Magnetic Carbon Foam Adorned with Co/Fe Nanoneedles as an Efficient Activator of Oxone for Oxidative Environmental Remediation: Roles of Surficial and Chemical Enhancement
Abstract
:1. Introduction
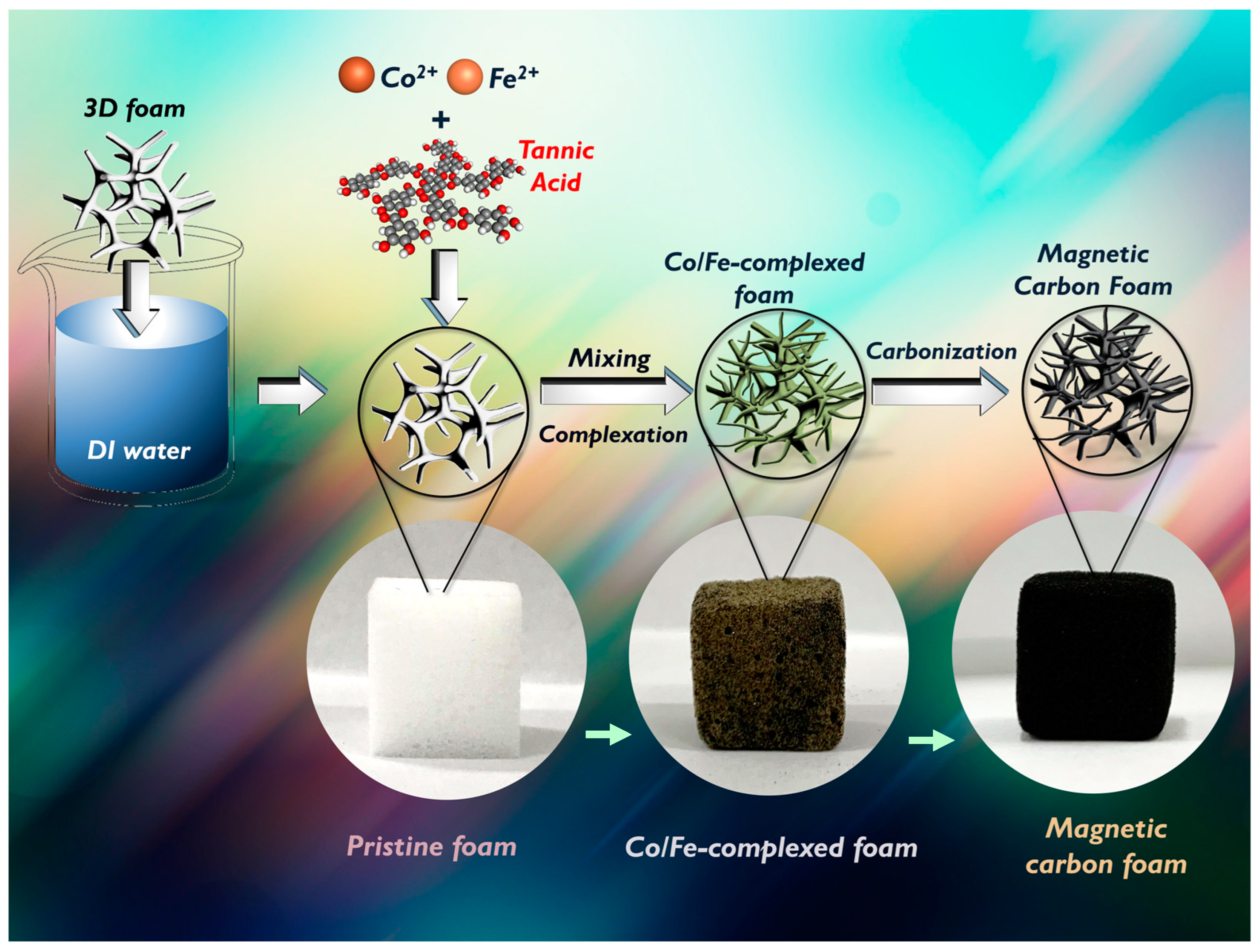
2. Experimental
3. Results and Discussion
3.1. Properties of MCF
Morphologies and Compositional Analyses
3.2. Degradation of BHPM by MCF + Oxone
3.3. BHPM by MCF + Oxone: Other Effects
3.4. Mechanistic Studies by MCF + Oxone
3.5. Degradation Process of BHPM
3.6. Eco-Toxicity Evaluation of BHP Degradation Intermediates
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lin, K.-Y.A.; Lin, J.-T.; Lu, X.-Y.; Hung, C.; Lin, Y.-F. Electrospun magnetic cobalt-embedded carbon nanofiber as a heterogeneous catalyst for activation of oxone for degradation of Amaranth dye. J. Colloid Interface Sci. 2017, 505, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Alvi, S.; Jayant, V.; Ali, R. Applications of Oxone® in Organic Synthesis: An Emerging Green Reagent of Modern Era. ChemistrySelect 2022, 7, e202200704. [Google Scholar] [CrossRef]
- Cui, K.-P.; He, Y.-Y.; Xu, K.-J.; Zhang, Y.; Chen, C.-B.; Xu, Z.-J.; Chen, X. Degradation of Tetracycline Hydrochloride by Cu-Doped MIL-101(Fe) Loaded Diatomite Heterogeneous Fenton Catalyst. Nanomaterials 2022, 12, 811. [Google Scholar] [CrossRef] [PubMed]
- Eslami, A.; Mehdipour, F.; Lin, K.-Y.A.; Sharifi Maleksari, H.; Mirzaei, F.; Ghanbari, F. Sono-photo activation of percarbonate for the degradation of organic dye: The effect of water matrix and identification of by-products. J. Water Process Eng. 2020, 33, 100998. [Google Scholar] [CrossRef]
- Kohantorabi, M.; Moussavi, G.; Giannakis, S. A review of the innovations in metal- and carbon-based catalysts explored for heterogeneous peroxymonosulfate (PMS) activation, with focus on radical vs. non-radical degradation pathways of organic contaminants. Chem. Eng. J. 2021, 411, 127957. [Google Scholar] [CrossRef]
- Lin, K.-Y.A.; Lin, J.-T.; Jochems, A.P. Oxidation of amaranth dye by persulfate and peroxymonosulfate activated by ferrocene. J. Chem. Technol. Biotechnol. 2017, 92, 163–172. [Google Scholar] [CrossRef]
- Gasim, M.F.; Lim, J.W.; Low, S.C.; Lin, K.Y.A.; Oh, W.D. Can biochar and hydrochar be used as sustainable catalyst for persulfate activation? Chemosphere 2022, 287, 132458. [Google Scholar] [CrossRef]
- Yang, S.Y.; Wang, P.; Yang, X.; Shan, L.; Zhang, W.Y.; Shao, X.T.; Niu, R. Degradation efficiencies of azo dye Acid Orange 7 by the interaction of heat, UV and anions with common oxidants: Persulfate, peroxymonosulfate and hydrogen peroxide. J. Hazard. Mater. 2010, 179, 552–558. [Google Scholar] [CrossRef]
- Zhang, B.T.; Xiang, W.X.; Jiang, X.M.; Zhang, Y.; Teng, Y.G. Oxidation of Dyes by Alkaline-Activated Peroxymonosulfate. J. Environ. Eng. 2016, 142, 04016003. [Google Scholar] [CrossRef]
- Wei, G.L.; Liang, X.L.; He, Z.S.; Liao, Y.S.; Xie, Z.Y.; Liu, P.; Ji, S.C.; He, H.P.; Li, D.Q.; Zhang, J. Heterogeneous activation of Oxone by substituted magnetites Fe3-xMxO4 (Cr, Mn, Co, Ni) for degradation of Acid Orange II at neutral pH. J. Mol. Catal. A-Chem. 2015, 398, 86–94. [Google Scholar] [CrossRef]
- Cai, C.; Zhang, H.; Zhong, X.; Hou, L. Ultrasound enhanced heterogeneous activation of peroxymonosulfate by a bimetallic Fe–Co/SBA-15 catalyst for the degradation of Orange II in water. J. Hazard. Mater. 2015, 283, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Giannakis, S.; Lin, K.-Y.A.; Ghanbari, F. A review of the recent advances on the treatment of industrial wastewaters by Sulfate Radical-based Advanced Oxidation Processes (SR-AOPs). Chem. Eng. J. 2021, 406, 127083. [Google Scholar] [CrossRef]
- Anipsitakis, G.P.; Stathatos, E.; Dionysiou, D.D. Heterogeneous Activation of Oxone Using Co3O4. J. Phys. Chem. B 2005, 109, 13052–13055. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Li, X.; Feng, J.; Tian, X. Oxone/Co2+ oxidation as an advanced oxidation process: Comparison with traditional Fenton oxidation for treatment of landfill leachate. Water Res. 2009, 43, 4363–4369. [Google Scholar] [CrossRef] [PubMed]
- Andrew Lin, K.-Y.; Hsu, F.-K. Magnetic iron/carbon nanorods derived from a metal organic framework as an efficient heterogeneous catalyst for the chemical oxidation process in water. RSC Adv. 2015, 5, 50790–50800. [Google Scholar] [CrossRef]
- Adil, S.F.; Ashraf, M.; Khan, M.; Assal, M.E.; Shaik, M.R.; Kuniyil, M.; Al-Warthan, A.; Siddiqui, M.R.H.; Tremel, W.; Tahir, M.N. Advances in Graphene/Inorganic Nanoparticle Composites for Catalytic Applications. Chem. Rec. 2022, 22, e202100274. [Google Scholar] [CrossRef]
- Xu, L.J.; Chu, W.; Gan, L. Environmental application of graphene-based CoFe2O4 as an activator of peroxymonosulfate for the degradation of a plasticizer. Chem. Eng. J. 2015, 263, 435–443. [Google Scholar] [CrossRef]
- Liang, X.; Zhao, Y.; Liu, J.; Yang, Z.; Yang, Q. Highly efficient activation of peroxymonosulfate by cobalt ferrite anchored in P-doped activated carbon for degradation of 2,4-D: Adsorption and electron transfer mechanism. J. Colloid Interface Sci. 2023, 642, 757–770. [Google Scholar] [CrossRef]
- Liu, Y.; Tao, F.; Xing, Y.; Pei, Y.; Ren, F. Melamine Foam-Derived Carbon Scaffold for Dendrite-Free and Stable Zinc Metal Anode. Molecules 2023, 28, 1742. [Google Scholar] [CrossRef]
- Duan, X.G.; Sun, H.Q.; Wang, Y.X.; Kang, J.; Wang, S.B. N-Doping-Induced Nonradical Reaction on Single-Walled Carbon Nanotubes for Catalytic Phenol Oxidation. ACS Catal. 2015, 5, 553–559. [Google Scholar] [CrossRef]
- Hu, H.; Guan, B.Y.; Lou, X.W. Construction of Complex CoS Hollow Structures with Enhanced Electrochemical Properties for Hybrid Supercapacitors. Chem 2016, 1, 102–113. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, X.; Hu, X.; Zhou, W.; Zhao, Y. Effect of Formic Acid Treatment on the Structure and Catalytic Activity of Co3O4 for N2O Decomposition. Catal. Lett. 2019, 149, 1026–1036. [Google Scholar] [CrossRef]
- Li, G.; Sun, J.H.; Hou, W.P.; Jiang, S.D.; Huang, Y.; Geng, J.X. Three-dimensional porous carbon composites containing high sulfur nanoparticle content for high-performance lithium-sulfur batteries. Nat. Commun. 2016, 7, 10601. [Google Scholar] [CrossRef] [PubMed]
- Hasan, Z.; Cho, D.W.; Chon, C.M.; Yoon, K.; Song, H. Reduction of p-nitrophenol by magnetic Co-carbon composites derived from metal organic frameworks. Chem. Eng. J. 2016, 298, 183–190. [Google Scholar] [CrossRef]
- He, P.; Wu, Y.; Chen, H.; Zhu, Z.; Liu, H.; Gao, J.; Xu, H. Hierarchical bimetal embedded in carbon nanoflower electrocatalysts derived from metal-organic frameworks for efficient oxygen evolution reaction. J. Alloys Compd. 2020, 813, 152192. [Google Scholar] [CrossRef]
- Liu, D.; Wu, C.; Yan, M.; Wang, J. Correlating the microstructure, growth mechanism and magnetic properties of FeSiAl soft magnetic composites fabricated via HNO3 oxidation. Acta Mater. 2018, 146, 294–303. [Google Scholar] [CrossRef]
- Osadchii, D.Y.; Olivos-Suarez, A.I.; Bavykina, A.V.; Gascon, J. Revisiting Nitrogen Species in Covalent Triazine Frameworks. Langmuir 2017, 33, 14278–14285. [Google Scholar] [CrossRef]
- Jiang, X.-Y.; Park, Y.-K.; Wen, J.-C.; Bui, H.M.; Lin, Y.-F.; Sirivithayapakorn, S.; Khiem, T.C.; Munagapati, V.S.; Lin, K.-Y.A. Hollow-architected Co3O4 for enhancing Oxone activation to eliminate an anesthetic, benzocaine, from water: A structure-property investigation with degradation pathway and eco-toxicity. J. Taiwan Inst. Chem. Eng. 2023, 150, 105042. [Google Scholar] [CrossRef]
- Miao, J.; Geng, W.; Alvarez, P.J.J.; Long, M. 2D N-Doped Porous Carbon Derived from Polydopamine-Coated Graphitic Carbon Nitride for Efficient Nonradical Activation of Peroxymonosulfate. Environ. Sci. Technol. 2020, 54, 8473–8481. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, X.; Sun, Z. CoNi alloy anchored onto N-doped porous carbon for the removal of sulfamethoxazole: Catalyst, mechanism, toxicity analysis, and application. Chemosphere 2022, 308, 136291. [Google Scholar] [CrossRef]
- Sun, X.; Qi, H.; Mao, S.; Sun, Z. Atrazine removal by peroxymonosulfate activated with magnetic CoFe alloy@N-doped graphitic carbon encapsulated in chitosan carbonized microspheres. Chem. Eng. J. 2021, 423, 130169. [Google Scholar] [CrossRef]
- Cong Khiem, T.; Duan, X.; Liu, W.-J.; Park, Y.-K.; Manh Bui, H.; Oh, W.-D.; Ghotekar, S.; Fai Tsang, Y.; Andrew Lin, K.-Y. MOF-templated Hollow Cobalt Sulfide as an Enhanced Oxone Activator for Degradation of UV Absorber: Key Role of Sulfur Vacancy-Induced Highly Active CoII Sites. Chem. Eng. J. 2022, 453, 139699. [Google Scholar] [CrossRef]
- Yuan, R.; Yin, X.; Gu, S.; Chang, J.; Li, H. Flexible double-wall carbon foam/Cu-Co oxides based symmetric supercapacitor with ultrahigh energy density. J. Mater. Sci. Technol. 2023, 160, 109–117. [Google Scholar] [CrossRef]
- Li, Y.; Li, F.-M.; Meng, X.-Y.; Li, S.-N.; Zeng, J.-H.; Chen, Y. Ultrathin Co3O4 Nanomeshes for the Oxygen Evolution Reaction. ACS Catal. 2018, 8, 1913–1920. [Google Scholar] [CrossRef]
- Yue, L.; Zhang, S.; Zhao, H.; Feng, Y.; Wang, M.; An, L.; Zhang, X.; Mi, J. One-pot synthesis CoFe2O4/CNTs composite for asymmetric supercapacitor electrode. Solid State Ion. 2019, 329, 15–24. [Google Scholar] [CrossRef]
- Othman, I.; Hisham Zain, J.; Abu Haija, M.; Banat, F. Catalytic activation of peroxymonosulfate using CeVO4 for phenol degradation: An insight into the reaction pathway. Appl. Catal. B 2020, 266, 118601. [Google Scholar] [CrossRef]
- Liu, W.-J.; Yang, H.; Park, Y.-K.; Kwon, E.; Huang, C.-W.; Thanh, B.X.; Khiem, T.C.; You, S.; Ghanbari, F.; Lin, K.-Y.A. Enhanced degradation of ultra-violet stabilizer Bis(4-hydroxy)benzophenone using oxone catalyzed by hexagonal nanoplate-assembled CoS 3-dimensional cluster. Chemosphere 2022, 288, 132427. [Google Scholar] [CrossRef]
- Guo, W.; Su, S.; Yi, C.; Ma, Z. Degradation of antibiotics amoxicillin by Co3O4-catalyzed peroxymonosulfate system. Environ. Prog. Sustain. Energy 2013, 32, 193–197. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, Y.; Wang, Y.; Le Roux, J.; Yang, Y.; Croué, J.-P. Efficient Peroxydisulfate Activation Process Not Relying on Sulfate Radical Generation for Water Pollutant Degradation. Environ. Sci. Technol. 2014, 48, 5868–5875. [Google Scholar] [CrossRef]
- Mostafa, S.; Rosario-Ortiz, F.L. Singlet oxygen formation from wastewater organic matter. Environ. Sci. Technol. 2013, 47, 8179–8186. [Google Scholar] [CrossRef]











Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-C.; Jiang, X.-Y.; Thanh, B.X.; Lin, J.-Y.; Wang, H.; Huang, C.-W.; Yang, H.; Ebrahimi, A.; Sirivithayapakorn, S.; Lin, K.-Y. Magnetic Carbon Foam Adorned with Co/Fe Nanoneedles as an Efficient Activator of Oxone for Oxidative Environmental Remediation: Roles of Surficial and Chemical Enhancement. C 2023, 9, 107. https://doi.org/10.3390/c9040107
Chen Y-C, Jiang X-Y, Thanh BX, Lin J-Y, Wang H, Huang C-W, Yang H, Ebrahimi A, Sirivithayapakorn S, Lin K-Y. Magnetic Carbon Foam Adorned with Co/Fe Nanoneedles as an Efficient Activator of Oxone for Oxidative Environmental Remediation: Roles of Surficial and Chemical Enhancement. C. 2023; 9(4):107. https://doi.org/10.3390/c9040107
Chicago/Turabian StyleChen, Yi-Chun, Xin-Yu Jiang, Bui Xuan Thanh, Jia-Yin Lin, Haitao Wang, Chao-Wei Huang, Hongta Yang, Afshin Ebrahimi, Sanya Sirivithayapakorn, and Kun-Yi (Andrew) Lin. 2023. "Magnetic Carbon Foam Adorned with Co/Fe Nanoneedles as an Efficient Activator of Oxone for Oxidative Environmental Remediation: Roles of Surficial and Chemical Enhancement" C 9, no. 4: 107. https://doi.org/10.3390/c9040107
APA StyleChen, Y. -C., Jiang, X. -Y., Thanh, B. X., Lin, J. -Y., Wang, H., Huang, C. -W., Yang, H., Ebrahimi, A., Sirivithayapakorn, S., & Lin, K. -Y. (2023). Magnetic Carbon Foam Adorned with Co/Fe Nanoneedles as an Efficient Activator of Oxone for Oxidative Environmental Remediation: Roles of Surficial and Chemical Enhancement. C, 9(4), 107. https://doi.org/10.3390/c9040107









_Lin.png)
