Changes of Antioxidant and Functional Components in Various Salt-Aged and Fresh Radishes after Fermentation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Materials
2.3. Fermentation
2.4. Yeast Count Test and Identification
2.5. Total Phenolic Content (TPC) Determination
2.6. Total Flavonoid Content (TFC) Determination
2.7. ABTS (2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) Radical Scavenging Assay
2.8. DPPH (Di(phenyl)-(2,4,6-trinitrophenyl)iminoazanium) Radical Scavenging Assay
2.9. pH Value and Total Titratable Acidity (TTA) Determination
2.10. Organic Acid and Carbohydrate Analysis
2.11. GABA Measurements
2.12. ACE Inhibition Activity Assay
2.13. Statistical Analysis
3. Results and Discussion
3.1. Variability in Yeast Counts and Identification after Fermentation
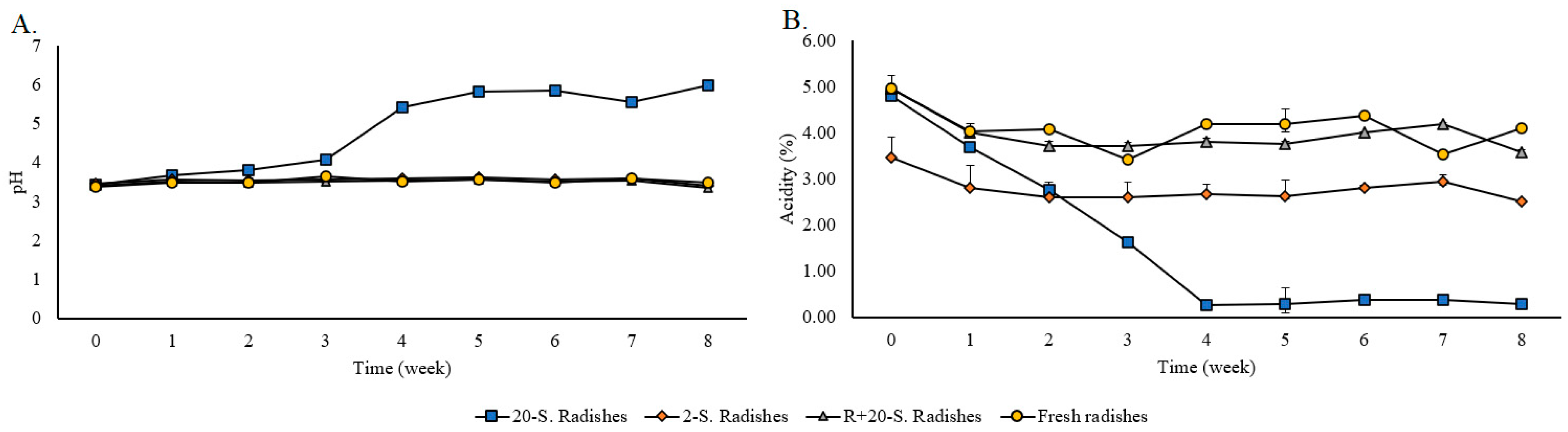
3.2. Variation of pH, Acidity, Glucose, Fructose, and Acetic Acid
3.3. Performance of Functional Components
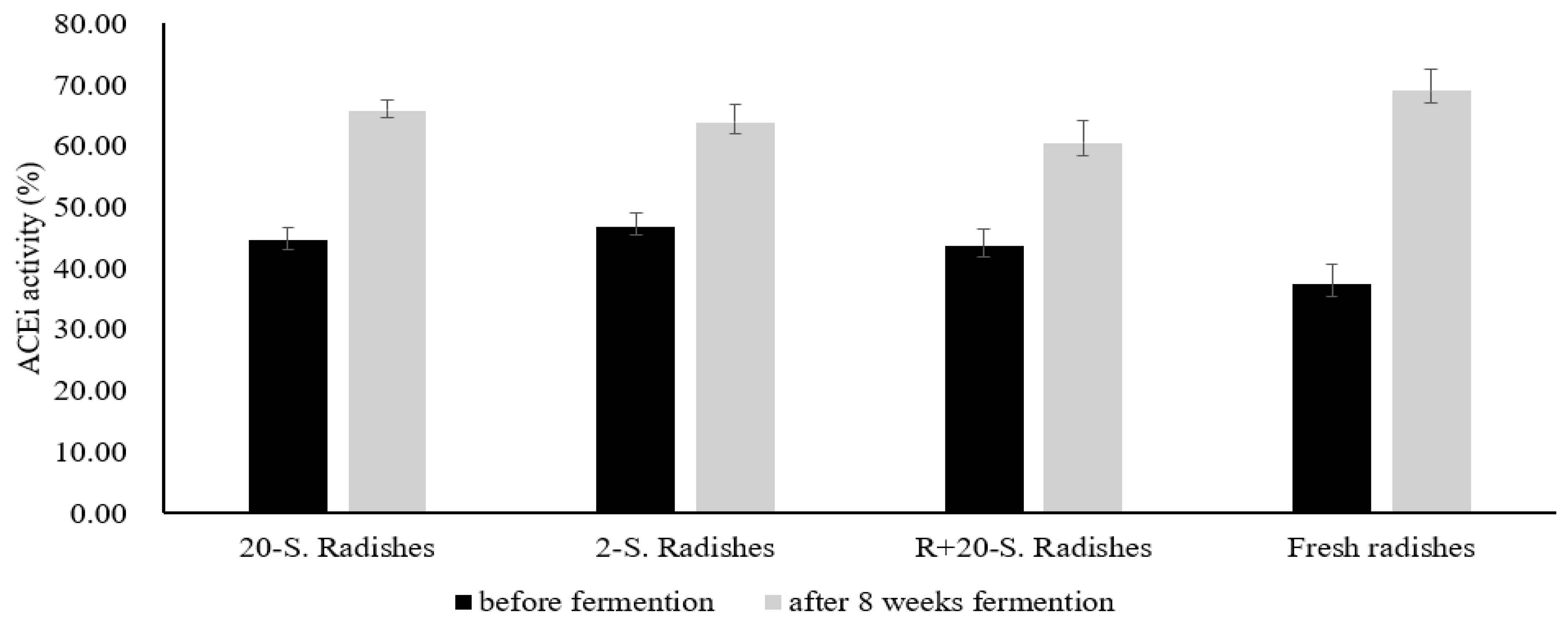
3.3.1. Effects of ACE Inhibition Capacity
3.3.2. Effects of GABA Concentration
3.3.3. Effects of Glucuronic Acid Concentration
3.4. Performance of Antioxidant Capacity
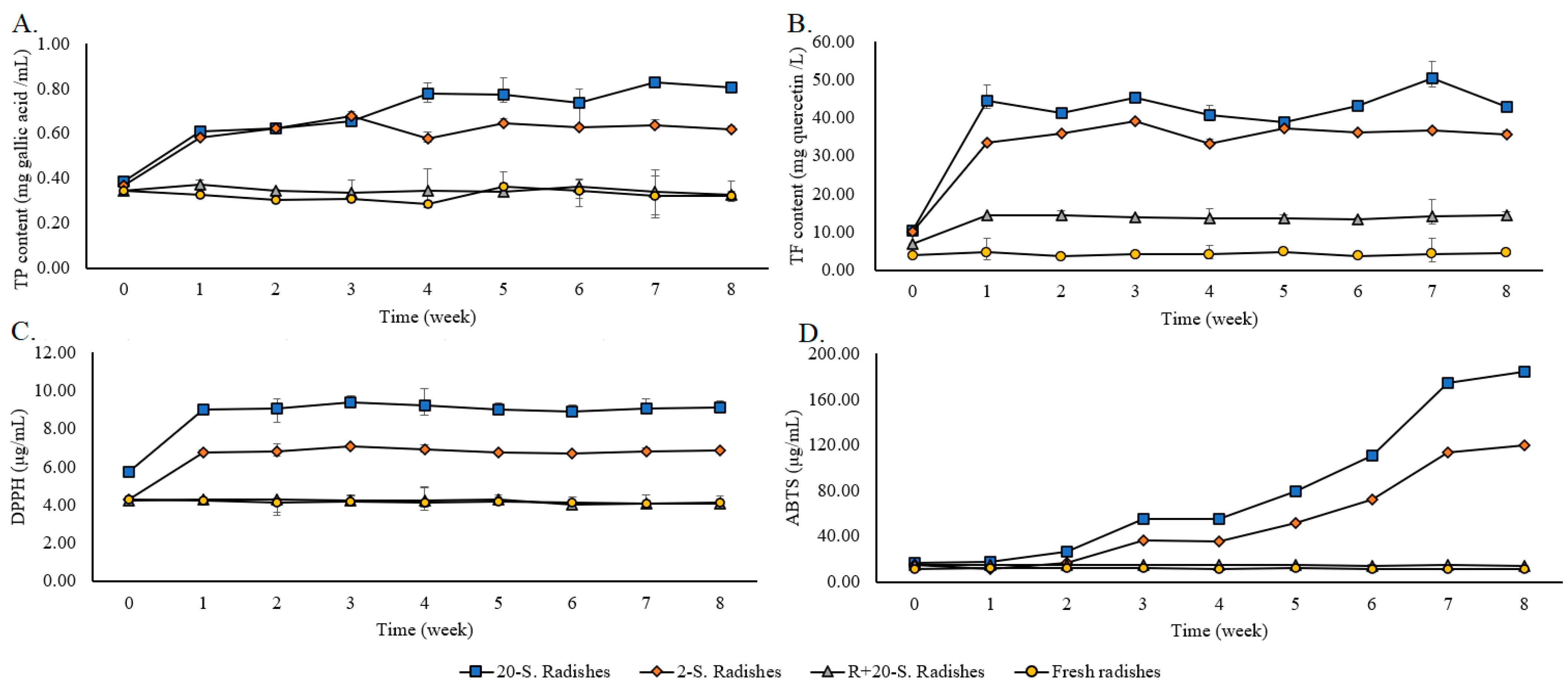
3.4.1. Effects of TPC Concentration
3.4.2. Effects of TFC Concentration
3.4.3. Effects of ABTS Radical Scavenging Activity
3.4.4. Effects of DPPH Radical Scavenging Activity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Meyer, C. Vegetarian Medicines; Spanner Books: Morgantown, WV, USA, 1981; ISBN 0914875493. [Google Scholar]
- Lim, H.-W.; Song, K.-Y.; Chon, J.-W.; Jeong, D.; Seo, K.-H. Antimicrobial Action of Raphanus raphanistrum Subsp. Sativus (Radish) Extracts against Foodborne Bacteria Present in Various Milk Products: A Preliminary Study. J. Milk Sci. Biotechnol. 2019, 37, 187–195. [Google Scholar] [CrossRef]
- Sevindik, M.; Onat, C.; Mohammed, F.S.; Uysal, İ.; Koçer, O. Antioxidant and Antimicrobial Activities of White Radish. Turkish J. Agric.-Food Sci. Technol. 2023, 11, 372–375. [Google Scholar] [CrossRef]
- Shukla, S.; Chatterji, S.; Mehta, S.; Rai, P.K.; Singh, R.K.; Yadav, D.K.; Watal, G. Antidiabetic Effect of Raphanus Sativus Root Juice. Pharm. Biol. 2011, 49, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Ippoushi, K.; Takeuchi, A.; Ito, H.; Horie, H.; Azuma, K. Antioxidative Effects of Daikon Sprout (Raphanus sativus L.) and Ginger (Zingiber Officinale Roscoe) in Rats. Food Chem. 2007, 102, 237–242. [Google Scholar] [CrossRef]
- Li, J.; Huang, S.Y.; Deng, Q.; Li, G.; Su, G.; Liu, J.; David Wang, H.M. Extraction and Characterization of Phenolic Compounds with Antioxidant and Antimicrobial Activities from Pickled Radish. Food Chem. Toxicol. 2020, 136, 111050. [Google Scholar] [CrossRef]
- Jing, P.; Song, L.H.; Shen, S.Q.; Zhao, S.J.; Pang, J.; Qian, B.J. Characterization of Phytochemicals and Antioxidant Activities of Red Radish Brines during Lactic Acid Fermentation. Molecules 2014, 19, 9675–9688. [Google Scholar] [CrossRef]
- Sawada, K.; Koyano, H.; Yamamoto, N.; Yamada, T. The Effects of Vegetable Pickling Conditions on the Dynamics of Microbiota and Metabolites. PeerJ 2021, 9, e11123. [Google Scholar] [CrossRef]
- Gong, L.; Li, T.; Feng, J.; Yin, J.; Zou, X.; Wang, J.; Wang, B. Enhanced DPPH Radical Scavenging Activity and Enriched γ-Aminobutyric Acid in Mulberry Juice Fermented by the Probiotic Lactobacillus Brevis S3. Fermentation 2023, 9, 829. [Google Scholar] [CrossRef]
- Tashiro, K.; Sakakibara, H.; Yokoyama, D.; Uehara, T.; Matsuura, Y.; Sakono, M. Consumption of Salted Pickles of Sun-Dried Radish Roots (Raphanus Sativus Cv. YR-Hyuga-Risou) Attenuates Blood Pressure in Spontaneously Hypertensive Rats. Food Sci. Technol. Res. 2017, 23, 757–763. [Google Scholar] [CrossRef]
- Wang, C.F.; Huang, C.R.; Lu, Y.C. Changes in Bio-Functional Compounds, ACE Inhibition, and Antioxidant Capacity after Mixed Fermentation of Eight Whole Grains. Fermentation 2023, 9, 209. [Google Scholar] [CrossRef]
- Lee, D.H.; Kim, S.H.; Lee, C.Y.; Jo, H.W.; Lee, W.H.; Kim, E.H.; Choi, B.K.; Huh, C.K. Screening of Acetic Acid Bacteria Isolated from Various Sources for Use in Kombucha Production. Fermentation 2024, 10, 18. [Google Scholar] [CrossRef]
- Samsonowicz, M.; Regulska, E.; Karpowicz, D.; Leśniewska, B. Antioxidant Properties of Coffee Substitutes Rich in Polyphenols and Minerals. Food Chem. 2019, 278, 101–109. [Google Scholar] [CrossRef] [PubMed]
- de Souza Gois Barbosa, M.; dos Santos Scholz, M.B.; Kitzberger, C.S.G.; Benassi, M.d.T. Correlation between the Composition of Green Arabica Coffee Beans and the Sensory Quality of Coffee Brews. Food Chem. 2019, 292, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.F.; Huang, C.R.; Lu, Y.C. Changes in the Bio-Compounds and Biological Activities of Eight Whole Grains Fermentation Starter with Different Oxidized Chin-Shin Oolong Teas. Foods 2023, 12, 1643. [Google Scholar] [CrossRef]
- Hayat, A.; Jahangir, T.M.; Khuhawar, M.Y.; Alamgir, M.; Hussain, Z.; Haq, F.U.; Musharraf, S.G. HPLC Determination of Gamma Amino Butyric Acid (GABA) and Some Biogenic Amines (BAs) in Controlled, Germinated, and Fermented Brown Rice by Pre-Column Derivatization. J. Cereal Sci. 2015, 64, 56–62. [Google Scholar] [CrossRef]
- Hayat, A.; Jahangir, T.M.; Khuhawar, M.Y.; Alamgir, M.; Siddiqui, A.J.; Musharraf, S.G. Simultaneous HPLC Determination of Gamma Amino Butyric Acid (GABA) and Lysine in Selected Pakistani Rice Varieties by Pre-Column Derivatization with 2-Hydroxynaphthaldehyde. J. Cereal Sci. 2014, 60, 356–360. [Google Scholar] [CrossRef]
- Je, J.; Park, J.; Jung, W.; Park, P.; Kim, S. Isolation of Angiotensin I Converting Enzyme (ACE) Inhibitor from Fermented Oyster Sauce, Crassostrea Gigas. Food Chem. 2005, 90, 809–814. [Google Scholar] [CrossRef]
- Rai, A.K.; Pandey, A.; Sahoo, D. Biotechnological Potential of Yeasts in Functional Food Industry. Trends Food Sci. Technol. 2019, 83, 129–137. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, S.; Liu, Z.; Wang, T.; Cai, S.; Chu, C.; Hu, X.; Yi, J. Effect of Inoculating Pichia Spp. Starters on Flavor Formation of Fermented Chili Pepper: Metabolomics and Genomics Approaches. Food Res. Int. 2023, 173, 113397. [Google Scholar] [CrossRef]
- Greppi, A.; Saubade, F.; Botta, C.; Humblot, C.; Guyot, J.P.; Cocolin, L. Potential Probiotic Pichia Kudriavzevii Strains and Their Ability to Enhance Folate Content of Traditional Cereal-Based African Fermented Food. Food Microbiol. 2017, 62, 169–177. [Google Scholar] [CrossRef]
- Hong, F.; Ming, L.; Yi, S.; Zhanxia, L.; Yongquan, W.; Chi, L. The Antihypertensive Effect of Peptides: A Novel Alternative to Drugs? Peptides 2008, 29, 1062–1071. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.F.; Tung, Y.T.; Wu, C.L.; Lee, B.H.; Hsu, W.H.; Pan, T.M. Antihypertensive Effects of Lactobacillus-Fermented Milk Orally Administered to Spontaneously Hypertensive Rats. J. Agric. Food Chem. 2011, 59, 4537–4543. [Google Scholar] [CrossRef] [PubMed]
- Vīna, I.; Linde, R.; Patetko, A.; Semjonovs, P. Glucuronic Acid from Fermented Beverages: Biochemical Functions in Humans and Its Role in Health Protection. Int. J. Recent Res. Appl. Stud. 2013, 14, 217–230. [Google Scholar]
- Hung, R.J.; Chien, H.S.; Lin, R.Z.; Lin, C.T.; Vatsyayan, J.; Peng, H.L.; Chang, H.Y. Comparative Analysis of Two UDP-Glucose Dehydrogenases in Pseudomonas Aeruginosa PAO1. J. Biol. Chem. 2007, 282, 17738–17748. [Google Scholar] [CrossRef]
- Villaño, D.; Lettieri-Barbato, D.; Guadagni, F.; Schmid, M.; Serafini, M. Effect of Acute Consumption of Oolong Tea on Antioxidant Parameters in Healthy Individuals. Food Chem. 2012, 132, 2102–2106. [Google Scholar] [CrossRef]
- Kim, J.S.; Kang, O.J.; Gweon, O.C. Comparison of Phenolic Acids and Flavonoids in Black Garlic at Different Thermal Processing Steps. J. Funct. Foods 2013, 5, 80–86. [Google Scholar] [CrossRef]
- Elosta, A.; Slevin, M.; Rahman, K.; Ahmed, N. Aged Garlic Has More Potent Antiglycation and Antioxidant Properties Compared to Fresh Garlic Extract in Vitro. Sci. Rep. 2017, 7, 39613. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Ahmed, O.K.; El-Desouky, W. Effect of Low Doses γ-Irradiation on Oxidative Stress and Secondary Metabolites Production of Rosemary (Rosmarinus officinalis L.) Callus Culture. Radiat. Phys. Chem. 2011, 80, 968–976. [Google Scholar] [CrossRef]

| Week | 20-S. Radishes | 2-S. Radishes | R + 20-S. Radishes | Fresh Radishes |
|---|---|---|---|---|
| 0 | 1.40 ± 0.02 aC | 1.43 ± 0.03 fC | 1.32 ± 0.03 aB | 1.26 ± 0.01 aA |
| 1 | 8.06 ± 0.06 bB | 1.30 ± 0.01 abA | 1.48 ± 0.02 bcA | 1.43 ± 0.02 deA |
| 2 | 9.42 ± 0.06 cB | 1.34 ± 0.02 bcA | 1.50 ± 0.02 cdA | 1.40 ± 0.02 bcA |
| 3 | 9.99 ± 0.03 dC | 1.28 ± 0.01 aA | 1.46 ± 0.02 bB | 1.46 ± 0.02 eB |
| 4 | 10.39 ± 0.01 deD | 1.40 ± 0.03 eB | 1.34 ± 0.04 aA | 1.56 ± 0.02 fC |
| 5 | 10.49 ± 0.04 efB | 1.51 ± 0.02 gA | 1.53 ± 0.01 dA | 1.53 ± 0.03 fA |
| 6 | 10.87 ± 0.02 fgD | 1.38 ± 0.02 deA | 1.50 ± 0.02 cdC | 1.41 ± 0.02 cdB |
| 7 | 11.07 ± 0.04 gD | 1.52 ± 0.02 gB | 1.45 ± 0.02 bA | 1.56 ± 0.02 fC |
| 8 | 11.08 ± 0.03 gC | 1.36 ± 0.02 cdA | 1.51 ± 0.02 cdB | 1.38 ± 0.01 bA |
| Week | 20-S. Radishes | 2-S. Radishes | R + 20-S. Radishes | Fresh Radishes |
|---|---|---|---|---|
| 0 | 63.31 ± 0.42 d | 53.33 ± 0.99 d | 56.14 ± 0.66 e | 69.60 ± 0.59 e |
| 1 | 51.16 ± 0.61 abc | 55.43 ± 2.56 d | 58.34 ± 3.84 e | 67.95 ± 3.45 e |
| 2 | 42.52 ± 3.25 a | 34.45 ± 0.69 bc | 36.26 ± 0.58 cd | 48.62 ± 0.88 c |
| 3 | 52.52 ± 0.83 c | 37.12 ± 3.20 c | 28.55 ± 2.56 abc | 58.46 ± 2.30 d |
| 4 | 48.19 ± 1.76 abc | 38.87 ± 0.65 c | 30.39 ± 5.51 bcd | 42.07 ± 0.50 b |
| 5 | 51.63 ± 3.26 bc | 34.42 ± 2.20 c | 36.23 ± 3.26 d | 37.23 ± 2.93 b |
| 6 | 53.33 ± 0.86 bc | 20.56 ± 0.36 a | 21.64 ± 1.57 a | 29.03 ± 1.43 a |
| 7 | 42.63 ± 0.81 a | 26.69 ± 2.66 ab | 28.10 ± 2.68 abc | 34.68 ± 2.41 ab |
| 8 | 45.68 ± 0.50 ab | 20.81 ± 0.33 a | 21.90 ± 0.08 ab | 28.55 ± 0.45 a |
| Week | 20-S. Radishes | 2-S. Radishes | R + 20-S. Radishes | Fresh Radishes |
|---|---|---|---|---|
| 0 | 798.46 ± 17.01 a | 740.79 ± 22.40 a | 779.78 ± 36.94 a | 798.03 ± 60.31 ab |
| 1 | 867.34 ± 27.69 bcd | 747.79 ± 23.68 a | 787.14 ± 20.05 a | 754.81 ± 42.92 a |
| 2 | 832.92 ± 40.29 abc | 740.86 ± 33.09 a | 779.85 ± 79.71 a | 956.76 ± 36.36 d |
| 3 | 916.13 ± 80.82 d | 818.86 ± 28.91 b | 861.96 ± 19.70 a | 755.00 ± 72.73 a |
| 4 | 763.06 ± 8.57 a | 821.46 ± 21.73 b | 833.11 ± 59.16 a | 775.98 ± 7.71 a |
| 5 | 913.45 ± 26.16 cd | 811.07 ± 14.66 b | 853.75 ± 31.58 a | 897.54 ± 23.55 cd |
| 6 | 927.25 ± 3.53 d | 782.37 ± 44.89 ab | 823.55 ± 58.42 a | 853.51 ± 3.18 bc |
| 7 | 953.06 ± 1.93 d | 777.53 ± 30.46 ab | 818.45 ± 14.12 a | 789.37 ± 1.73 ab |
| 8 | 819.17 ± 16.72 ab | 793.43 ± 39.73 ab | 835.19 ± 10.81 a | 788.94 ± 15.05 ab |
| Week | 20-S. Radishes | 2-S. Radishes | R + 20-S. Radishes | Fresh Radishes |
|---|---|---|---|---|
| 0 | 21,505.70 ± 21.13 e | 20,947.74 ± 47.01 f | 22,050.25 ± 89.71 f | 20,433.12 ± 90.92 f |
| 1 | 18,796.49 ± 18.67 d | 14,211.13 ± 89.14 b | 14,959.08 ± 75.64 b | 15,632.74 ± 16.80 de |
| 2 | 13,593.60 ± 42.04 c | 14,872.83 ± 25.55 c | 15,655.61 ± 52.61 c | 15,248.65 ± 78.39 cd |
| 3 | 12,606.09 ± 23.73 b | 15,576.34 ± 13.68 de | 16,396.14 ± 25.94 d | 15,194.00 ± 21.57 bcd |
| 4 | 12,578.19 ± 77.35 b | 15,528.11 ± 22.73 d | 16,345.38 ± 33.07 d | 15,011.49 ± 96.18 abc |
| 5 | 13,516.56 ± 61.32 c | 15,993.26 ± 40.15 e | 16,835.01 ± 99.41 e | 15,906.17 ± 55.19 e |
| 6 | 13,423.70 ± 39.47 c | 13,500.57 ± 23.12 a | 14,211.13 ± 65.42 a | 15,007.43 ± 55.28 abc |
| 7 | 13,267.86 ± 34.50 c | 14,129.19 ± 51.31 b | 14,872.83 ± 89.66 b | 14,638.70 ± 31.54 ab |
| 8 | 10,089.53 ± 71.49 a | 14,797.52 ± 45.07 c | 15,576.34 ± 38.59 c | 14,586.24 ± 64.34 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.-F.; Huang, C.-R.; Lu, Y.-C. Changes of Antioxidant and Functional Components in Various Salt-Aged and Fresh Radishes after Fermentation. Fermentation 2024, 10, 479. https://doi.org/10.3390/fermentation10090479
Wang C-F, Huang C-R, Lu Y-C. Changes of Antioxidant and Functional Components in Various Salt-Aged and Fresh Radishes after Fermentation. Fermentation. 2024; 10(9):479. https://doi.org/10.3390/fermentation10090479
Chicago/Turabian StyleWang, Chih-Feng, Cui-Rou Huang, and Ying-Chen Lu. 2024. "Changes of Antioxidant and Functional Components in Various Salt-Aged and Fresh Radishes after Fermentation" Fermentation 10, no. 9: 479. https://doi.org/10.3390/fermentation10090479





