Production of Porous Agarose-Based Structures: Freeze-Drying vs. Supercritical CO2 Drying
Abstract
:1. Introduction
2. Results and Discussion
2.1. Morphology of Agarose-Based Cryogels Obtained by a Fast-Cooling Step
2.2. Morphology of Agarose-Based Cryogels Obtained by a Slow-Cooling Step
2.3. Morphology of Agarose-Based Aerogels
2.4. DSC and TGA Results
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Hydrogel Preparation
4.2.2. Cryogel Preparation
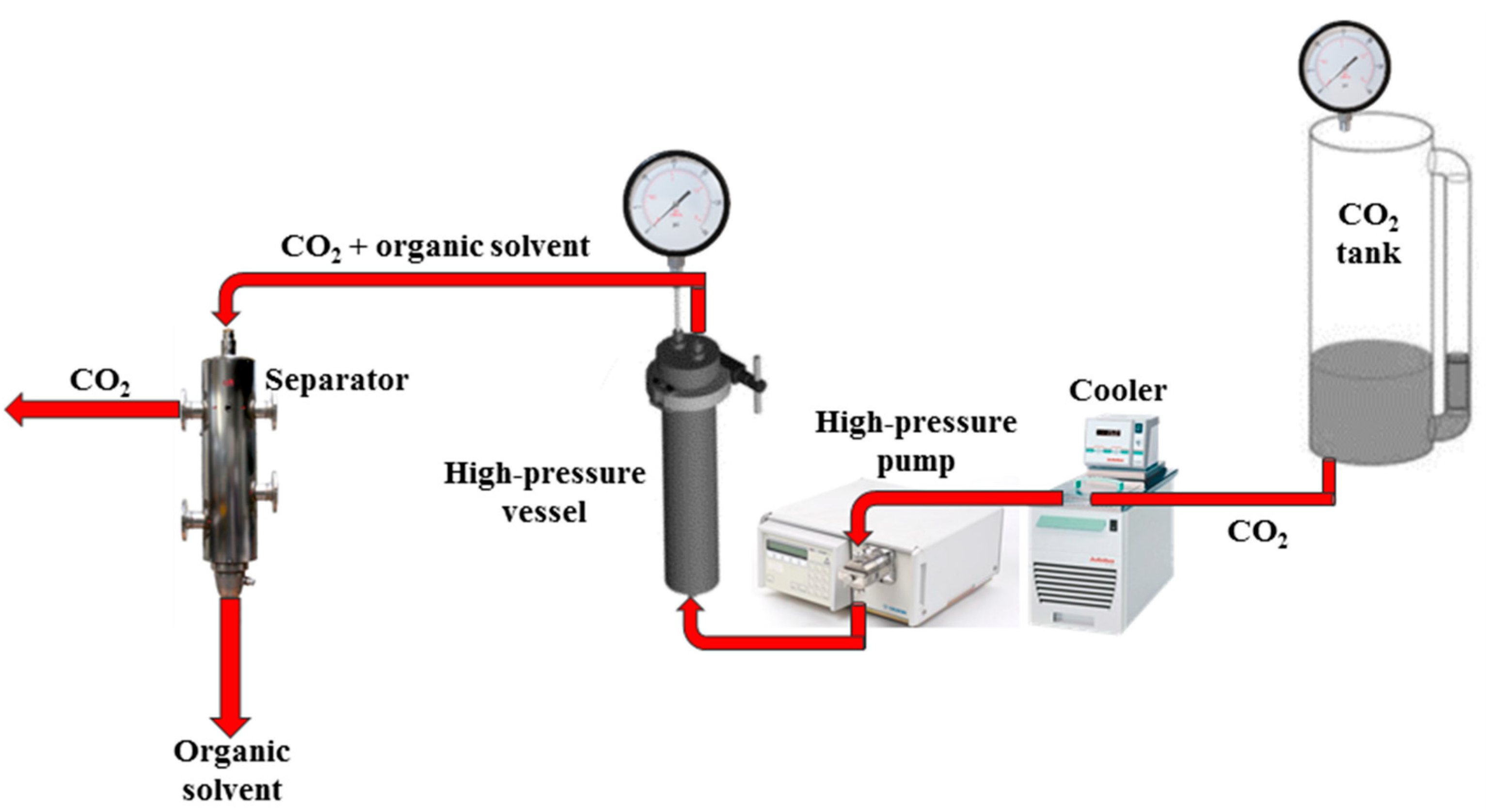
4.2.3. Aerogel Preparation
4.3. Characterizations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Wen, Y.; Oh, J.K. Recent strategies to develop polysaccharide-based nanomaterials for biomedical applications. Macromol. Rapid Commun. 2014, 35, 1819–1832. [Google Scholar] [CrossRef] [PubMed]
- Guastaferro, M.; Reverchon, E.; Baldino, L. Agarose, Alginate and Chitosan Nanostructured Aerogels for Pharmaceutical Applications: A Short Review. Front. Bioeng. Biotechnol. 2021, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Guastaferro, M.; Reverchon, E.; Baldino, L. Polysaccharide-based aerogel production for biomedical applications: A comparative review. Materials 2021, 14, 1631. [Google Scholar] [CrossRef]
- Malviya, R.; Sharma, P.K.; Dubey, S.K. Modification of polysaccharides: Pharmaceutical and tissue engineering applications with commercial utility (patents). Mater. Sci. Eng. C 2016, 68, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Le Goff, K.J.; Gaillard, C.; Helbert, W.; Garnier, C.; Aubry, T. Rheological study of reinforcement of agarose hydrogels by cellulose nanowhiskers. Carbohydr. Polym. 2015, 116, 117–123. [Google Scholar] [CrossRef]
- Mozafari, M. Synthesis and characterisation of poly(lactide-coglycolide) nanospheres using vitamin E emulsifier prepared through one-step oil-in-water emulsion and solvent evaporation techniques. IET Nanobiotechnol. 2014, 8, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Zarrintaj, P.; Manouchehri, S.; Ahmadi, Z.; Saeb, M.R.; Urbanska, A.M.; Kaplan, D.L.; Mozafari, M. Agarose-based biomaterials for tissue engineering. Carbohydr. Polym. 2018, 187, 66–84. [Google Scholar] [CrossRef]
- Murdan, S. Electro-responsive drug delivery from hydrogels. J. Control. Release. 2003, 92, 1–17. [Google Scholar] [CrossRef]
- Kumar, N.; Desagani, D.; Chandran, G.; Ghosh, N.N.; Karthikeyan, G.; Waigaonkar, S.; Ganguly, A. Biocompatible agarose-chitosan coated silver nanoparticle composite for soft tissue engineering applications. Artif. Cells Nanomed. Biotechnol. 2018, 46, 637–649. [Google Scholar] [CrossRef] [Green Version]
- Luo, J.; Zhang, X.; Ong’achwa MacHuki, J.; Dai, C.; Li, Y.; Guo, K.; Gao, F. Three-Dimensionally N-Doped Graphene-Hydroxyapatite/Agarose as an Osteoinductive Scaffold for Enhancing Bone Regeneration. ACS Appl. Bio Mater. 2019, 2, 299–310. [Google Scholar] [CrossRef]
- Gao, M.; Lu, P.; Bednark, B.; Lynam, D.; Conner, J.M.; Sakamoto, J.; Tuszynski, M.H. Templated agarose scaffolds for the support of motor axon regeneration into sites of complete spinal cord transection. Biomaterials 2013, 34, 1529–1536. [Google Scholar] [CrossRef] [Green Version]
- Lynam, D.A.; Shahriari, D.; Wolf, K.J.; Angart, P.A.; Koffler, J.; Tuszynski, M.H.; Chan, C.; Walton, P.; Sakamoto, J. Brain derived neurotrophic factor release from layer-by-layer coated agarose nerve guidance scaffolds. Acta Biomater. 2015, 18, 128–131. [Google Scholar] [CrossRef] [PubMed]
- Fey, T.; Zierath, B.; Greil, P.; Potoczek, M. Microstructural, mechanical and thermal characterization of alumina gel-cast foams manufactured with the use of agarose as gelling agent. J. Porous Mater. 2015, 22, 1305–1312. [Google Scholar] [CrossRef]
- Normand, V.; Lootens, D.L.; Amici, E.; Plucknett, K.P.; Aymard, P. New insight into agarose gel mechanical properties. Biomacromolecules 2000, 1, 730–738. [Google Scholar] [CrossRef]
- Shu, P. Gelation mechanism of chromium(III). Am. Chem. Soc. Div. Pet. Chem. Prepr. 1988, 33, 43–48. [Google Scholar] [CrossRef]
- Kara, S.; Arda, E.; Dolastir, F.; Pekcan, Ö. Thermal phase transitions of agarose in various compositions: A fluorescence study. J. Fluoresc. 2011, 21, 1871–1877. [Google Scholar] [CrossRef] [Green Version]
- Ramya, J.R.; Arul, K.T.; Asokan, K.; Ilangovan, R. Enhanced microporous structure of gamma irradiated agarose-gelatin-HAp flexible films for IR window and microelectronic applications. Mater. Today Commun. 2020, 24, 101215. [Google Scholar] [CrossRef]
- Witzler, M.; Ottensmeyer, P.F.; Gericke, M.; Heinze, T.; Tobiasch, E.; Schulze, M. Non-Cytotoxic Agarose/Hydroxyapatite composite scaffolds for drug release. Int. J. Mol. Sci. 2019, 20, 3565. [Google Scholar] [CrossRef] [Green Version]
- Kazimierczak, P.; Benko, A.; Nocun, M.; Przekora, A. Novel chitosan/agarose/hydroxyapatite nanocomposite scaffold for bone tissue engineering applications: Comprehensive evaluation of biocompatibility and osteoinductivity with the use of osteoblasts and mesenchymal stem cells. Int. J. Nanomed. 2019, 14, 6615–6630. [Google Scholar] [CrossRef] [Green Version]
- Sivashankari, P.R.; Prabaharan, M. Three-dimensional porous scaffolds based on agarose/chitosan/graphene oxide composite for tissue engineering. Int. J. Biol. Macromol. 2020, 146, 222–231. [Google Scholar] [CrossRef]
- Yuan, Y.; Wang, L.; Mu, R.J.; Gong, J.; Wang, Y.; Li, Y.; Ma, J.; Pang, J.; Wu, C. Effects of konjac glucomannan on the structure, properties, and drug release characteristics of agarose hydrogels. Carbohydr. Polym. 2018, 190, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Zhang, S.; Ying, Z.; Liu, J.; Zhou, Y.; Chen, F. Engineering of aerogel-based biomaterials for biomedical applications. Int. J. Nanomed. 2020, 15, 2363–2378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maleki, H.; Durães, L.; García-González, C.A.; del Gaudio, P.; Portugal, A.; Mahmoudi, M. Synthesis and biomedical applications of aerogels: Possibilities and challenges. Adv. Colloid Interface Sci. 2016, 236, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Stergar, J.; Maver, U. Review of aerogel-based materials in biomedical applications. J. Sol-Gel Sci. Technol. 2016, 77, 738–752. [Google Scholar] [CrossRef]
- Tabernero, A.; Baldino, L.; Misol, A.; Cardea, S.; Martin del Valle, E. Role of rheological properties on physical chitosan aerogels obtained by supercritical drying. Carbohydr. Polym. 2020, 233, 115850. [Google Scholar] [CrossRef]
- Zhou, Y.; Gao, H.L.; Shen, L.L.; Pan, Z.; Mao, L.B.; Wu, T.; He, J.C.; Zou, D.H.; Zhang, Z.Y.; Yu, S.H. Chitosan microspheres with an extracellular matrix-mimicking nanofibrous structure as cell-carrier building blocks for bottom-up cartilage tissue engineering. Nanoscale 2016, 8, 309–317. [Google Scholar] [CrossRef]
- Li, W.J.; Laurencin, C.T.; Caterson, E.J.; Tuan, R.S.; Ko, F.K. Electrospun nanofibrous structure: A novel scaffold for tissue engineering. J. Biomed. Mater. Res. 2002, 60, 613–621. [Google Scholar] [CrossRef]
- Baldino, L.; Cardea, S.; Reverchon, E. Nanostructured chitosan–gelatin hybrid aerogels produced by supercritical gel drying. Polym. Eng. Sci. 2018, 58, 1494–1499. [Google Scholar] [CrossRef]
- Trucillo, P.; Cardea, S.; Baldino, L.; Reverchon, E. Production of liposomes loaded alginate aerogels using two supercritical CO2 assisted techniques. J. CO2 Util. 2020, 39, 101161. [Google Scholar] [CrossRef]
- Tabernero, A.; Baldino, L.; Cardea, S.; Martin del Valle, E.; Reverchon, E. A phenomenological approach to study mechanical properties of polymeric porous structures processed using supercritical CO2. Polymers 2019, 11, 485. [Google Scholar] [CrossRef] [Green Version]
- Baldino, L.; Cardea, S.; Reverchon, E. Natural aerogels production by supercritical gel drying. Chem. Eng. Trans. 2015, 43, 739–744. [Google Scholar] [CrossRef]
- Reverchon, E.; Cardea, S.; Rapuano, C. A new supercritical fluid-based process to produce scaffolds for tissue replacement. J. Supercrit. Fluids 2008, 45, 365–373. [Google Scholar] [CrossRef]
- Reverchon, E.; Cardea, S. Supercritical fluids in 3-D tissue engineering. J. Supercrit. Fluids 2012, 69, 97–107. [Google Scholar] [CrossRef]
- Baudron, V.; Gurikov, P.; Smirnova, I.; Whitehouse, S. Porous starch materials via supercritical-and freeze-drying. Gels 2019, 5, 12. [Google Scholar] [CrossRef] [Green Version]
- O’Brien, F.J.; Harley, B.A.; Yannas, I.V.; Gibson, L. Influence of freezing rate on pore structure in freeze-dried collagen-GAG scaffolds. Biomaterials 2004, 25, 1077–1086. [Google Scholar] [CrossRef]
- Qian, L.; Zhang, H. Controlled freezing and freeze drying: A versatile route for porous and micro-/nano-structured materials. J. Chem. Technol. Biotechnol. 2011, 86, 172–184. [Google Scholar] [CrossRef]
- Silva-Espinoza, M.A.; Ayed, C.; Foster, T.; Del Mar Camacho, M.; Martínez-Navarrete, N. The impact of freeze-drying conditions on the physico-chemical properties and bioactive compounds of a freeze-dried orange puree. Foods 2020, 9, 32. [Google Scholar] [CrossRef] [Green Version]
- Searles, J.A.; Carpenter, J.F.; Randolph, T.W. The ice nucleation temperature determines the primary drying rate of lyophilization for samples frozen on a temperature-controlled shelf. J. Pharm. Sci. 2001, 90, 860–871. [Google Scholar] [CrossRef]
- Fang, R.; Tanaka, K.; Mudhivarthi, V.; Bogner, R.H.; Pikal, M.J. Effect of Controlled Ice Nucleation on Stability of Lactate Dehydrogenase during Freeze-Drying. J. Pharm. Sci. 2018, 107, 824–830. [Google Scholar] [CrossRef]
- Assegehegn, G.; Brito-de la Fuente, E.; Franco, J.M.; Gallegos, C. The Importance of Understanding the Freezing Step and Its Impact on Freeze-Drying Process Performance. J. Pharm. Sci. 2019, 108, 1378–1395. [Google Scholar] [CrossRef] [PubMed]
- Ullrich, S.; Seyferth, S.; Lee, G. Measurement of shrinkage and cracking in lyophilized amorphous cakes. Part I: Final-product assessment. J. Pharm. Sci. 2015, 104, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Salati, M.A.; Khazai, J.; Tahmuri, A.M.; Samadi, A.; Taghizadeh, A.; Taghizadeh, M.; Zarrintaj, P.; Ramsey, J.D.; Habibzadeh, S.; Seidi, F.; et al. Agarose-Based biomaterials: Opportunities and challenges in cartilage tissue engineering. Polymers 2020, 12, 1150. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J.; Zhang, H.; Lin, J.; Ge, Z.; Zou, X. Macroporous interpenetrating network of polyethylene glycol (PEG) and gelatin for cartilage regeneration. Biomed. Mater. 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- Flégeau, K.; Pace, R.; Gautier, H.; Rethore, G.; Guicheux, J.; Le Visage, C.; Weiss, P. Toward the development of biomimetic injectable and macroporous biohydrogels for regenerative medicine. Adv. Colloid Interface Sci. 2017, 247, 589–609. [Google Scholar] [CrossRef] [PubMed]
- Rybacki, E.; Reinicke, A.; Meier, T.; Makasi, M.; Dresen, G. What controls the mechanical properties of shale rocks?—Part I: Strength and Young’s modulus. J. Pet. Sci. Eng. 2015, 135, 702–722. [Google Scholar] [CrossRef]
- Choren, J.A.; Heinrich, S.M.; Silver-Thorn, M.B. Young’s modulus and volume porosity relationships for additive manufacturing applications. J. Mater. Sci. 2013, 48, 5103–5112. [Google Scholar] [CrossRef]
- Alnaief, M.; Obaidat, R.; Mashaqbeh, H. Effect of processing parameters on preparation of carrageenan aerogel microparticles. Carbohydr. Polym. 2018, 180, 264–275. [Google Scholar] [CrossRef]
- Jin, H.; Nishiyama, Y.; Wada, M.; Kuga, S. Nanofibrillar cellulose aerogels. Colloids Surfaces A Physicochem. Eng. Asp. 2004, 240, 63–67. [Google Scholar] [CrossRef]
- Johnson, T.D.; Lin, S.Y.; Christman, K.L. Tailoring material properties of a nanofibrous extracellular matrix derived hydrogel. Nanotechnology 2011, 22, 494015. [Google Scholar] [CrossRef] [Green Version]
- Lei, B.; Shin, K.H.; Noh, D.Y.; Jo, I.H.; Koh, Y.H.; Choi, W.Y.; Kim, H.E. Nanofibrous gelatin-silica hybrid scaffolds mimicking the native extracellular matrix (ECM) using thermally induced phase separation. J. Mater. Chem. 2012, 22, 14133–14140. [Google Scholar] [CrossRef]
- Trivedi, T.J.; Rao, K.S.; Kumar, A. Facile preparation of agarose-chitosan hybrid materials and nanocomposite ionogels using an ionic liquid via dissolution, regeneration and sol-gel transition. Green Chem. 2014, 16, 320–330. [Google Scholar] [CrossRef] [Green Version]
- Moritaka, H.; Nishinari, K.; Horiuchi, H.; Watase, M. Rheological Properties of Aqueous Agarose-Gelatin Gels. J. Texture Stud. 1980, 11, 257–270. [Google Scholar] [CrossRef]
- Corazzari, I.; Nisticò, R.; Turci, F.; Faga, M.G.; Franzoso, F.; Tabasso, S.; Magnacca, G. Advanced physico-chemical characterization of chitosan by means of TGA coupled on-line with FTIR and GCMS: Thermal degradation and water adsorption capacity. Polym. Degrad. Stab. 2015, 112, 1–9. [Google Scholar] [CrossRef]
- Baldino, L.; Zuppolini, S.; Cardea, S.; Diodato, L.; Borriello, A.; Reverchon, E.; Nicolais, L. Production of biodegradable superabsorbent aerogels using a supercritical CO2 assisted drying. J. Supercrit. Fluids 2020, 156, 104681. [Google Scholar] [CrossRef]
- Baldino, L.; Aragón, J.; Mendoza, G.; Irusta, S.; Cardea, S.; Reverchon, E. Production, characterization and testing of antibacterial PVA membranes loaded with HA-Ag3PO4 nanoparticles, produced by SC-CO2 phase inversion. J. Chem. Tech. Biotech. 2019, 94, 98–108. [Google Scholar] [CrossRef] [Green Version]
- Baldino, L.; Cardea, S.; Reverchon, E. Loaded silk fibroin aerogel production by supercritical gel drying process for nanomedicine applications. Chem. Eng. Trans. 2016, 49, 343–348. [Google Scholar] [CrossRef]
- Firouzabadi, H.; Iranpoor, N.; Gholinejad, M.; Akbari, S.; Jeddi, N. Palladium nanoparticles supported on agarose-functionalized magnetic nanoparticles of Fe3O4 as a recyclable catalyst for C-C bond formation via Suzuki-Miyaura, Heck-Mizoroki and Sonogashira-Hagihara coupling reactions. RSC Adv. 2014, 4, 17060–17070. [Google Scholar] [CrossRef]








| Freeze-Drying, Fast Cooling Rate | Sample Shrinkage, % | Bulk Density, g/cm3 | Specific Surface Area, m2/g |
| 1 wt% agarose cryogel | 98 | 0.54 | 9 |
| 3 wt% agarose cryogel | 52 | 0.06 | 13 |
| 5 wt% agarose cryogel | 28 | 0.11 | 13 |
| 8 wt% agarose cryogel | 28 | 0.15 | 14 |
| Freeze-Drying, Slow Cooling Rate | Sample Shrinkage, % | Bulk Density, g/cm3 | Specific Surface Area, m2/g |
| 1 wt% agarose cryogel | 68 | 0.03 | 10 |
| 3 wt% agarose cryogel | 36 | 0.05 | 11 |
| 5 wt% agarose cryogel | 24 | 0.07 | 13 |
| 8 wt% agarose cryogel | 24 | 0.14 | 21 |
| SC-CO2 Drying | Sample Shrinkage, % | Bulk Density, g/cm3 | Specific Surface Area, m2/g |
| 1 wt% agarose aerogel | 28 | 0.02 | 87 |
| 3 wt% agarose aerogel | 20 | 0.02 | 154 |
| 5 wt% agarose aerogel | 21 | 0.07 | 156 |
| 8 wt% agarose aerogel | 21 | 0.13 | 170 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guastaferro, M.; Baldino, L.; Reverchon, E.; Cardea, S. Production of Porous Agarose-Based Structures: Freeze-Drying vs. Supercritical CO2 Drying. Gels 2021, 7, 198. https://doi.org/10.3390/gels7040198
Guastaferro M, Baldino L, Reverchon E, Cardea S. Production of Porous Agarose-Based Structures: Freeze-Drying vs. Supercritical CO2 Drying. Gels. 2021; 7(4):198. https://doi.org/10.3390/gels7040198
Chicago/Turabian StyleGuastaferro, Mariangela, Lucia Baldino, Ernesto Reverchon, and Stefano Cardea. 2021. "Production of Porous Agarose-Based Structures: Freeze-Drying vs. Supercritical CO2 Drying" Gels 7, no. 4: 198. https://doi.org/10.3390/gels7040198









