Effect of Micro-Nanobubbles on Arsenic Removal by Trichoderma atroviride for Bioscorodite Generation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganism and Growth Conditions
2.2. Set Up of the Micro-Nanobubbles Aeration
2.3. Determination of Culture Parameters of the Fungus T. atroviride
2.4. Biochemical Parameters
2.4.1. Residual Glucose
2.4.2. Glucose Oxidase Enzyme Activity
2.4.3. Biomass
2.5. Physicochemical Parameters
2.6. Scorodite Production
2.6.1. Chemical Scorodite
2.6.2. Bioscorodite
2.7. Characterization of Chemical Scorodite and Bioscorodite
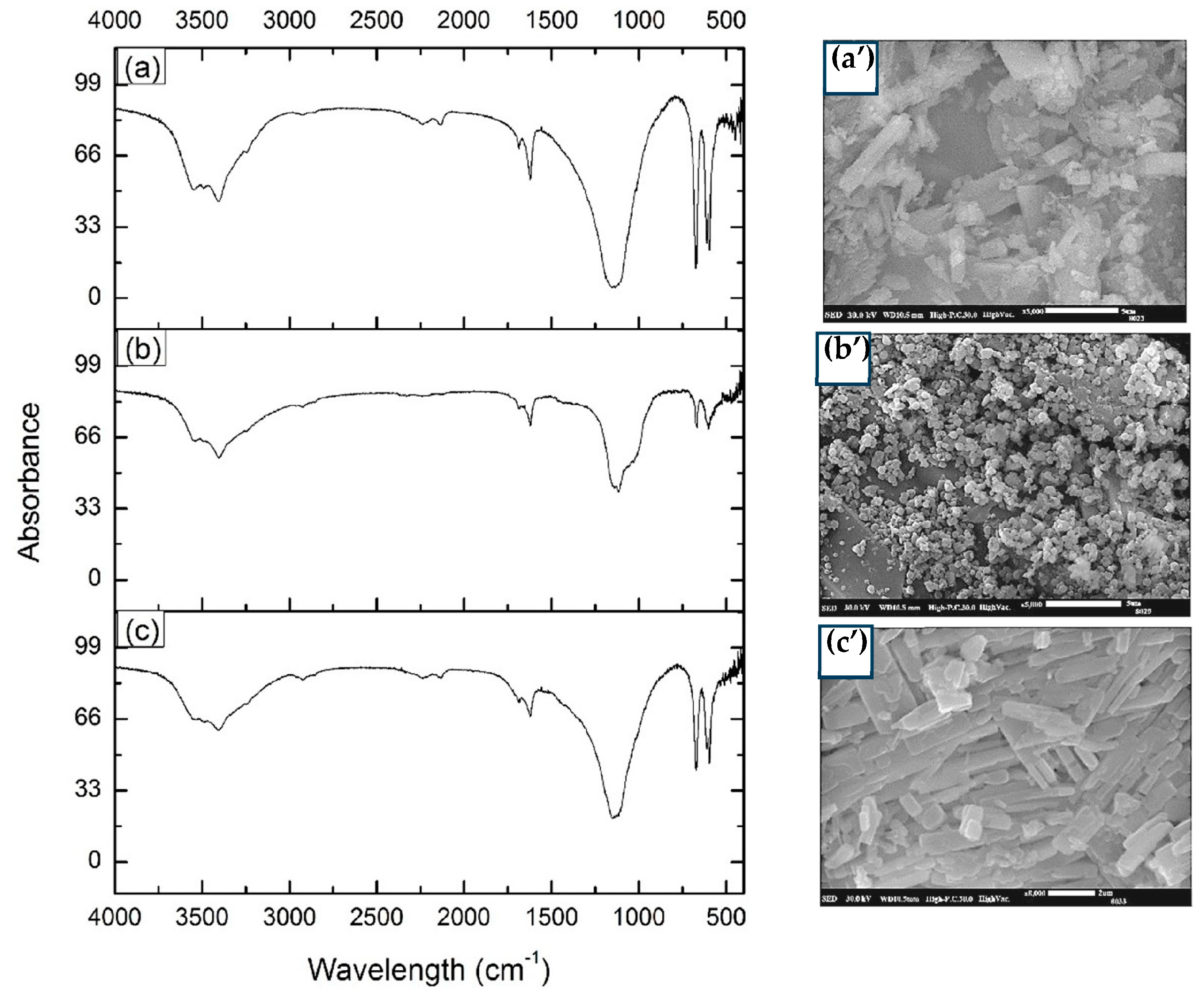
2.7.1. Fourier Transformation Infrared Spectrophotometry (FTIR)
2.7.2. Scanning Electronic Microscopy (SEM)
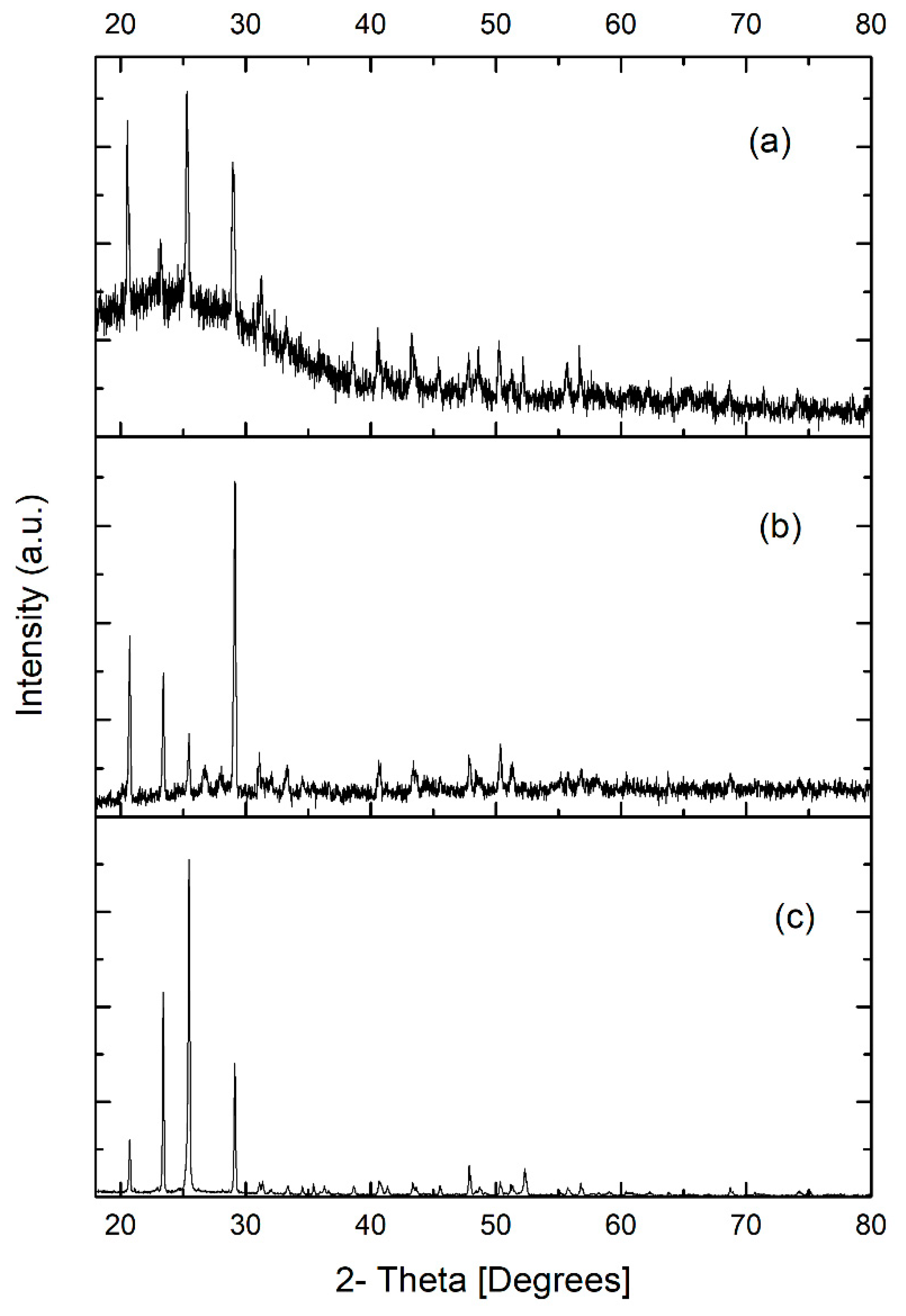
2.7.3. X-ray Diffraction Analysis
2.8. As Determination
2.9. Statistical Analysis
3. Results
3.1. Nanobubbling Size Determination in the TR-MNBs System
3.2. Glucose Consumption, Glucose Oxidase Activity and Biomass Produced
3.3. Determination of Physicochemical Parameters
3.4. Bioscorodite Characterization
3.4.1. Fourier Transform Infrared Spectroscopy
3.4.2. Scanning Electron Microscopy (SEM)
3.4.3. X-ray Diffraction
3.5. Determination of As in the Culture Medium of T. atroviride
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rathi, B.S.; Kumar, P.S. A review on sources, identification and treatment strategies for the removal of toxic Arsenic from water system. J. Hazard. Mater. 2021, 418, 126299. [Google Scholar] [CrossRef] [PubMed]
- Mohd, S.; Kushwaha, A.S.; Shukla, J.; Mandrah, K.; Shankar, J.; Arjaria, N.; Saxena, P.N.; Khare, P.; Narayan, R.; Dixit, S.; et al. Fungal mediated biotransformation reduces toxicity of arsenic to soil dwelling microorganism and plant. Ecotoxicol. Environ. Saf. 2019, 176, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Qin, X.; Tang, J.; Liu, W.; Yang, H. Review of arsenic geochemical characteristics and its significance on arsenic pollution studies in karst groundwater, Southwest China. Appl. Geochem. 2017, 77, 80–88. [Google Scholar] [CrossRef]
- Fang, Y.; Chen, M.; Liu, C.; Dong, L.; Zhou, J.; Yi, X.; Ji, D.; Qiao, J.; Tong, H. Arsenic release from microbial reduction of scorodite in the presence of electron shuttle in flooded soil. J. Environ. Sci. 2023, 126, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Nam, I.-H.; Murugesan, K.; Ryu, J.; Kim, J.H. Arsenic (As) Removal Using Talaromyces sp. KM-31 Isolated from As-Contaminated Mine Soil. Minerals 2019, 9, 568. [Google Scholar] [CrossRef]
- Raju, N.J. Arsenic in the geo-environment: A review of sources, geochemical processes, toxicity and removal technologies. Environ. Res. 2022, 203, 111782. [Google Scholar] [CrossRef]
- Beni, C.; Marconi, S.; Boccia, P.; Ciampa, A.; Diana, G.; Aromolo, R.; Sturchio, E.; Neri, U.; Sequi, P.; Valentini, M. Use of arsenic contaminated irrigation water for lettuce cropping: Effects on Soil, Groundwater, and Vegetal. Biol. Trace Element Res. 2011, 143, 518–529. [Google Scholar] [CrossRef]
- Smedley, P.L.; Kinniburgh, D.G. A review of the source, behaviour and distribution of arsenic in natural waters. Appl. Geochem. 2002, 17, 517–519. [Google Scholar] [CrossRef]
- Cubadda, F.; Jackson, B.P.; Cottingham, K.L.; Van Horne, Y.O.; Kurzius-Spencer, M. Human exposure to dietary inorganic arsenic and other arsenic species: State of knowledge, gaps and uncertainties. Sci. Total. Environ. 2017, 579, 1228–1239. [Google Scholar] [CrossRef]
- Okibe, N.; Koga, M.; Morishita, S.; Tanaka, M.; Heguri, S.; Asano, S.; Sasaki, K.; Hirajima, T. Microbial formation of crystalline scorodite for treatment of As(III)-bearing copper refinery process solution using Acidianus brierleyi. Hydrometallurgy 2014, 143, 34–41. [Google Scholar] [CrossRef]
- Gonzalez-Contreras, P.; Weijma, J.; Buisman, C.J.N. Bioscorodite crystallization in an airlift reactor for arsenic removal. Cryst. Growth Des. 2012, 12, 2699–2706. [Google Scholar] [CrossRef]
- Gonzalez-Contreras, P.; Weijma, J.; van der Weijden, R.; Buisman, C.J.N. Biogenic scorodite crystallization by Acidianus sulfidivorans for arsenic removal. Environ. Sci. Technol. 2010, 44, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Pérez, J.L.; Vázquez, R.R. Removal of orange G dye by Aspergillus niger and its effect on organic acid production. Prep. Biochem. Biotechnol. 2022, 53, 860–871. [Google Scholar] [CrossRef]
- Jaiswal, V.; Saxena, S.; Kaur, I.; Dubey, P.; Nand, S.; Naseem, M.; Singh, S.; Srivastava, P.; Barik, S.K. Application of four novel fungal strains to remove arsenic from contaminated water in batch and column modes. J. Hazard. Mater. 2018, 356, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Vala, A.K. Tolerance and removal of arsenic by a facultative marine fungus Aspergillus candidus. Bioresour. Technol. 2010, 101, 2565–2567. [Google Scholar] [CrossRef] [PubMed]
- Granchinho, S.C.R.; Franz, C.M.; Polishchuk, E.; Cullen, W.R.; Reimer, K.J. Transformation of arsenic(V) by the fungus Fusarium oxysporum melonis isolated from the alga Fucus gardneri. Appl. Organomet. Chem. 2002, 16, 721–726. [Google Scholar] [CrossRef]
- Srivastava, P.K.; Vaish, A.; Dwivedi, S.; Chakrabarty, D.; Singh, N.; Tripathi, R.D. Biological removal of arsenic pollution by soil fungi. Sci. Total. Environ. 2011, 409, 2430–2442. [Google Scholar] [CrossRef]
- Tripathi, P.; Singh, P.C.; Mishra, A.; Chauhan, P.S.; Dwivedi, S.; Bais, R.T.; Tripathi, R.D. Trichoderma: A potential bioremediator for environmental clean up. Clean Technol. Environ. Policy 2013, 15, 541–550. [Google Scholar] [CrossRef]
- Ramírez-Castillo, J.A.; Rodríguez-Vázquez, R.; Aguilar-López, R.; Zúñiga-Silva, J.R. Bioscorodite production from As(III) and Fe(II) salts under oxidizing and acidic conditions of Trichoderma atroviride culture. Water 2023, 15, 1905. [Google Scholar] [CrossRef]
- Levitsky, I.; Tavor, D.; Gitis, V. Micro and nanobubbles in water and wastewater treatment: A state-of-the-art review. J. Water Process. Eng. 2022, 47, 102688. [Google Scholar] [CrossRef]
- Batagoda, J.H.; Hewage, S.D.A.; Meegoda, J.N. Nano-ozone bubbles for drinking water treatment. J. Environ. Eng. Sci. 2019, 14, 57–66. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, K.; Cen, C.; Wu, X.; Mao, R.; Zheng, Y. Role of bulk nanobubbles in removing organic pollutants in wastewater treatment. AMB Express 2021, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, A.; Etchepare, R.; Calgaroto, S.; Rubio, J. Aqueous dispersions of nanobubbles: Generation, properties and features. Miner. Eng. 2016, 94, 29–37. [Google Scholar] [CrossRef]
- Khan, P.; Zhu, W.; Huang, F.; Gao, W.; Khan, N.A. Micro–nanobubble technology and water-related application. Water Supply 2020, 20, 2021–2035. [Google Scholar] [CrossRef]
- Haris, S.; Qiu, X.; Klammler, H.; Mohamed, M.M. The use of micro-nano bubbles in groundwater remediation: A comprehensive review. Groundw. Sustain. Dev. 2020, 11, 100463. [Google Scholar] [CrossRef]
- Cortés-Espinosa, D.; Fernández, F.J.; Ainhoa-Arana, A.; Rodríguez-Vázquez, R. Selection and identification of fungi isolated from sugarcane bagasse and their application for phenanthrene removal from soil. J. Environ. Sci. Health Part A Toxic/Hazard. Subst. Environ. Eng. 2006, 41, 475–486. [Google Scholar] [CrossRef]
- Chávez-Gómez, B.; Quintero, R.; Esparza-García, F.; Mesta-Howard, A.; de la Serna, F.Z.D.; Hernández-Rodríguez, C.; Gillén, T.; Poggi-Varaldo, H.; Barrera-Cortés, J.; Rodríguez-Vázquez, R. Removal of phenanthrene from soil by co-cultures of bacteria and fungi pregrown on sugarcane bagasse pith. Bioresour. Technol. 2003, 89, 177–183. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Lira-Pérez, J.; Hidalgo-Lara, M.; Melendez-Estrada, J.; Jesus, B.G.-D.; Rodríguez-Vazquéz, R. The contribution of H2O2 produced by Aspergillus niger in vat blue dye discoloration: Enhancement by a statistical optimization methodology. Rev. Mex. Ing. Química 2019, 18, 701–714. [Google Scholar] [CrossRef]
- Temesgen, T.; Bui, T.T.; Han, M.; Kim, T.-I.; Park, H. Micro and nanobubble technologies as a new horizon for water-treatment techniques: A review. Adv. Colloid Interface Sci. 2017, 246, 40–51. [Google Scholar] [CrossRef]
- Bauer, J.A.; Zámocká, M.; Majtán, J.; Bauerová-Hlinková, V. Glucose oxidase, an enzyme “Ferrari”: Its Structure, Function, Production and Properties in the Light of Various Industrial and Biotechnological Applications. Biomolecules 2022, 12, 472. [Google Scholar] [CrossRef] [PubMed]
- Rossi-Rodrigues, B.C.; Brochetto-Braga, M.R.; Tauk-Tornisielo, S.M.; Carmona, E.C.; Arruda, V.M.; Netto, J.C. Comparative growth of Trichoderma strains in different nutritional sources, using bioscreen c automated system. Braz. J. Microbiol. 2009, 40, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Lund, P.A.; De Biase, D.; Liran, O.; Scheler, O.; Mira, N.P.; Cetecioglu, Z.; Fernández, E.N.; Bover-Cid, S.; Hall, R.; Sauer, M.; et al. Understanding how microorganisms respond to acid pH is central to their control and successful exploitation. Front. Microbiol. 2020, 11, 556140. [Google Scholar] [CrossRef] [PubMed]
- Tec-Caamal, E.N.; Rodríguez-Vázquez, R.; Weijma, J.; Aguilar-López, R. Simulation platform for in-situ Fe(II) oxidation and bioscorodite crystallization in a one-step process for As(V) immobilization from acid wastewater. Miner. Eng. 2021, 172, 107170. [Google Scholar] [CrossRef]
- Liaud, N.; Giniés, C.; Navarro, D.; Fabre, N.; Crapart, S.; Gimbert, I.H.; Levasseur, A.; Raouche, S.; Sigoillot, J.-C. Exploring fungal biodiversity: Organic acid production by 66 strains of filamentous fungi. Fungal Biol. Biotechnol. 2014, 1, 1–10. [Google Scholar] [CrossRef]
- Scholz, S.A.; Graves, I.; Minty, J.J.; Lin, X.N. Production of cellulosic organic acids via synthetic fungal consortia. Biotechnol. Bioeng. 2018, 115, 1096–1100. [Google Scholar] [CrossRef]
- Li, D.; Mi Zou, M.; Jiang, L. Dissolved oxygen control strategies for water treatment: A review. Water Sci. Technol. 2022, 86, 1444–1466. [Google Scholar] [CrossRef] [PubMed]
- Boyd, C.E. Dissolved oxygen and redox potential. In Water Quality; Springer: Boston, MA, USA, 2000. [Google Scholar] [CrossRef]
- Rong, Z.; Tang, X.; Wu, L.; Chen, X.; Dang, W.; Wang, Y. A novel method to synthesize scorodite using ferrihydrite and its role in removal and immobilization of arsenic. J. Mater. Res. Technol. 2020, 9, 5848–5857. [Google Scholar] [CrossRef]
- Johnson, D.B.; Dybowska, A.; Schofield, P.F.; Herrington, R.J.; Smith, S.L.; Santos, A.L. Bioleaching of arsenic-rich cobalt mineral resources, and evidence for concurrent biomineralization of scorodite during oxidative bio-processing of skutterudite. Hydrometallurgy 2020, 195, 105395. [Google Scholar] [CrossRef]
- Li, G.; Qi, X.; Shi, J.; Yan, G.; Wang, H.; Zhang, A. Removal of arsenic from smelting wastewater using Fe3O4 as an in situ Fe source: The effect of pre-dissolution and the evolution process of scorodite. Environ. Sci. Water Res. Technol. 2022, 8, 2796–2806. [Google Scholar] [CrossRef]
- Yang, J.; Chai, L.; Yue, M.; Li, Q. Complexation of arsenate with ferric ion in aqueous solutions. RSC Adv. 2015, 5, 103936–103942. [Google Scholar] [CrossRef]
- Ma, X.; Qi, F.; Gomez, M.A.; Su, R.; Yan, Z.; Yao, S.; Wang, S.; Jia, Y. Spectroscopic study on the local structure of sulfate (SO42−) incorporated in scorodite (FeAsO4·2H2O) lattice: Implications for understanding the Fe(III)-As(V)-SO42−-bearing minerals formation. Am. Miner. 2022, 107, 1840–1849. [Google Scholar] [CrossRef]
- Vega-Hernandez, S.; Sanchéz-Andrea, I.; Weijma, J.; Buisman, C.J. An integrated green methodology for the continuous biological removal and fixation of arsenic from acid wastewater through the GAC-catalyzed As(III) oxidation. Chem. Eng. J. 2021, 421, 127758. [Google Scholar] [CrossRef]
- Qi, X.; Li, Y.; Wei, L.; Hao, F.; Zhu, X.; Wei, Y.; Li, K.; Wang, H. Disposal of high-arsenic waste acid by the stepwise formation of gypsum and scorodite. RSC Adv. 2020, 10, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Adachi, K.; Anezaki, T.; Karube, T.; Iizuka, A.; Shibata, E. pH dependance of scorodite formation in As(V) solution using magnetite as the solid iron source. Mater. Trans. 2022, 63, 1287–1293. [Google Scholar] [CrossRef]
- Su, R.; Ma, X.; Yin, X.; Zhao, X.; Yan, Z.; Lin, J.; Zeng, X.; Zhang, D.; Wang, S.; Jia, Y. Arsenic removal from hydrometallurgical waste sulfuric acid via scorodite formation using siderite (FeCO3). Chem. Eng. J. 2021, 424, 130552. [Google Scholar] [CrossRef]
- Kitamura, Y.; Okawa, H.; Kato, T.; Sugawara, K. Effect of ultrasound intensity on the size and morphology of synthesized scorodite particles. Adv. Powder Technol. 2016, 27, 891–897. [Google Scholar] [CrossRef]
- Iizuka, A.; Adachi, K.; Shibata, E. Investigation of the scorodite formation mechanism in As(V) solution containing Fe(II) with hematite addition using a stable iron isotope. Mater. Trans. 2022, 63, 655–661. [Google Scholar] [CrossRef]
- Tabelin, C.B.; Corpuz, R.D.; Igarashi, T.; Villacorte-Tabelin, M.; Ito, M.; Hiroyoshi, N. Hematite-catalyzed scorodite formation as a noel arsenic immobilization strategy under ambient conditions. Chemosphere. 2019, 233, 946–953. [Google Scholar] [CrossRef]
- Castro, L.d.S.; Pedersoli, W.R.; Antoniêto, A.C.C.; Steindorff, A.S.; Silva-Rocha, R.; Martinez-Rossi, N.M.; Rossi, A.; Brown, N.A.; Goldman, G.H.; Faça, V.M.; et al. Comparative metabolism of cellulose, sophorose and glucose in Trichoderma reeseiusing high-throughput genomic and proteomic analyses. Biotechnol. Biofuels. 2014, 7, 41. [Google Scholar] [CrossRef]
- Sati, S.; Bisht, S. Utilization of various carbon sources for the growth of waterborne conidial fungi. Mycologia 2006, 98, 678–681. [Google Scholar] [CrossRef] [PubMed]
- Verma, M.; Brar, S.K.; Tyagi, R.D.; Surampalli, R.Y.; Valéro, J.R. Dissolved oxygen as principal parameter for conidia production of biocontrol fungi Trichoderma viride in non-Newtonian wastewater. J. Ind. Microbiol. Biotechnol. 2006, 33, 941–952. [Google Scholar] [CrossRef] [PubMed]
- Prakash, V.; Basu, K. Mass multiplication of Trichoderma in bioreactors. In Trichoderma: Agricultural Applications and Beyond; Soil Biology; Manoharachary, C., Singh, H.B., Varma, A., Eds.; Springer: Cham, Switzerland, 2020; Volume 61. [Google Scholar] [CrossRef]
- Hernández-Peñaranda, A.M.; Salazar-Montoya, J.A.; Rodríguez-Vázquez, R.; Ramos-Ramírez, E.G. Rheological behavior of Phanerochaete chrysosporium broth during lignin degradation. J. Environ. Sci. Health Part A 2001, 36, 1983–1996. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Yuan, S.; Song, H.; Li, X. Micro-nano bubbles production using a swirling-type venturi bubble generator. Chem. Eng. Process. Intensif. 2022, 170, 108697. [Google Scholar] [CrossRef]
- Kumar, A.; Yadav, A.N.; Mondal, R.; Kour, D.; Subrahmanyam, G.; Shabnam, A.A.; Khan, S.A.; Yadav, K.K.; Sharma, G.K.; Cabral-Pinto, M.; et al. Myco-remediation: A mechanistic understanding of contaminants alleviation from natural environment and future prospect. Chemosphere 2021, 284, 131325. [Google Scholar] [CrossRef] [PubMed]
- Mobilian, C.; Craft, C.B. Wetland Soils: Physical and Chemical Properties and Biogeochemical Processes. In Encyclopedia of Inland Waters, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar] [CrossRef]
- Huang, L.; Ji, X.; Nan, B.; Yang, P.; Shi, H.; Wu, Y.; Yu, D.; Wu, H.; Zhang, Y.; Xiao, P. Enhanced photoelectrochemical water oxidation by Micro− Nano Bubbles: Measurements and Mechanisms. J. Alloys Compd. 2023, 965, 171449. [Google Scholar] [CrossRef]
- Sakr, M.; Mohamed, M.M.; Maraqa, M.A.; Hamouda, M.A.; Hassan, A.A.; Ali, J.; Jung, J. A critical review of the recent developments in micro–nano bubbles applications for domestic and industrial wastewater treatment. Alex. Eng. J. 2021, 61, 6591–6612. [Google Scholar] [CrossRef]
- Jain, P.; Singh, P.; Singh, M.B. Fundamental of advanced oxidation processes. Adv. Oxid. Process. Dye.-Contain. Wastewater 2022, 1, 1–19. [Google Scholar] [CrossRef]
- Mushtaq, S.; Bareen, F.; Tayyeb, A. Equilibrium kinetics and thermodynamic studies on biosorption of heavy metals by metal-resistant strains of Trichoderma isolated from tannery solid waste. Environ. Sci. Pollut. Res. 2023, 30, 10925–10954. [Google Scholar] [CrossRef]
- Coelho, E.; Reis, T.A.; Cotrim, M.; Mullan, T.K.; Corrêa, B. Resistant fungi isolated from contaminated uranium mine in Brazil shows a high capacity to uptake uranium from water. Chemosphere 2020, 248, 126068. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S.; Duc, P.A.; Rangasamy, G. Strategies for microbial bioremediation of environmental pollutants from industrial wastewater: A sustainable approach. Chemosphere 2022, 313, 137323. [Google Scholar] [CrossRef] [PubMed]
- Barahona, S.; Herrera, E.; Jara, A.; Castro-Severyn, J.; Gallardo, K.; Fuentes, G.; Dorador, C.; Saavedra, C.; Remonsellez, F. Arsenopyrite dissolution and bioscorodite precipitation by Acidithiobacillus ferrivorans ACH under Mesophilic Condition. Minerals 2022, 12, 520. [Google Scholar] [CrossRef]
- Ke, P.-C.; Liu, Z.-H. Synthesis, in-situ coating and characterization of scorodite with high leaching stability. Trans. Nonferrous Met. Soc. China 2019, 29, 876–892. [Google Scholar] [CrossRef]
- Vega-Hernandez, S.; Weijma, J.; Buisman, C.J. Particle size control of biogenic scorodite during the GAC-catalysed As(III) oxidation for efficient arsenic removal in acid wastewaters. Water Resour. Ind. 2020, 3, 100128. [Google Scholar] [CrossRef]
- Singhania, S.; Wang, Q.; Filippou, D.; Demopoulos, G.P. Temperature and seeding effects on the precipitation of scorodite from sulfate solutions under atmospheric-pressure conditions. Met. Mater. Trans. B 2005, 36, 327–333. [Google Scholar] [CrossRef]
- Shi, J.; Duan, X.; Qi, X.; Li, G.; Yan, G.; Wang, H. Removal of arsenic from copper smelting wastewater using zinc slag to synthesize scorodite. J. Mater. Sci. Mater. Electron. 2023, 34, 973. [Google Scholar] [CrossRef]
- Tanaka, M.; Okibe, N. Factors to enable crystallization of environmentally stable bioscorodite from dilute As(III)-contaminated waters. Minerals 2018, 8, 23. [Google Scholar] [CrossRef]
- Fujita, T.; Taguchi, R.; Abumiya, M.; Matsumoto, M.; Shibata, E.; Nakamura, T. Effect of pH on atmospheric scorodite synthesis by oxidation of ferrous ions: Physical properties and stability of the scorodite. Hydrometallurgy 2009, 96, 189–198. [Google Scholar] [CrossRef]
- Min, X.-B.; Liao, Y.-P.; Chai, L.-Y.; Yang, Z.-H.; Xiong, S.; Liu, L.; Li, Q.-Z. Removal and stabilization of arsenic from anode slime by forming crystal scorodite. Trans. Nonferrous Met. Soc. China 2015, 25, 1298–1306. [Google Scholar] [CrossRef]
- Guo, F.; Demopoulos, G.P. Development of an encapsulation process to extend the stability of scorodite under wider pH and redox potential range conditions. In Extraction 2018: Proceedings of the First Global Conference on Extractive Metallurgy; Springer International Publishing: New York, NY, USA, 2018; Volume 1, pp. 1411–1420. [Google Scholar] [CrossRef]
- Majzlan, J.; Alpers, C.N.; Koch, C.B.; McCleskey, R.B.; Myneni, S.C.; Neil, J.M. Vibrational, X-ray absorption, and Mössbauer spectra of sulfate minerals from the weathered massive sulfide deposit at Iron Mountain, California. Chem. Geol. 2011, 284, 296–305. [Google Scholar] [CrossRef]
- Mauro, D.; Biagioni, C.; Pasero, M.; Zaccarini, F. Crystal-chemistry of sulfates from Apuan Alps (Tuscany, Italy). II. Crystal structure and hydrogen bonding system of römerite, Fe2+Fe3+2(SO4)4(H2O)14. Atti Soc. Tosc. Sci. Nat. Mem. Ser. A 2018, 125, 5–11. [Google Scholar] [CrossRef]
- Tabelin, C.B.; Veerawattananun, S.; Ito, M.; Hiroyoshi, N.; Igarashi, T. Pyrite oxidation in the presence of hematite and alumina: II. Effects on the cathodic and anodic half-cell reactions. Sci. Total. Environ. 2017, 581–582, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Voegelin, A.; Hug, S.J. Catalyzed oxidation of arsenic(III) by hydrogen peroxide on the surface of ferrihydrite: An in Situ ATR-FTIR study. Environ. Sci. Technol. 2003, 37, 972–978. [Google Scholar] [CrossRef] [PubMed]

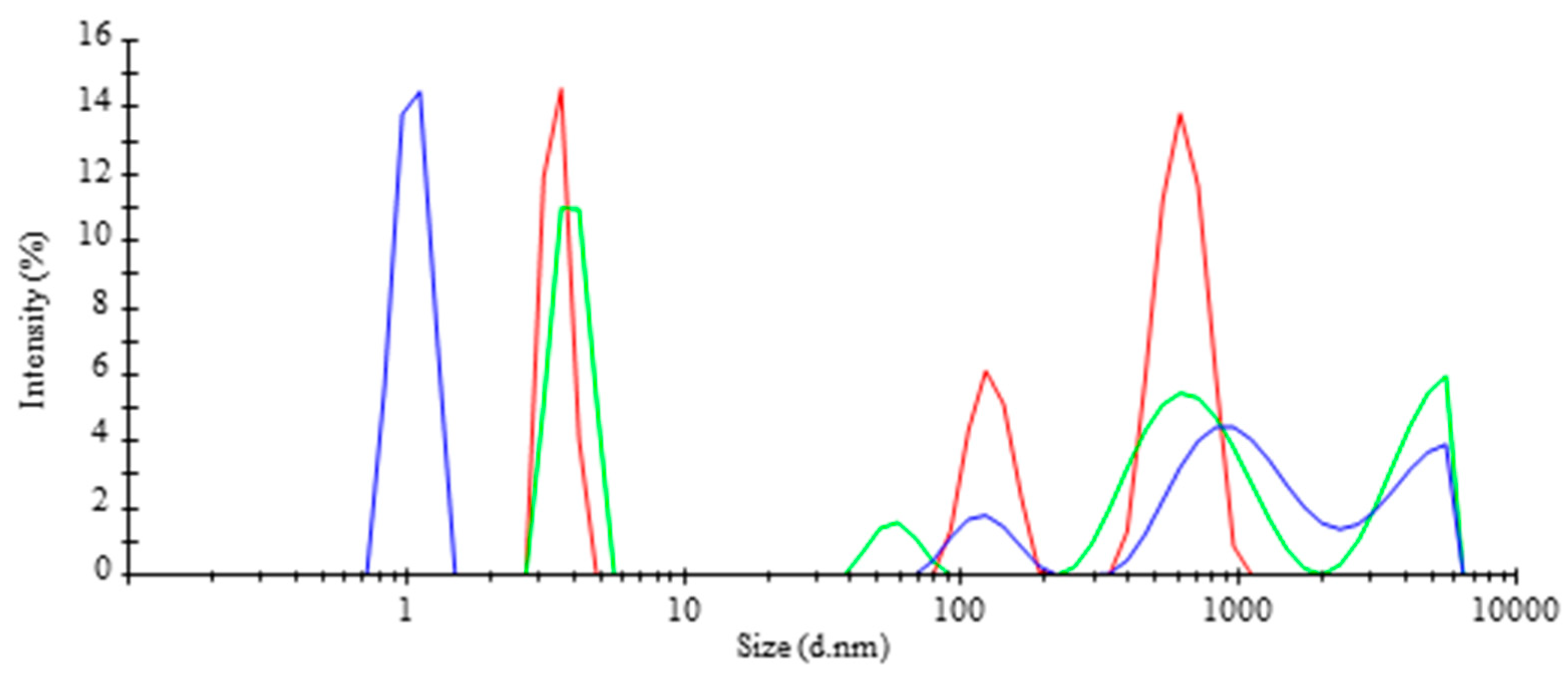
 ), TR-MNBs system (
), TR-MNBs system ( ). * Indicates significant difference (p < 0.05).
). * Indicates significant difference (p < 0.05).
 ), TR-MNBs system (
), TR-MNBs system ( ). * Indicates significant difference (p < 0.05).
). * Indicates significant difference (p < 0.05).
 ), TR-MNBs system (
), TR-MNBs system ( ). * Indicates significant difference (p < 0.05).
). * Indicates significant difference (p < 0.05).
 ), TR-MNBs system (
), TR-MNBs system ( ). * Indicates significant difference (p < 0.05).
). * Indicates significant difference (p < 0.05).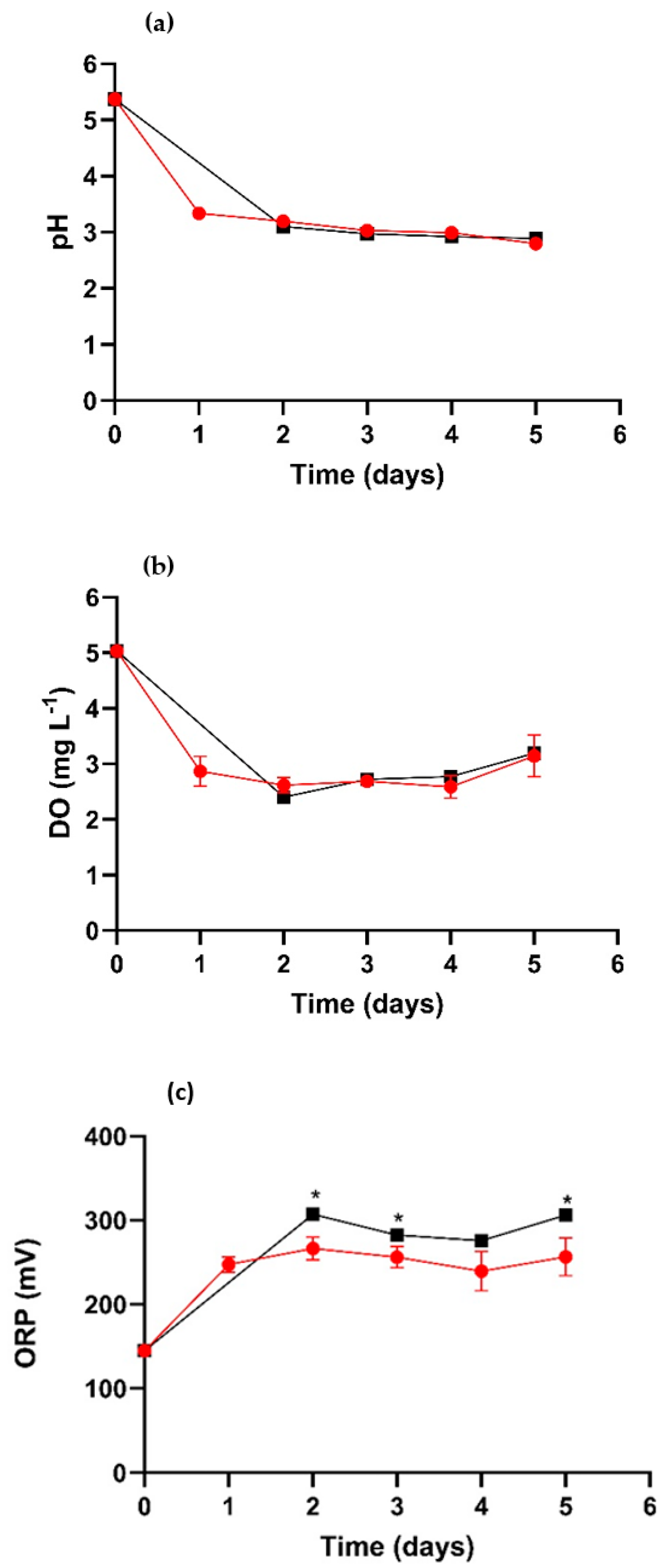

| Average of Size Diameter (nm) | Intensity (%) | SD (±) |
|---|---|---|
| 3.5 | 30.4 | 0.35 |
| 127.1 | 19.3 | 21.12 |
| 625.8 | 50.3 | 121.70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morales-Mendoza, A.G.; Flores-Trujillo, A.K.I.; Ramírez-Castillo, J.A.; Gallardo-Hernández, S.; Rodríguez-Vázquez, R. Effect of Micro-Nanobubbles on Arsenic Removal by Trichoderma atroviride for Bioscorodite Generation. J. Fungi 2023, 9, 857. https://doi.org/10.3390/jof9080857
Morales-Mendoza AG, Flores-Trujillo AKI, Ramírez-Castillo JA, Gallardo-Hernández S, Rodríguez-Vázquez R. Effect of Micro-Nanobubbles on Arsenic Removal by Trichoderma atroviride for Bioscorodite Generation. Journal of Fungi. 2023; 9(8):857. https://doi.org/10.3390/jof9080857
Chicago/Turabian StyleMorales-Mendoza, Asunción Guadalupe, Ana Karen Ivanna Flores-Trujillo, Jesús Adriana Ramírez-Castillo, Salvador Gallardo-Hernández, and Refugio Rodríguez-Vázquez. 2023. "Effect of Micro-Nanobubbles on Arsenic Removal by Trichoderma atroviride for Bioscorodite Generation" Journal of Fungi 9, no. 8: 857. https://doi.org/10.3390/jof9080857





