquaculture 2019, 510, 1–8. [Google Scholar] [CrossRef]
- Siddik, M.A.B.; Vatsos, I.N.; Rahman, M.A.; Pham, H.D. Selenium-Enriched Spirulina (SeE-SP) Enhance Antioxidant Response, Immunity, and Disease Resistance in Juvenile Asian Seabass, Lates calcarifer. Antioxidants 2022, 11, 1572. [Google Scholar] [CrossRef]
- Ahmadifar, M.; Esfahani, D.E.; Ahmadifar, E.; Sheikhzadeh, N.; Mood, S.M.; Moradi, S.Z. Combined effects of Spirulina platensis and Pediococcus acidilactici on the growth performance, digestive enzyme activity, antioxidative status, and immune genes in zebrafish. Ann. Anim. Sci. 2023, 23, 1159–1167. [Google Scholar] [CrossRef]
- Esmaeili, M. Blood Performance: A New Formula for Fish Growth and Health. Biology 2021, 10, 1236. [Google Scholar] [CrossRef]
- Fazio, F.; Saoca, C.; Vazzana, I.; Piccione, G. Influence of Body Size on Blood Hemogram in Rainbow Trout Oncorhynchus mykiss (Walbaum, 1792). Veter Med. Open J. 2017, 2, 91–94. [Google Scholar] [CrossRef]
- Acar, Ü.; Kesbiç, O.S.; İnanan, B.E.; Yılmaz, S. Effects of dietary Bergamot (Citrus bergamia) peel oil on growth, haematology and immune response of European sea bass (Dicentrarchus labrax) juveniles. Aquac. Res. 2019, 50, 3305–3312. [Google Scholar] [CrossRef]
- Vicente, I.; Fleuri, L.; Carvalho, P.; Gardim Guimarães, M.; Naliato, R.; Müller, H.; Sartori, M.M.; Pezzato, L.; Barros, M. Orange peel fragment improves antioxidant capacity and haematological profile of Nile tilapia subjected to heat/dissolved oxygen-induced stress. Aquac. Res. 2018, 50, 80–92. [Google Scholar] [CrossRef]
- Sayed, A.E.H.; Hamed, M.; El-Sayed, A.A.A.; Nunes, B.; Soliman, H.A.M. The mitigating effect of Spirulina (Arthrospira platensis) on the hemotoxicity of gibberellic acid on juvenile tilapia (Oreochromis niloticus). Environ. Sci. Pollut. Res. Int. 2023, 30, 25701–25711. [Google Scholar] [CrossRef] [PubMed]
- Arabi, H.; Gholipourkanani, H.; Shahsavani, D.; Harsij, M. Improving effect of Spirulina platensis on hematological parameters in Cyprinus carpio exposed to sublethal doses of cyanide. Comp. Clin. Path. 2016, 25, 335–342. [Google Scholar] [CrossRef]
- Magouz, F.; El-Din, M.; Amer, A.; Gewaily, M.; El-Dahdoh, W.; Dawood, M. A blend of herbal essential oils enhanced the growth performance, blood bio-immunology traits, and intestinal health of Nile tilapia (Oreochromis niloticus). Ann. Anim. Sci. 2021, 22, 000010247820210066. [Google Scholar] [CrossRef]
- Costa, R.; Dugo, P.; Navarra, M.; Raymo, V.; Dugo, G.; Mondello, L. Study on the chemical composition variability of some processed bergamot (Citrus bergamia) essential oils. Flavour. Fragr. J. 2010, 25, 4–12. [Google Scholar] [CrossRef]
- Wei, A.; Shibamoto, T. Antioxidant Activities and Volatile Constituents of Various Essential Oils. J. Agric. Food Chem. 2007, 55, 1737–1742. [Google Scholar] [CrossRef]
- Hassaan, M.S.; Mohammady, E.Y.; Soaudy, M.R.; Sabae, S.A.; Mahmoud, A.M.A.; El-Haroun, E.R. Comparative study on the effect of dietary β-carotene and phycocyanin extracted from Spirulina platensis on immune-oxidative stress biomarkers, genes expression and intestinal enzymes, serum biochemical in Nile tilapia, Oreochromis niloticus. Fish Shellfish Immunol. 2021, 108, 63–72. [Google Scholar] [CrossRef]
- Grover, P.; Bhatnagar, A.; Kumari, N.; Bhatt, A.; Nishad, D.; Purkayastha, J. C-Phycocyanin-a novel protein from Spirulina platensis- In Vivo toxicity, Antioxidant and Immunomodulatory Studies. Saudi J. Biol. Sci. 2020, 28, 1853–1859. [Google Scholar] [CrossRef]
- Abdel-Daim, M.M.; Dawood, M.A.O.; AlKahtane, A.A.; Abdeen, A.; Abdel-Latif, H.M.R.; Senousy, H.H.; Aleya, L.; Alkahtani, S. Spirulina platensis mediated the biochemical indices and antioxidative function of Nile tilapia (Oreochromis niloticus) intoxicated with aflatoxin B1. Toxicon 2020, 184, 152–157. [Google Scholar] [CrossRef]
- Lim, K.C.; Yusoff, F.M.; Karim, M.; Natrah, F.M.I. Carotenoids modulate stress tolerance and immune responses in aquatic animals. Rev Aquac. 2023, 15, 872–894. [Google Scholar] [CrossRef]
- Mokhbatly, A.-A.; Assar, D.; Ghazy, E.; Elbialy, Z.; Rizk, S.; Omar, A.; Gaafar, A.; Dawood, M. The protective role of spirulina and β-glucan in African catfish (Clarias gariepinus) against chronic toxicity of chlorpyrifos: Hemato-biochemistry, histopathology, and oxidative stress traits. Environ. Sci. Pollut. Res. 2020, 27, 31636–31651. [Google Scholar] [CrossRef]
- Kumar, S.; Moniruzzaman, M.; Chakraborty, A.; Sarbajna, A.; Chakraborty, S.B. Crosstalk between heat shock proteins, NRF2, NF-κB and different endogenous antioxidants during lead-induced hepatotoxicity in Puntius ticto. Aquat. Toxicol. 2021, 233, 105771. [Google Scholar] [CrossRef]
- Wardyn, J.D.; Ponsford, A.H.; Sanderson, C.M. Dissecting molecular cross-talk between Nrf2 and NF-κB response pathways. Biochem. Soc. Trans. 2015, 43, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Mokhtar, D.M.; Zaccone, G.; Alesci, A.; Kuciel, M.; Hussein, M.T.; Sayed, R.K.A. Main Components of Fish Immunity: An Overview of the Fish Immune System. Fishes 2023, 8, 93. [Google Scholar] [CrossRef]
- Mahmoud, M.; Elamie, M.; Kilany, O.; Dessouki, A. Spirulina (Arthrospira platensis) supplementation improves growth performance, feed utilization, immune response, and relieves oxidative stress in Nile tilapia (Oreochromis niloticus) challenged with Pseudomonas fluorescens. Fish Shellfish Immunol. 2017, 72, 291–300. [Google Scholar] [CrossRef]
- Güroy, B.; Güroy, D.; Bilen, S.; Kenanoğlu, O.N.; Şahin, I.; Terzi, E.; Karadal, O.; Mantoğlu, S. Effect of dietary Spirulina (Arthrospira platensis) on the growth performance, immune-related gene expression and resistance to Vibrio anguillarum in European seabass (Dicentrarchus labrax). Aquac. Res. 2022, 53, 2263–2274. [Google Scholar] [CrossRef]
- Yousefi, M.; Ahmadifar, M.; Mohammadzadeh, S.; Kalhor, N.; Esfahani, D.E.; Bagheri, A.; Mashhadizadeh, N.; Moghadam, M.S.; Ahmadifar, E. Individual and combined effects of the dietary Spirulina platensis and Bacillus licheniformis supplementation on growth performance, antioxidant capacity, innate immunity, relative gene expression and resistance of goldfish, Carassius auratus to Aeromonas hydrophila. Fish Shellfish Immunol. 2022, 127, 1070–1078. [Google Scholar] [CrossRef]
- Abdel-Latif, H.; Khafaga, A.; Dawood, M. Dietary oregano essential oil improved antioxidative status, immune-related genes, and resistance of common carp (Cyprinus carpio L.) to Aeromonas hydrophila infection. Fish Shellfish Immunol. 2020, 104, 1–7. [Google Scholar] [CrossRef]
- Baba, E.; Acar, Ü.; Öntaş, C.; Kesbic, O.; Yilmaz, S. Evaluation of Citrus limon peels essential oil on growth performance, immune response of Mozambique tilapia Oreochromis mossambicus challenged with Edwardsiella tarda. Aquaculture 2016, 465, 13–18. [Google Scholar] [CrossRef]
- Yaqoob, P.; Calder, P.C. Fatty acids and immune function: New insights into mechanisms. Br. J. Nutr. 2007, 98 (Suppl. S1), S41–S45. [Google Scholar] [CrossRef]
- Qian, X.; Yang, Z.; Mao, E.; Chen, E. Regulation of fatty acid synthesis in immune cells. Scand. J. Immunol. 2018, 88, e12713. [Google Scholar] [CrossRef]
- Carroll, R.; Zaslona, Z.; Galvan-Pena, S.; Koppe, E.; Sévin, D.; Angiari, S.; Triantafilou, M.; Triantafilou, K.; Modis, L.; O’Neill, L. An unexpected link between fatty acid synthase and cholesterol synthesis in proinflammatory macrophage activation. J. Biol. Chem. 2018, 293, jbc.RA118.001921. [Google Scholar] [CrossRef] [PubMed]
- Modrá, H.; Svobodová, Z.; Kolářová, J. Comparison of Differential Leukocyte Counts in Fish of Economic and Indicator Importance. Acta Vet. Brno 1998, 67, 215. [Google Scholar] [CrossRef]
- Shlenkina, T.M.; Romanova, E.M.; Lyubomirova, V.N.; Romanov, V.V.; Shadieva, L.A. The effects of the probiotic Subtilis on the peripheral blood system of Clarias gariepinus. BIO Web Conf. 2020, 27, 00133. [Google Scholar] [CrossRef]
- Vallejos-Vidal, E.; Reyes-López, F.; Teles, M.; MacKenzie, S. The response of fish to immunostimulant diets. Fish Shellfish Immunol. 2016, 56, 34–69. [Google Scholar] [CrossRef] [PubMed]
- Barman, D.; Nen, P.; Mandal, S.; Kumar, V. Immunostimulants for Aquaculture Health Management. JMSRD 2013, 3, 1–11. [Google Scholar] [CrossRef]
- Davis, A.K.; Maney, D.L.; Maerz, J.C. The use of leukocyte profiles to measure stress in vertebrates: A review for ecologists. Funct. Ecol. 2008, 22, 760–772. [Google Scholar] [CrossRef]
| Ingredient Composition | % | Chemical Analysis | % |
|---|---|---|---|
| Fish meal (72% CP) | 10 | Dry matter (DM %) | 93.00 |
| Soybean meal | 40 | Crude protein (CP %) | 30.45 |
| Yellow corn | 24 | Ether extract (EE %) | 7.940 |
| Wheat bran | 10 | Crude fiber (CF %) | 4.950 |
| Rice bran | 10 | Ash % | 8.660 |
| Corn oil | 3 | Nitrogen-free extract (NFE %) | 48.00 |
| Dicalcium phosphate | 1 | Calculated energy | value |
| Vitamin and mineral mix | 2 | Gross energy (kcal/kg) | 4496.36 |
| Total | 100 | Metabolizable energy (Kcal/kg) | 371.63 |
| Gene | Primers | Acc. No | Ref. |
|---|---|---|---|
| ef-1α | F: TCAACGCTCAGGTCATCATC R: ACGGTCGATCTTCTCAACCA | XM_003458541 | [49] |
| β-actin | F: CAGCAAGCAGGAGTACGATGAG R: TGTGTGGTGTGTGGTTGTTTTG | XM_003455949.2 | [50] |
| GHR1 | F: CAGACTTCTACGCTCAGGTC R: CTGGATTCTGAGTTGCTGTC | AY973232.1 | |
| IGF-1 | F: GTTTGTCTGTGGAGAGCGAGG R: GAAGCAGCACTCGTCCACG | Y10830.1 | |
| FABP3 | F: CAAGCCCACCACCATCATCT R: TTCCCGTCCTCTATCGTGACA | XM_003444047.5 | [51] |
| CD36 | F: CCCAAAGCGAACGTCACATT R: ATGTGATGCTGGAGGAAGCAA | XM_003452029.5 | |
| FAS | F: TGAAACTGAAGCCTTGTGTGCC R: TCCCTGTGAGCGGAGGTGATTA | GU433188 | [5] |
| LPL | F: TGCTAATGTGATTGTGGTGGAC R: GCTGATTTTGTGGTTGGTAAGG | NM_001279753.1 | |
| GPX | F: CCAAGAGAACTGCAAGAACGA R: CAGGACACGTCATTCCTACAC | DQ355022 | [4] |
| CAT | F: CCCAGCTCTTCATCCAGAAAC R: GCCTCCGCATTGTACTTCTT | JF801726.1 | |
| LZM | F: AAGGGAAGCAGCAGCAGTTGTG R: CGTCCATGCCGTTAGCCTTGAG | XM_003460550.2 | [52] |
| C3 | F: GGTGTGGATGCACCTGAGAA R: GGGAAATCGGTACTTGGCCT | XM_013274267.2 | |
| Muc | F: TGCCCAGGAGGTAGATATGC R: TACAGCATGAGCAGGAATGC | XM_005466350 | [53] |
| Pept1 | F: CAAAGCACTGGTGAAGGTCC R: CACTGCGTCAAACATGGTGA | XM_013271589 |
| Control | LEO | SP | LEO/SP | p-Value | |
|---|---|---|---|---|---|
| Initial wight (g) | 7.73 ± 0.11 a | 7.88 ± 0.10 a | 8.08 ± 0.06 a | 8.07 ± 0.15 a | 0.124 |
| Final weight (g) | 39.50 ± 1.64 b | 44.67 ± 1.02 ab | 47.67 ± 0.42 a | 48.17 ± 1.21 a | <0.01 |
| Weight gain (g) | 31.90 ± 1.68 b | 36.82 ± 1.01 ab | 39.58 ± 0.42 a | 39.77 ± 1.08 a | <0.05 |
| Feed intake (g) | 60.07 ± 0.87 b | 61.93 ± 0.06 ab | 63.95 ± 0.33 a | 63.53 ± 0.36 a | <0.01 |
| FCR | 2.03 ± 0.13 a | 1.68 ± 0.04 b | 1.63 ± 0.03 b | 1.61 ± 0.07 b | <0.01 |
| SGR (%/day) | 1.53 ± 0.05 b | 1.67 ± 0.02 ab | 1.69 ± 0.02 a | 1.71 ± 0.03 a | 0.014 |
| Body length (cm) | 9.80 ± 0.49 b | 11.27 ± 0.18 ab | 11.52 ± 0.50 a | 11.60 ± 0.27 a | 0.012 |
| Liver weight (g) | 0.77 ± 0.09 a | 0.79 ± 0.07 a | 0.79 ± 0.07 a | 0.98 ± 0.12 a | 0.393 |
| Intestine weight (g) | 0.79 ± 0.04 a | 1.03 ± 0.12 a | 1.02 ± 0.08 a | 0.98 ± 0.60 a | 0.408 |
| HSI (%) | 1.78 ± 0.14 a | 1.78 ± 0.16 a | 1.77 ± 0.25 a | 2.08 ± 0.28 a | 0.711 |
| Control | LEO | SP | LEO/SP | p-Value | |
|---|---|---|---|---|---|
| SOD (IU/L) | 7.95 ± 0.05 c | 8.75 ± 0.28 b | 10.07 ± 0.02 a | 9.96 ± 0.09 a | <0.0001 |
| GPX (IU/L) | 8.39 ± 0.31 c | 11.05 ± 0.10 b | 10.78 ± 0.27 b | 12.39 ± 0.21 a | <0.0001 |
| MDA (IU/L) | 18.54 ± 0.28 a | 15.55 ± 0.35 b | 15.31 ± 0.35 b | 15.11 ± 0.40 b | <0.001 |
| PA (%) | 11.14 ± 0.08 c | 13.14 ± 0.17 b | 11.88 ± 0.12 c | 15.32 ± 0.48 a | <0.0001 |
| PI | 0.98 ± 0.02 | 1.08 ± 0.03 | 1.07 ± 0.05 | 1.15 ± 0.038 | 0.1034 |
| LZM (Unite/mL) | 8.52 ± 0.31 c | 13.16 ± 0.22 a | 11.48 ± 0.19 b | 12.90 ± 0.11 a | <0.0001 |
| Control | LEO | SP | LEO/SP | p-Values | |
|---|---|---|---|---|---|
| RBCs (×106/mm3) | 3.20 ± 0.07 c | 3.77 ± 0.04 a | 3.57 ± 0.014 ab | 3.49 ± 0.015 b | <0.001 |
| Hb % | 9.74 ± 0.15 c | 11.44 ± 0.07 a | 10.88 ± 0.06 b | 10.65 ± 0.06 b | <0.0001 |
| PCV (%) | 31.00 ± 0.57 c | 37.00 ± 0.57 a | 34.67 ± 0.33 b | 34.33 ± 0.33 b | <0.0001 |
| WBCs (×103/mm3) | 10.15 ± 0.14 c | 11.19 ± 0.18 b | 11.54 ± 0.27 ab | 12.13 ± 0.15 a | <0.001 |
| Heterophils (%) | 16.00 ± 0.57 a | 9.67 ± 0.33 b | 10.67 ± 0.33 b | 10.33 ± 0.33 b | <0.0001 |
| Lymphocytes (%) | 74.33 ± 0.88 b | 82.67 ± 0.66 a | 80.33 ± 0.33 a | 80.67 ± 0.33 a | <0.0001 |
| H/L ratio | 0.215 ± 0.01 a | 0.117 ± 0.01 b | 0.133 ± 0.01 b | 0.128 ± 0.01 b | <0.0001 |
| Control | LEO | SP | LEO/SP | p-Values | |
|---|---|---|---|---|---|
| TG (mg/dL) | 95.47 ± 3.78 | 104.3 ± 3.93 | 105.5 ± 5.51 | 100.5 ± 1.42 | 0.325 |
| CHOL (mg/dL) | 106.3 ± 0.52 | 103.3 ± 2.31 | 102.4 ± 2.40 | 101.7 ± 1.89 | 0.254 |
| AST (U/L) | 29.59 ± 0.32 | 28.54 ± 1.09 | 28.47 ± 0.42 | 27.95 ± 1.03 | 0.553 |
| ALT (U/L) | 31.70 ± 0.75 | 30.59 ± 0.47 | 30.50 ± 0.47 | 29.37 ± 0.23 | 0.073 |
| Glucose (mg/dL) | 2.34 ± 0.09 | 2.68 ± 0.077 | 2.52 ± 0.05 | 2.64 ± 0.11 | 0.102 |
| Albumin (g/dL) | 1.51 ± 0.05 | 1.50 ± 0.009 | 1.54 ± 0.03 | 1.47 ± 0.04 | 0.729 |
| Total protein (g/dL) | 3.85 ± 0.13 | 4.19 ± 0.070 | 4.06 ± 0.04 | 4.12 ± 0.14 | 0.223 |
| Items | Control | LEO | SP | LEO/SP | p-Value | |
|---|---|---|---|---|---|---|
| Anterior segment | Villus height | 135.6 ± 6.9 c | 165.3 ± 9.8 bc | 147.1 ± 12.8 b | 216.8 ± 7.5 a | <0.001 |
| Villus width | 45.11 ± 2.8 c | 58.81 ± 1.9 bc | 68.95 ± 4.1 ab | 82.8 ± 4.2 a | <0.001 | |
| Crypt depth | 25.74 ± 1.7 d | 46.73 ± 4.3 b | 43.86 ± 1.4 c | 66.12 ± 1.9 a | <0.0001 | |
| Middle segment | Villus height | 110.3 ± 6.6 d | 143.3 ± 7.2 c | 189.6 ± 7.5 b | 286.9 ± 4.5 a | <0.0001 |
| Villus width | 60.6 ± 0.8 c | 64.30 ± 3.5 b | 67.7 ± 2.4 b | 91.4 ± 2.6 a | <0.0001 | |
| Crypt depth | 31.8 ± 3.6 c | 40.19 ± 2.6 b | 42.19 ± 2.3 b | 65.13 ± 3.9 a | <0.001 | |
| Posterior segment | Villus height | 90.72 ± 3.7 c | 106 ± 5.4 c | 136.8 ± 4.8 b | 200.2 ± 6.1 a | <0.0001 |
| Villus width | 47.29 ± 3.9 c | 61.31 ± 1.7 b | 57.46 ± 3.9 bc | 77.59 ± 1.8 a | <0.001 | |
| Crypt depth | 22.27 ± 0.5 c | 25.46 ± 0.8 bc | 31.11 ± 2.9 b | 42.77 ± 2 a | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdo, S.E.; El-Nahas, A.F.; Abdellatif, R.E.; Mohamed, R.; Helal, M.A.; Azzam, M.M.; Di Cerbo, A.; El-Kassas, S. Combined Dietary Spirulina platensis and Citrus limon Essential Oil Enhances the Growth, Immunity, Antioxidant Capacity and Intestinal Health of Nile Tilapia. Vet. Sci. 2024, 11, 474. https://doi.org/10.3390/vetsci11100474
Abdo SE, El-Nahas AF, Abdellatif RE, Mohamed R, Helal MA, Azzam MM, Di Cerbo A, El-Kassas S. Combined Dietary Spirulina platensis and Citrus limon Essential Oil Enhances the Growth, Immunity, Antioxidant Capacity and Intestinal Health of Nile Tilapia. Veterinary Sciences. 2024; 11(10):474. https://doi.org/10.3390/vetsci11100474
Chicago/Turabian StyleAbdo, Safaa E., Abeer F. El-Nahas, Rabab E. Abdellatif, Radi Mohamed, Mohamed A. Helal, Mahmoud M. Azzam, Alessandro Di Cerbo, and Seham El-Kassas. 2024. "Combined Dietary Spirulina platensis and Citrus limon Essential Oil Enhances the Growth, Immunity, Antioxidant Capacity and Intestinal Health of Nile Tilapia" Veterinary Sciences 11, no. 10: 474. https://doi.org/10.3390/vetsci11100474
APA StyleAbdo, S. E., El-Nahas, A. F., Abdellatif, R. E., Mohamed, R., Helal, M. A., Azzam, M. M., Di Cerbo, A., & El-Kassas, S. (2024). Combined Dietary Spirulina platensis and Citrus limon Essential Oil Enhances the Growth, Immunity, Antioxidant Capacity and Intestinal Health of Nile Tilapia. Veterinary Sciences, 11(10), 474. https://doi.org/10.3390/vetsci11100474








