Characterization and Pathogenicity of Colletotrichum truncatum Causing Hylocereus undatus Anthracnose through the Changes of Cell Wall-Degrading Enzymes and Components in Fruits
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fruit Sample Collection
2.2. Pathogen Isolation and Purification
2.3. Isolate Pathogenicity Determination
2.4. Morphological Characteristics of the Isolate Identification
2.5. Molecular Characteristics of the Isolate Identification
2.6. Effect of Isolate Infection on the Physiological-Biochemical Characteristics of H. undatus Fruits
2.6.1. Effect of the Isolate Infection on the Activities of Cell Wall-Degrading Enzymes of H. undatus Fruits
2.6.2. Effect of the Isolate Infection on the Contents of Cell Wall Components of H. undatus Fruits
2.7. Statistical Analysis
3. Results
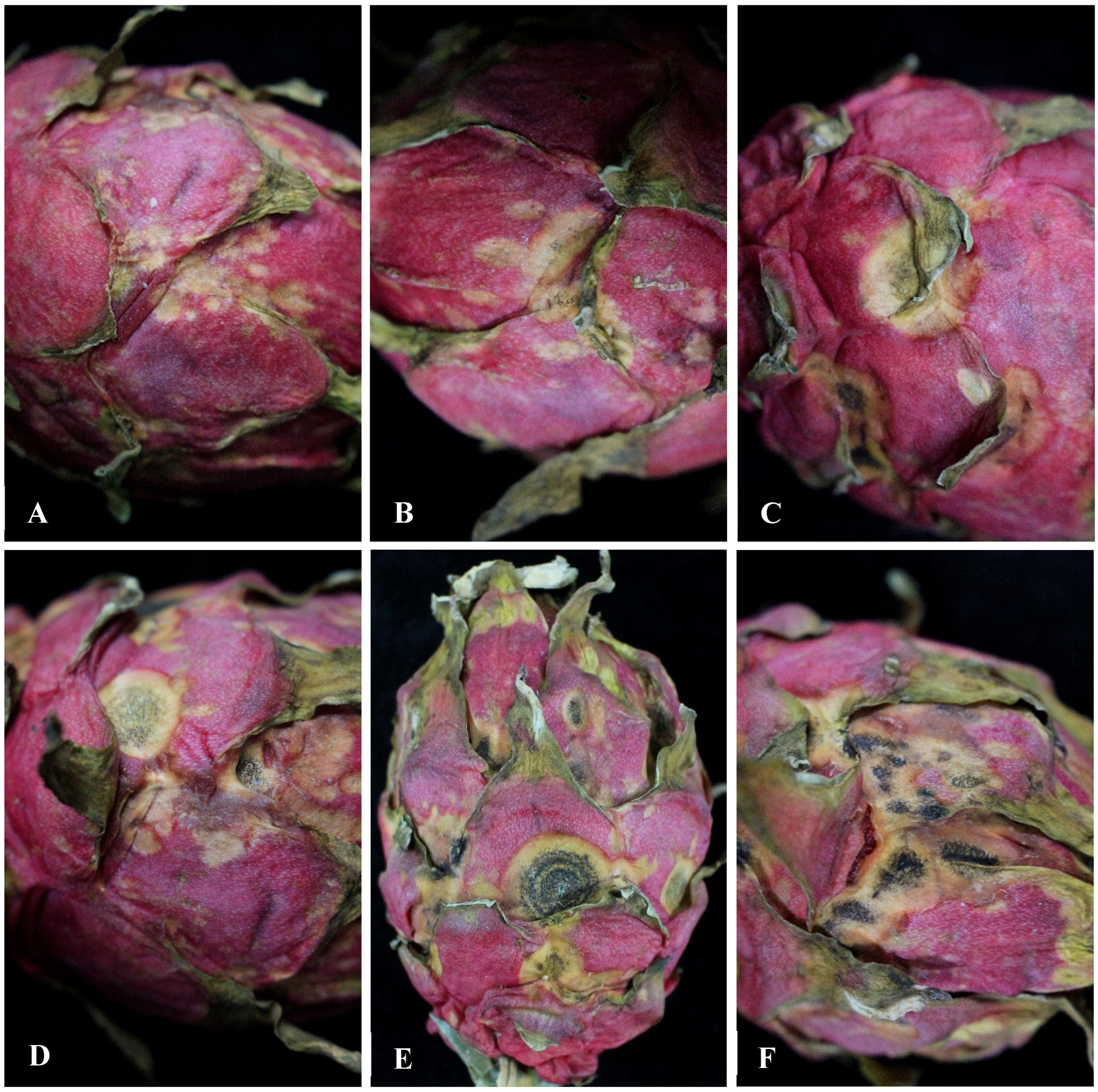
3.1. Observation of the Symptoms of H. undatus Fruits Anthracnose in Greenhouse
3.2. Isolation and Determination of the Pathogenicity of the Isolates
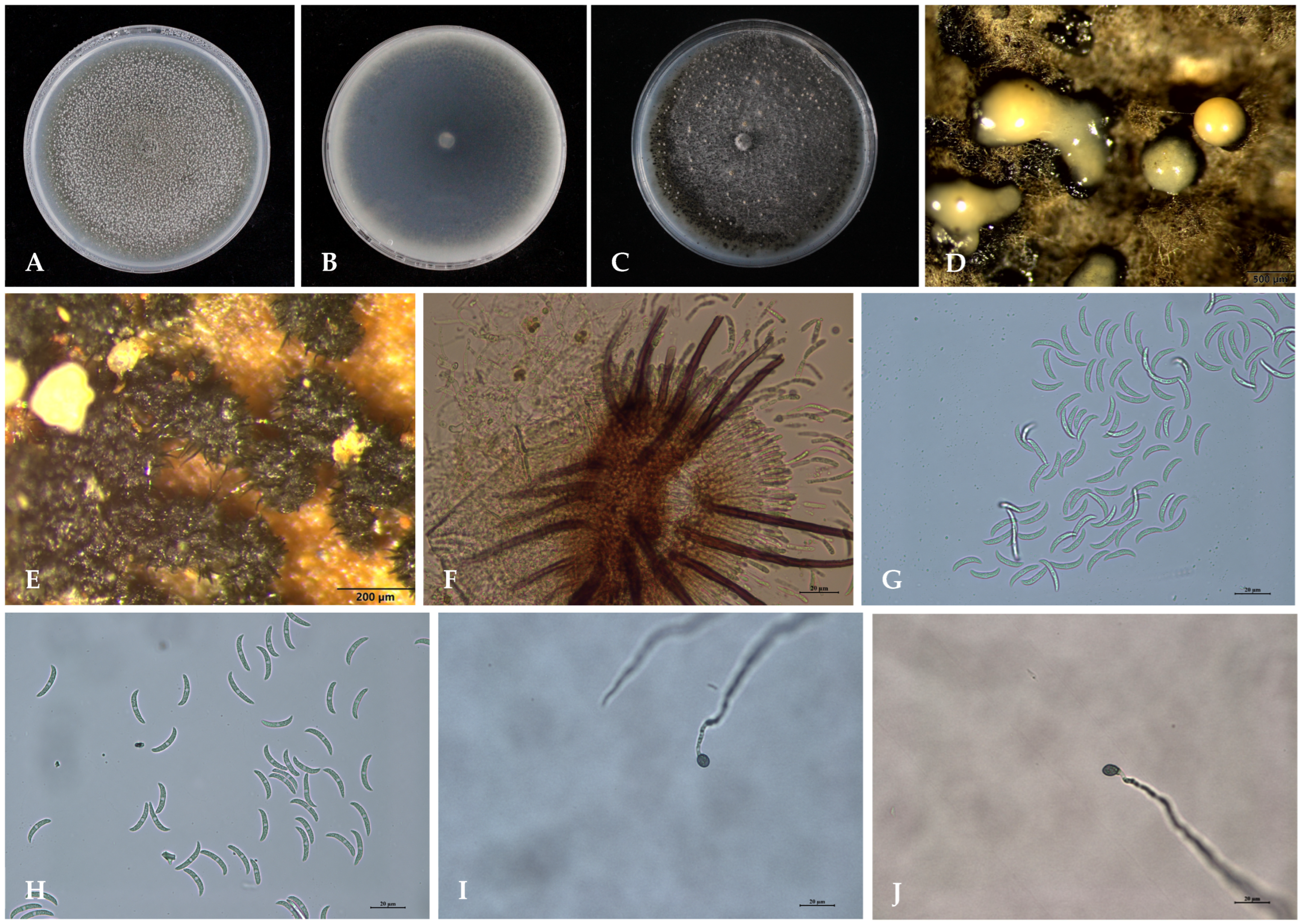
3.3. Morphological Characteristics of the Isolate
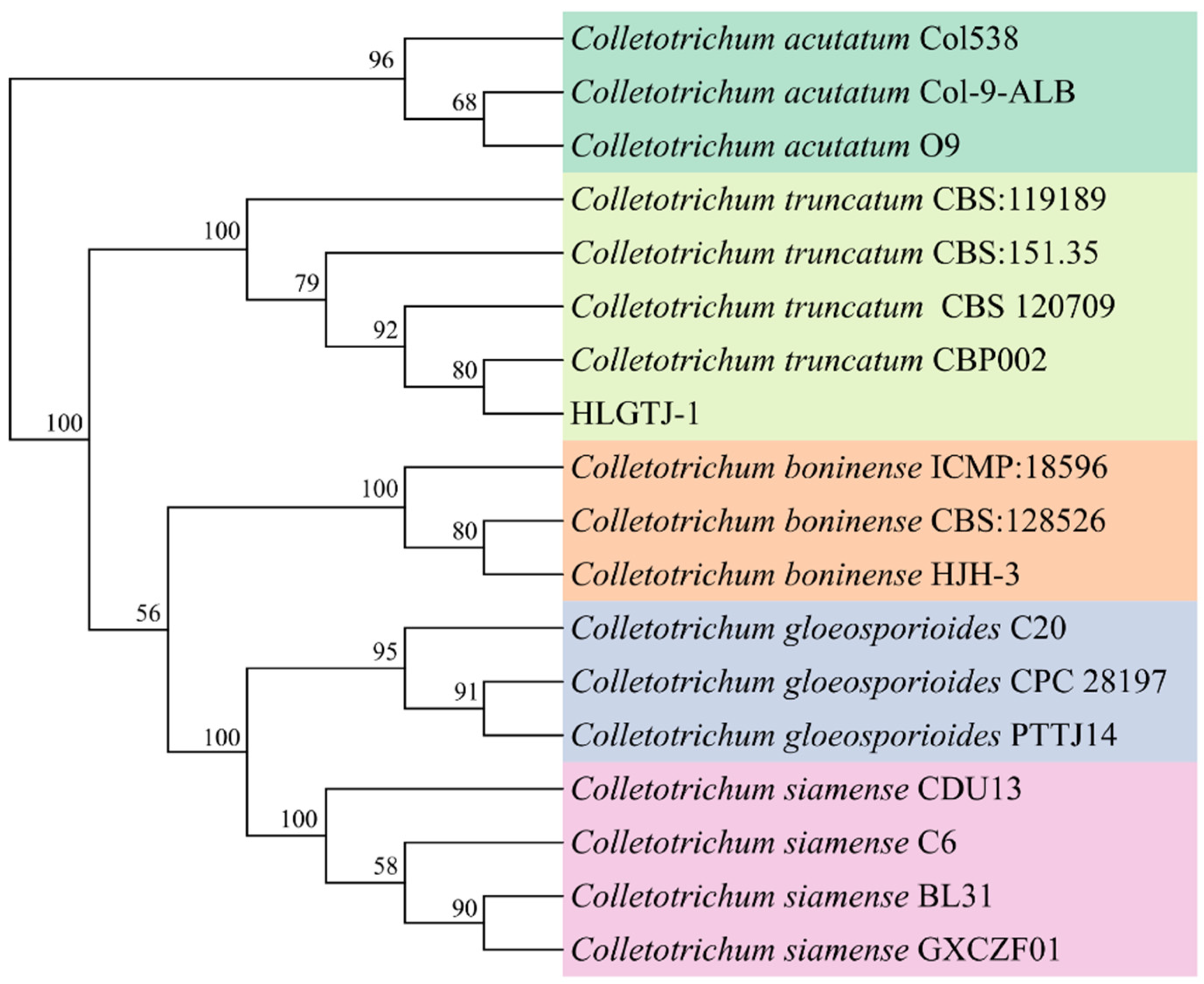
3.4. Molecular Identification of the Isolate
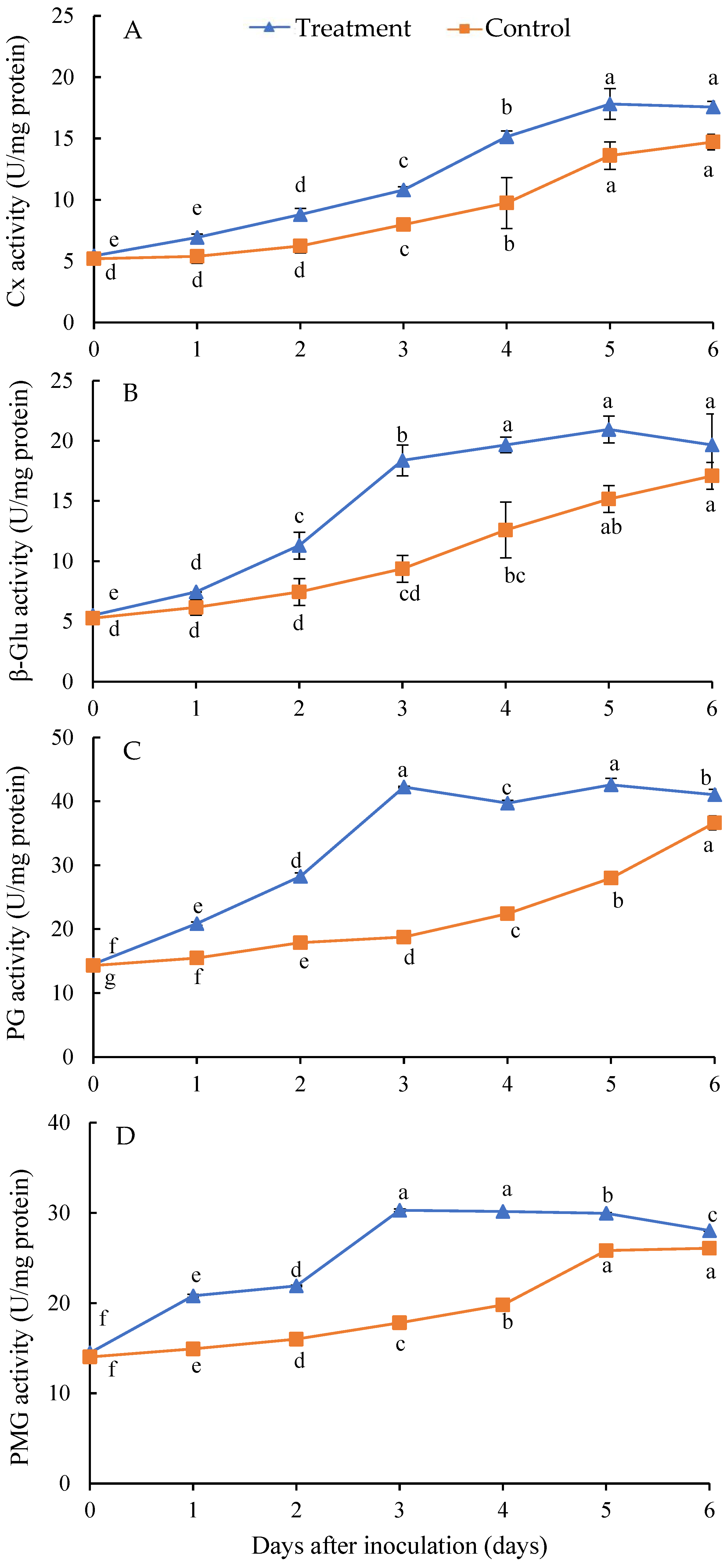
3.5. Effect of Isolate Infection on the Activities of Cell Wall-Degrading Enzymes of H. undatus Fruits
3.6. Effect of Isolate Infection on the Contents of Cell Wall Components of H. undatus Fruits
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elobeidy, A.A. Mass propagation of pitaya (dragon fruit). Fruits 2006, 61, 313–319. [Google Scholar] [CrossRef]
- Jitareerat, P.; Sripong, K.; Masaya, K.; Aiamla, S.; Uthairatanakij, A. Combined effects of food additives and heat treatment on fruit rot disease and quality of harvested dragon fruit. Agric. Nat. Resour. 2018, 52, 543–549. [Google Scholar] [CrossRef]
- Fan, P.; Huber, D.J.; Su, Z.; Hu, M.J.; Gao, Z.; Li, M.; Shi, X.; Zhang, Z. Effect of postharvest spray of apple polyphenols on the quality of fresh-cut red pitaya fruit during shelf life. Food Chem. 2018, 243, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.J.; Paull, R.E. Overall Dragon Fruit Production and Global Marketing. FFTC Agricultural Policy Platform (FFTC-AP). 2018. Available online: https://ap.fftc.org.tw/article/1596 (accessed on 14 August 2024).
- Luu, T.; Phi, Q.; Nguyen, T.; Dinh, V.; Pham, B.; Do, T. Antagonistic activity of endophytic bacteria isolated from weed plant against stem end rot pathogen of pitaya in Vietnam. Egypt. J. Biol. Pest Control 2021, 31, 14. [Google Scholar] [CrossRef]
- Ali, A.; Zahid, N.; Manickam, N.; Siddiqui, Y.; Alderson, P.G.; Maqbool, M. Effectiveness of submicron chitosan dispersions in controlling anthracnose and maintaining quality of dragon fruit. Postharvest Biol. Technol. 2013, 86, 147–153. [Google Scholar] [CrossRef]
- Ali, A.; Zahid, N.; Manickam, S.; Siddiqui, Y.; Alderson, P.G.; Maqbool, M. Induction of lignin and pathogenesis related proteins in dragon fruit plants in response to submicron chitosan dispersions. Crop Prot. 2014, 63, 83–88. [Google Scholar] [CrossRef]
- Zahid, N.; Ali, A.; Manickam, S.; Siddiqui, Y.; Alderson, P.G.; Maqbool, M. Efficacy of curative applications of SCD on anthracnose intensity and vegetative growth of dragon fruit plants. Crop Prot. 2014, 62, 129–134. [Google Scholar] [CrossRef]
- Inokuti, E.M.; Silva, D.E.M.; Almeida, M.M.M.; Oliveira, J.R.; Andrade, I.L.; Silva, C.F.B.; Corrêa, M.C.M.; Lima, C.S. First report of Colletotrichum tropicale causing anthracnose on pitaya (Hylocereus costaricensis) in Brazil. Plant Dis. 2024, 108, 797. [Google Scholar] [CrossRef]
- Nascimento, M.B.; Bellé, C.; Azambuja, R.M.; Maich, S.L.P.; Neves, C.G.; Souza-Junior, I.T.; Jacobsen, C.R.F.; Barros, D.R. First report of Colletotrichum karstii causing anthracnose spot on pitaya (Hylocereus undatus) in Brazil. Plant Dis. 2019, 103, 2137. [Google Scholar] [CrossRef]
- Masyahit, M.; Sijam, K.; Awang, Y.; Mohd Satar, M.G. The first report of the occurrence of anthracnose disease caused by Colletotrichum gloeosporioides (penz.) Penz. & Sacc. on dragon fruit (Hylocereus spp.) in Peninsular Malaysia. Am. J. Appl. Sci. 2009, 6, 902–912. [Google Scholar]
- Zahid, N.; Ali, A.; Manickam, S.; Siddiqui, Y.; Maqbool, M. Potential of chitosan loaded nanoemulsions to control different Colletotrichum spp. and maintain quality of tropical fruits during cold storage. J. Appl. Microbiol. 2012, 113, 925–939. [Google Scholar] [CrossRef] [PubMed]
- Palmateer, A.J.; Ploetz, R.C.; van Santen, E.; Correll, J.C. First occurrence of anthracnose caused by Colletotrichum gloeosporioides on Pitahaya. Plant Dis. 2007, 91, 631. [Google Scholar] [CrossRef]
- Taba, S.; Mikami, D.; Takaesu, K.; Ooshiro, A.; Moromizato, Z.; Nakasone, S.; Kawano, S. Anthracnose of pitaya (Hylocereus undatus) by Colletotrichum gloeosporioides. Jpn. J. Phytopathol. 2006, 72, 25–27. [Google Scholar] [CrossRef]
- Takahashi, L.M.; Rosa, D.D.; Basseto, M.A.; de Souza, H.G.; Furtado, E.L. First report of Colletotrichum gloeosporioides on Hylocereus megalanthus in Brazil. Australas. Plant Dis. 2008, 3, 96–97. [Google Scholar]
- Suzianti, I.V.; Intan Sakinah, M.A.; Latiffah, Z. Characterization and pathogenicity of Colletotrichum truncatum causing stem anthracnose of red-fleshed dragon fruit (Hylocereus polyrhizus) in Malaysia. J. Phytopathol. 2015, 163, 67–71. [Google Scholar]
- Salunkhe, V.N.; Bhagat, Y.S.; Lonkar, S.G.; Kakade, V.D.; Chavan, S.B.; Kochewad, S.A.; Nangare, D.D. First report of Colletotrichum truncatum causing anthracnose of dragon fruit (Hylocereus spp.) in India. Plant Dis. 2022, 106, 2754. [Google Scholar] [CrossRef]
- Abirami, K.; Sakthivel, K.; Sheoran, N.; Baskaran, V.; Gautam, R.K.; Jerard, B.A.; Kumar, A. Occurrence of anthracnose disease caused by Colletotrichum siamense on dragon fruit (Hylocereus undatus) in Andaman Islands, India. Plant Dis. 2019, 103, 768. [Google Scholar] [CrossRef]
- Nuñez-García, P.R.; Carrillo-Fasio, J.A.; Márquez-Licona, G.; Leyva-Madrigal, K.Y.; Lagunes-Fortiz, E.; Tovar-Pedraza, J.M. First report of Colletotrichum tropicale causing anthracnose on Pitahaya fruit in Mexico. Plant Dis. 2023, 106, 1750. [Google Scholar] [CrossRef] [PubMed]
- Meetum, P.; Leksomboon, C.; Kanjanamaneesathian, M. First report of Colletotrichum aenigma and C. siamense, the causal agents of anthracnose disease of dragon fruit in Thailand. J. Plant Pathol. 2015, 97, 391–403. [Google Scholar]
- Edzel, E.; John Darby, T.; Jennelyn, B.; Rodel, M.; Mark Angelo, B. First report of Colletotrichum fructicola, causing anthracnose of Hylocereus plants, in the Philippines. Czech Mycol. 2021, 73, 79–90. [Google Scholar]
- Ma, W.J.; Yang, X.; Wang, X.R.; Zeng, Y.S.; Liao, M.D.; Chen, C.J.; Sun, S.; Jia, D.M. First report of anthracnose disease on young stems of bawanghua (Hylocereus undatus) caused by Colletotrichum gloeosporioides in China. Plant Dis. 2014, 98, 991. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.J.; Chen, S.C.; Chen, Y.F.; Zou, C.C.; Wang, X.L.; Wang, Z.H.; Liu, A.R.; Ahammed, G.J. First report of red dragon fruit (Hylocereus polyrhizus) anthracnose caused by Colletotrichum siamense in China. Plant Dis. 2018, 6, 862. [Google Scholar] [CrossRef]
- Guo, L.W.; Wu, Y.X.; Ho, H.H.; Su, Y.Y.; Mao, Z.C.; He, P.F.; He, Y.Q. First report of dragon fruit (Hylocereus undatus) anthracnose caused by Colletotrichum truncatum in China. J. Phytopathol. 2014, 162, 272–275. [Google Scholar] [CrossRef]
- Lin, C.P.; Ann, P.J.; Huang, H.C.; Chang, T.T.; Tsai, J.N. Anthracnose of pitaya (Hylocereus spp.) caused by Colletotrichum spp., a new postharvest disease in Taiwan. J. Taiwan Agric. Res. 2017, 66, 171–183. [Google Scholar]
- Kubicek, C.P.; Starr, T.L.; Glass, N.L. Plant cell wall degrading enzymes and their secretion in plant-pathogenic fungi. Annu. Rev. Phytopathol. 2014, 52, 427–451. [Google Scholar] [CrossRef]
- Anand, T.; Bhaskaran, R.; Karthikeyan, T.R.G.; Rajesh, M.; Senthilraja, G. Production of cell wall degrading enzymes and toxins by Colletotrichum capsici and Alternaria alternata causing fruit rot of chillies. J. Plant Prot. Res. 2008, 48, 437–451. [Google Scholar] [CrossRef]
- Fernando, T.H.P.S.; Jayasinghe, C.K.; Wijesundera, R.L.C. Cell wall degrading enzyme secretion by Colletotrichum acutatum, the causative fungus of secondary leaf fall of Hevea brasiliensis. Mycol. Res. 2001, 105, 195–201. [Google Scholar] [CrossRef]
- Kimaru, S.K.; Monda, E.; Cheruiyot, R.C.; Mbaka, J.; Alakonya, A. Morphological and molecular identification of the causal agent of anthracnose disease of avocado in Kenya. Int. J. Microbiol. 2018, 10, 1925. [Google Scholar] [CrossRef] [PubMed]
- Almeida, D.P.F.; Huber, D.J. In vivo pectin solubility in ripening and chill-injured tomato fruit. Physiol. Plant 2007, 174, 174–182. [Google Scholar] [CrossRef]
- Zhang, S.; Xiang, D.; Sun, C.; Han, K.; Li, T.; Zhou, J.; Xu, B. Morphological and molecular identification of peach brown rot disease in Tibet and exploration of the biocontrol efficiency of Trichoderma. J. Fungi 2022, 8, 1174. [Google Scholar] [CrossRef]
- Damm, U.; Woudenberg, J.H.C.; Cannon, P.F.; Crous, P.W. Colletotrichum species with curved conidia from herbaceous hosts. Fung. Diver. 2009, 39, 45–87. [Google Scholar]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols; Academic Press: New York, NY, USA, 1990; Volume 35, pp. 315–322. [Google Scholar]
- Shi, M.; Xue, S.M.; Zhang, M.Y.; Li, S.P.; Huang, B.Z.; Huang, Q.; Liu, Q.B.; Liao, X.L.; Li, Y.Z. Colletotrichum truncatum-a new etiological anthracnose agent of sword bean (Canavalia gladiata) in southwestern China. Pathogens 2022, 11, 1463. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, Y.; Ali, A. Colletotrichum gloeosporioides (Anthracnose). In Postharvest Decay Control Strategies; Elsevier Inc.: Amsterdam, The Netherlands, 2014; pp. 337–371. [Google Scholar]
- Ford, R.; Banniza, S.; Photita, W.; Taylor, P.W.J. Morphological and molecular discrimination of Colletotrichum truncatum causing anthracnose on lentil in Canada. Australas. Plant Pathol. 2004, 33, 559–569. [Google Scholar] [CrossRef]
- García-León, E.; Cota-Barreras, G.A.; Beltrán-Peña, H.; Leyva-Madrigal, K.Y.; Valenzuela-Escoboza, F.A.; Cota-Barreras, C.I.; Tovar-Pedraza, J.M. First report of Colletotrichum truncatum causing anthracnose of guar (Cyamopsis tetragonoloba) in Mexico. Plant Dis. 2022, 106, 6. [Google Scholar] [CrossRef]
- Diao, Y.Z.; Zhang, C.; Lin, D.; Liu, X.L. First report of Colletotrichum truncatum causing anthracnose of tomato in China. Plant Dis. 2023, 98, 5. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.; Guo, W.; Zhang, G.J.; Liu, S.C.; Chen, Y. First report of Colletotrichum truncatum causing anthracnose of strawberry in China. Plant Dis. 2017, 101, 5. [Google Scholar] [CrossRef]
- Maharaj, A.; Rampersand, S.N. Genetic differentiation of Colletotrichum gloeosporioides and C. truncatum associated with anthracnose disease of papaya (Carica papaya L.) and bell pepper (Capsium annuum L.) based on ITS PCR-RFLP fingerprinting. Mol. Biotechnol. 2012, 50, 237–249. [Google Scholar] [CrossRef]
- Than, P.P.; Jeewon, R.; Hyde, K.D.; Pongsupasamit, S.; Mongkolporn, O.; Taylor, P.W.J. Characterization and pathogenicity of Colletotrichum species associated with anthracnose on chili (Capsicum spp.) in Thailand. Plant Pathol. 2008, 57, 562–572. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, S.; Lin, H.; Sun, J.; Lin, Y.; Wang, H.; Lin, M.; Shi, J. Phomopsis longanae chi-induced changes in activities of cell wall-degrading enzymes and contents of cell wall components in pericarp of harvested longan fruit and its relation to disease development. Front. Microbiol. 2018, 9, 1051. [Google Scholar] [CrossRef]
- Ramos, A.M.; Gally, M.; Szapiro, G.; Itzcovich, T.; Carabajal, M.; Levin, L. In vitro growth and cell wall degrading enzyme production by Argentinean isolates of Macrophomina phaseolina, the causative agent of charcoal rot in corn. Rev. Argent. Microbiol. 2016, 48, 267–273. [Google Scholar] [CrossRef]
- Huang, X.M.; Wang, H.C.; Gao, F.F.; Huang, H.B. A comparative study of the pericarp of litchi cultivars susceptible and resistant to fruit cracking. J. Hortic. Sci. Biotechnol. 1999, 74, 351–354. [Google Scholar] [CrossRef]
- Vorwerk, S.; Somerville, S.; Somerville, C. The role of plant cell wall polysaccharide composition in disease resistance. Trends Plant Sci. 2004, 4, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.W.; Cheng, G.P.; Yang, E.; Yi, C.; Ruenroengklin, N.; Lu, W.J.; Luo, Y.B.; Jiang, Y.M. Modification of pectin polysaccharides during ripening of postharvest banana fruit. Food Chem. 2008, 111, 144–149. [Google Scholar] [CrossRef]
- Zhou, R.; Li, Y.F.; Yan, L.P.; Xie, J. Effect of edible coatings on enzymes, cell-membrane integrity, and cell-wall constituents in relation to brittleness and firmness of Huanghua pears (Pyrus pyrifolia Nakai cv. Huanghua) during storage. Food Chem. 2011, 124, 569–575. [Google Scholar] [CrossRef]
- Chen, Y.H.; Hung, Y.C.; Chen, M.Y.; Lin, H.T. Effects of acidic electrolyzed oxidizing water on retarding cell wall degradation and delaying softening of blueberries during postharvest storage. LWT Food Sci. Technol. 2017, 84, 650–657. [Google Scholar] [CrossRef]
- Li, M.; Gao, Z.Y.; Hu, M.J.; Zhou, S.; Yang, D.P.; Yang, B.; Yi, J.X.; Yang, F.Z. Pathogenicity of cell wall degrading enzymes produced by Botryodiplodia theobromae Pat. against mangoes. Agric. Biotechnol. 2012, 1, 18–21. [Google Scholar]


| Region or Genes | Primer Name | Primer Sequence (5′–3′) | Annealing Temperature (°C) |
|---|---|---|---|
| ITS | HJ-ITS4 HJ-ITS5 | TCCTCCGCTTATTGATATGC GGAAGTAAAAGTCGTAACAAGG | 56 |
| GAPDH | HJ-GAPDHF HJ-GAPDHR | GCCGTCAACGACCCCTTCATTG GGGTGGAGTCGTACTTGAGCAT | 56 |
| HIS3 | HJ-HIS3F HJ-HIS3R | AGGTCCACTGGTGGCAAG AGCTGGATGTCCTTGGACTG | 54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Liu, Y.; Liu, J.; Li, E.; Xu, B. Characterization and Pathogenicity of Colletotrichum truncatum Causing Hylocereus undatus Anthracnose through the Changes of Cell Wall-Degrading Enzymes and Components in Fruits. J. Fungi 2024, 10, 652. https://doi.org/10.3390/jof10090652
Zhang S, Liu Y, Liu J, Li E, Xu B. Characterization and Pathogenicity of Colletotrichum truncatum Causing Hylocereus undatus Anthracnose through the Changes of Cell Wall-Degrading Enzymes and Components in Fruits. Journal of Fungi. 2024; 10(9):652. https://doi.org/10.3390/jof10090652
Chicago/Turabian StyleZhang, Shuwu, Yun Liu, Jia Liu, Enchen Li, and Bingliang Xu. 2024. "Characterization and Pathogenicity of Colletotrichum truncatum Causing Hylocereus undatus Anthracnose through the Changes of Cell Wall-Degrading Enzymes and Components in Fruits" Journal of Fungi 10, no. 9: 652. https://doi.org/10.3390/jof10090652






