The Polyamine Signaling Pathway in Response to Waterlogging Stress of Paeonia lactiflora
Abstract
:1. Introduction
- A preliminary analysis of the polyamine signaling pathway of herbaceous peony in response to waterlogging was carried out, and five key genes of the polyamine signaling pathway were screened: OR552626, OR552627, OR552628, OR552629, and OR552630. Functional validation analyses were performed on the five screened genes at a later stage;
- This study provides a basis for further in-depth research on the mechanism of waterlogging tolerance in herbaceous peony, selection and breeding of excellent waterlogging-tolerant varieties, and promotion of herbaceous peony.
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Treatment and Sampling
2.3. Test Methods
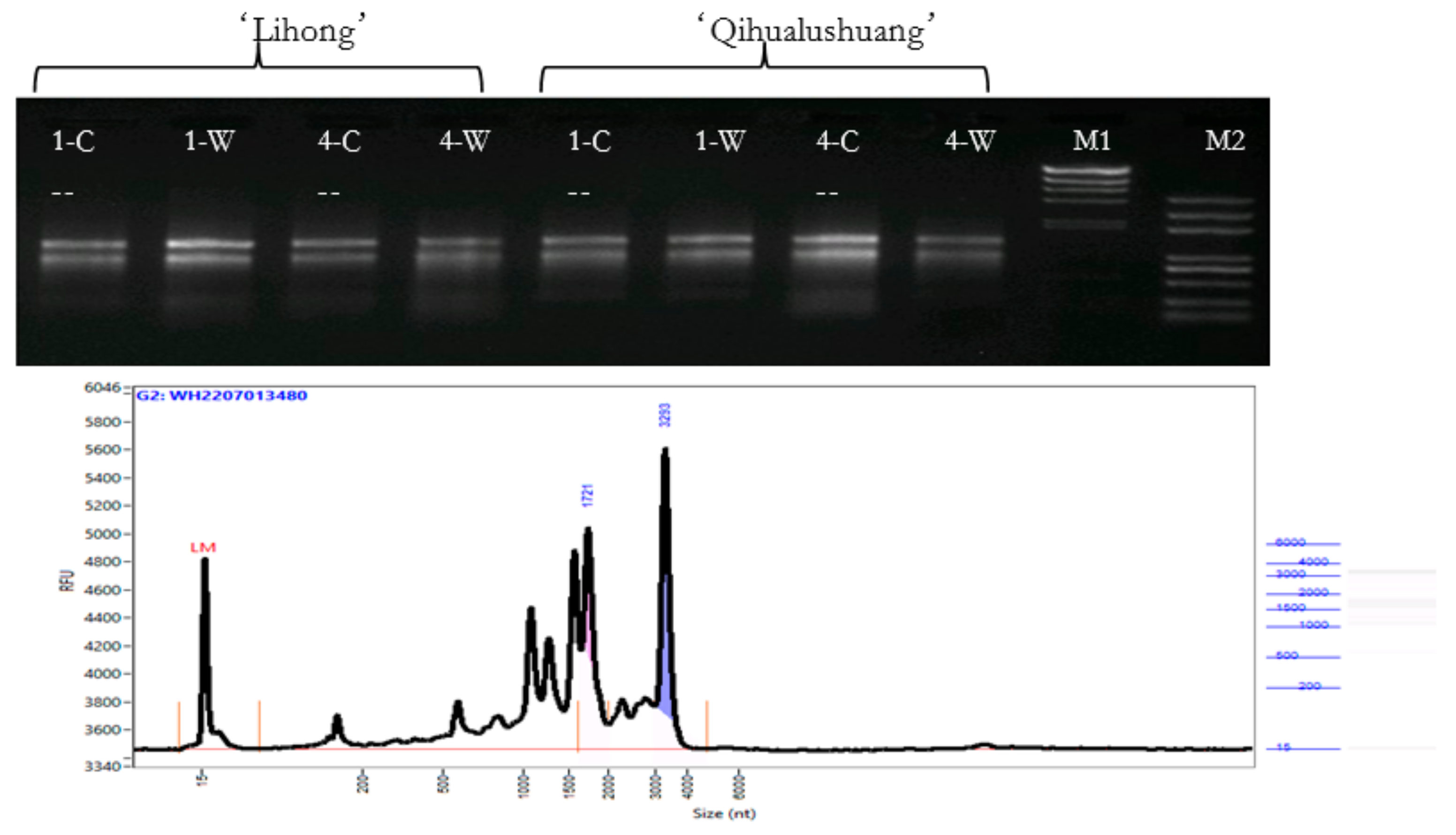
2.3.1. Transcriptome Sequencing Analysis
- (1)
- Total RNA extraction from herbaceous peonies
- (2)
- Construction and Sequencing of cDNA Libraries
- (3)
- Fluorescence quantitative PCR expression validation
2.3.2. Determination of Endogenous Polyamine Content
2.3.3. Determination of Growth Hormone and Abscisic Acid Content
2.3.4. Data Processing and Analysis
3. Results and Analysis
3.1. Morphological Changes of Herbaceous Peony under Waterlogging Stress
3.2. Transcriptome Sequencing Analysis
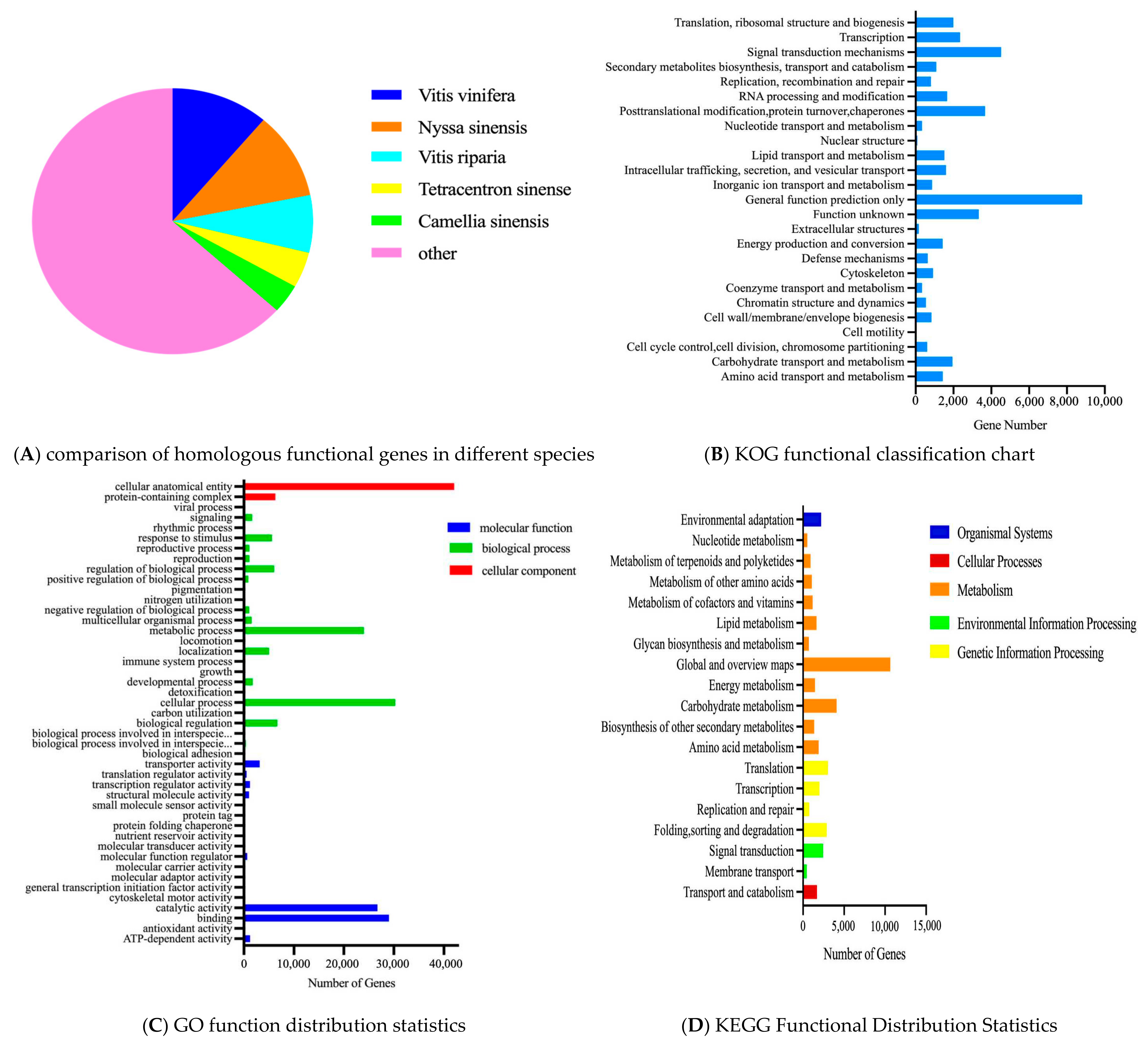
3.2.1. Functional Annotation and Classification of Unigenes
- (1)
- Comparison of homologous functional genes between different species
- (2)
- KOG functional classification
- (3)
- GO functional analysis
- (4)
- Functional analysis of KEGG pathways
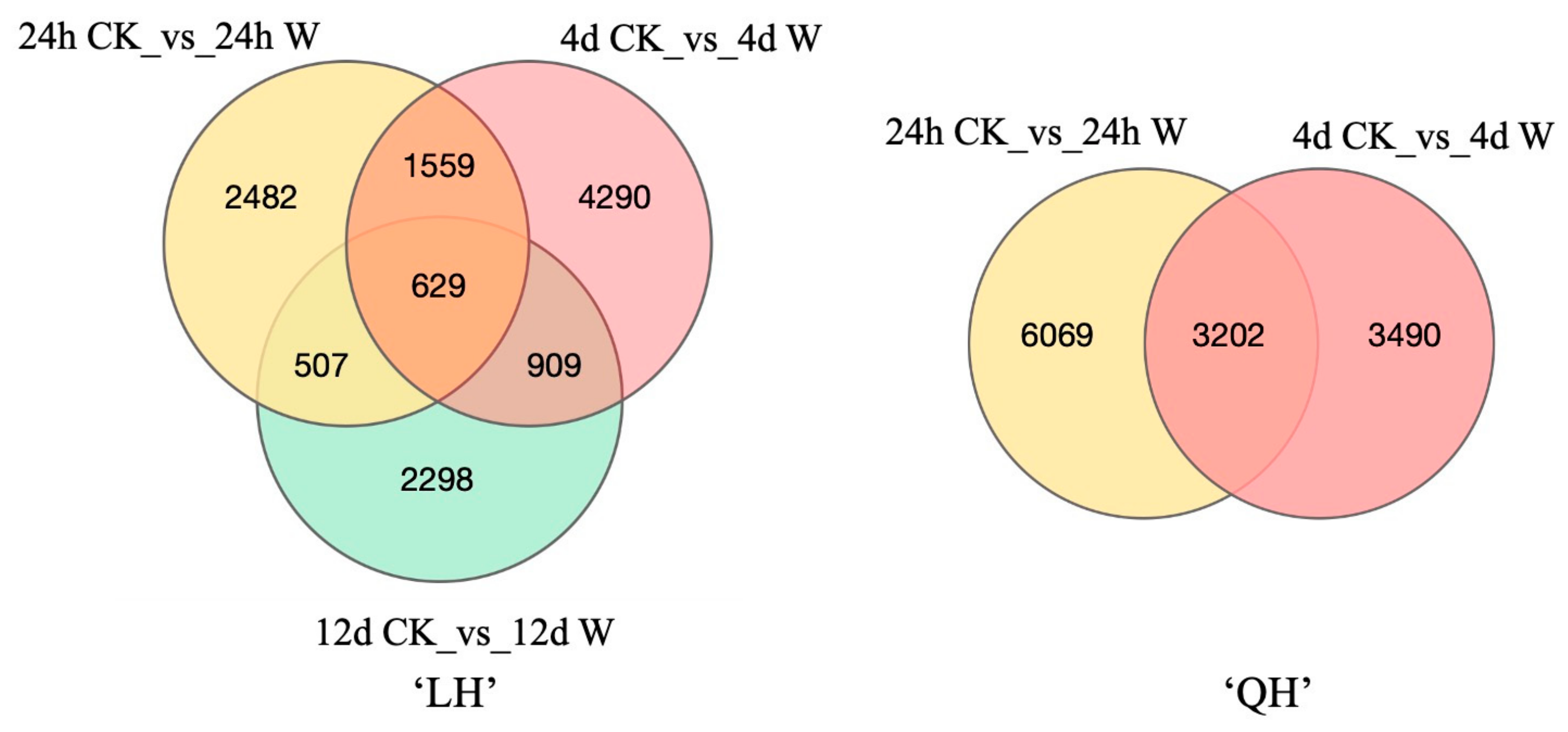
3.2.2. Screening and Analysis of Differentially Expressed Genes
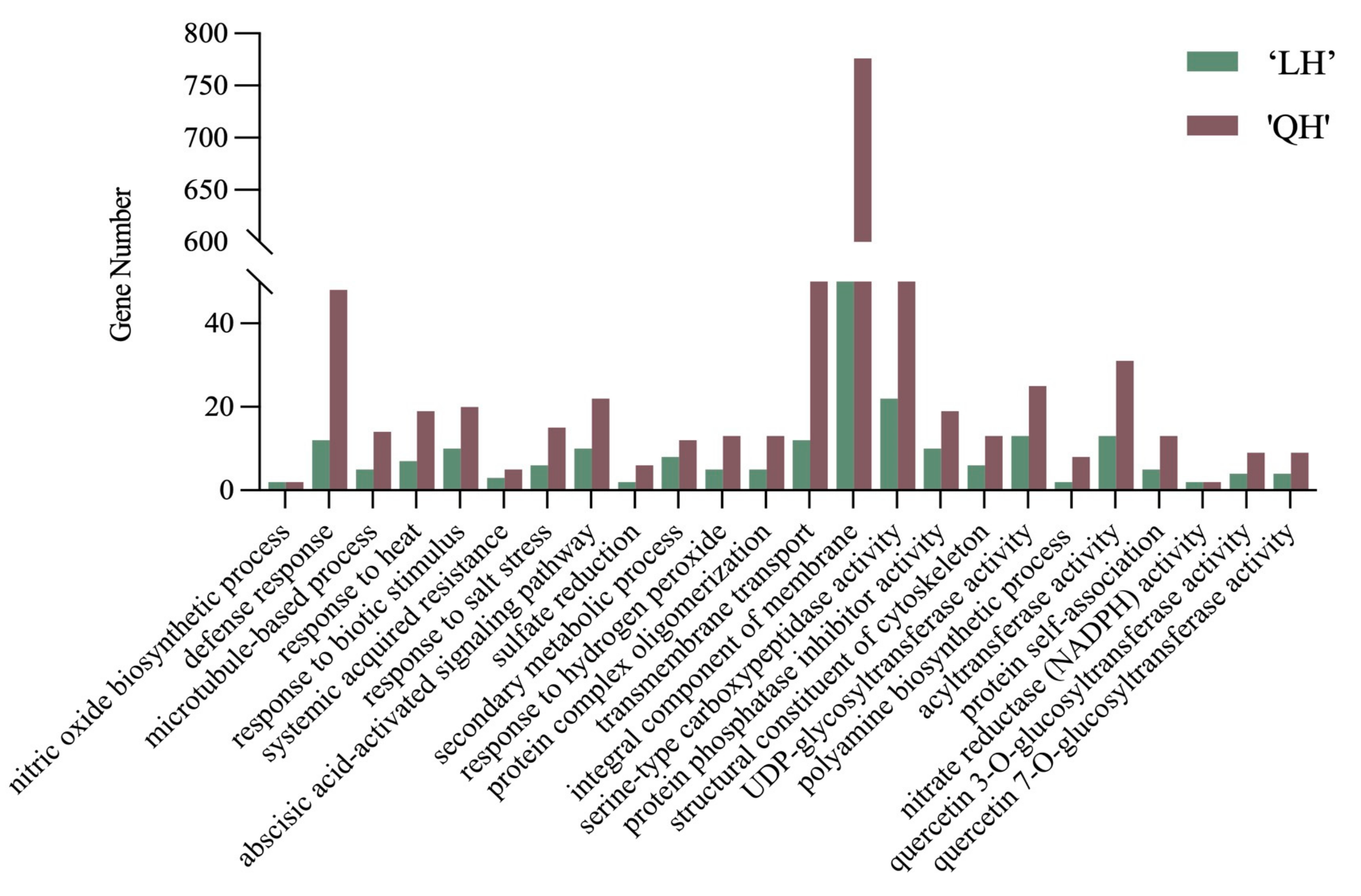
3.2.3. GO Pathway Enrichment Analysis of Differentially Expressed Genes in Herbaceous Peony in Response to Waterlogging Stress
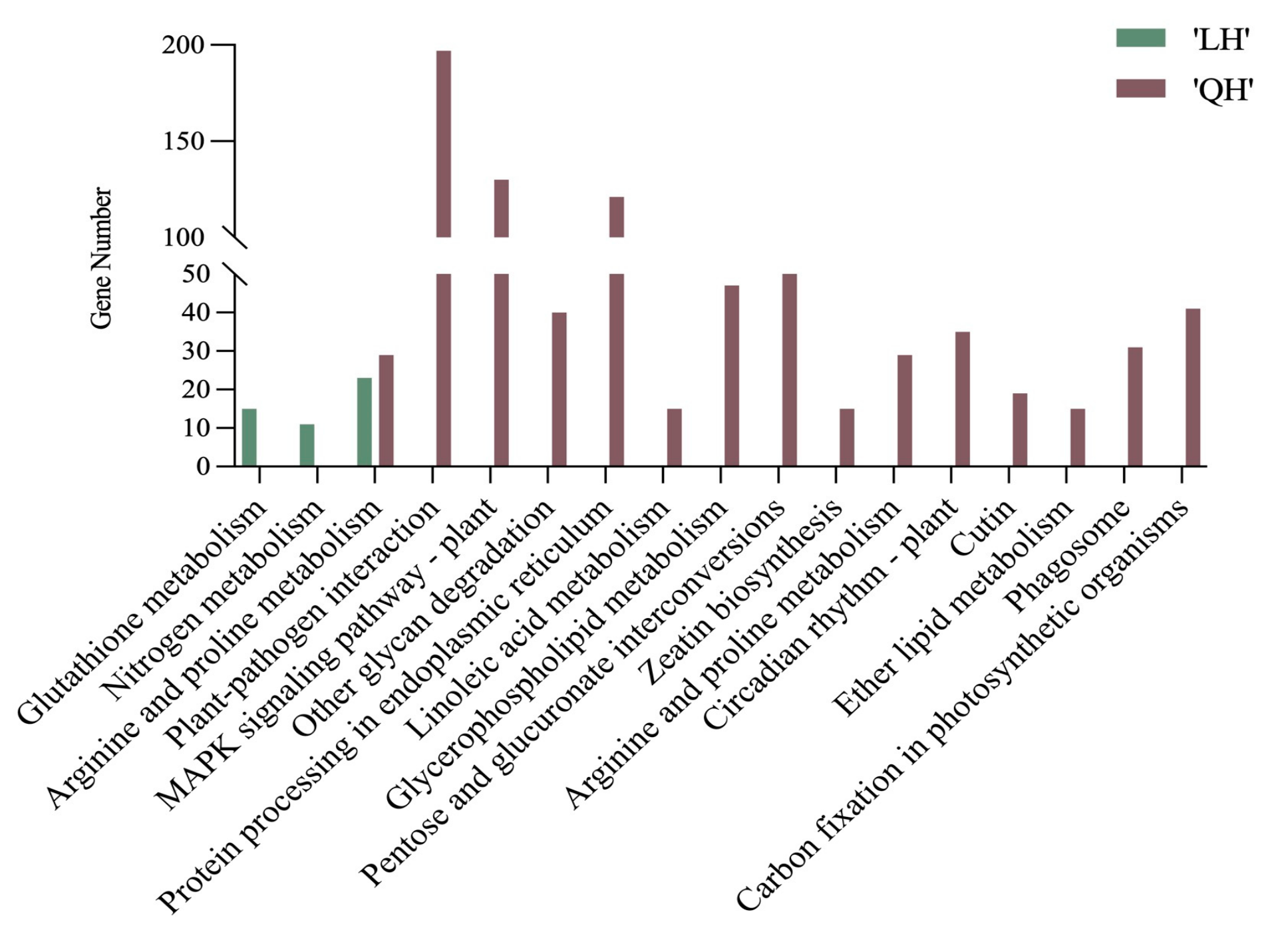
3.2.4. KEGG Pathway Enrichment Analysis of Differentially Expressed Genes in Paeonia in Response to Waterlogging Stress
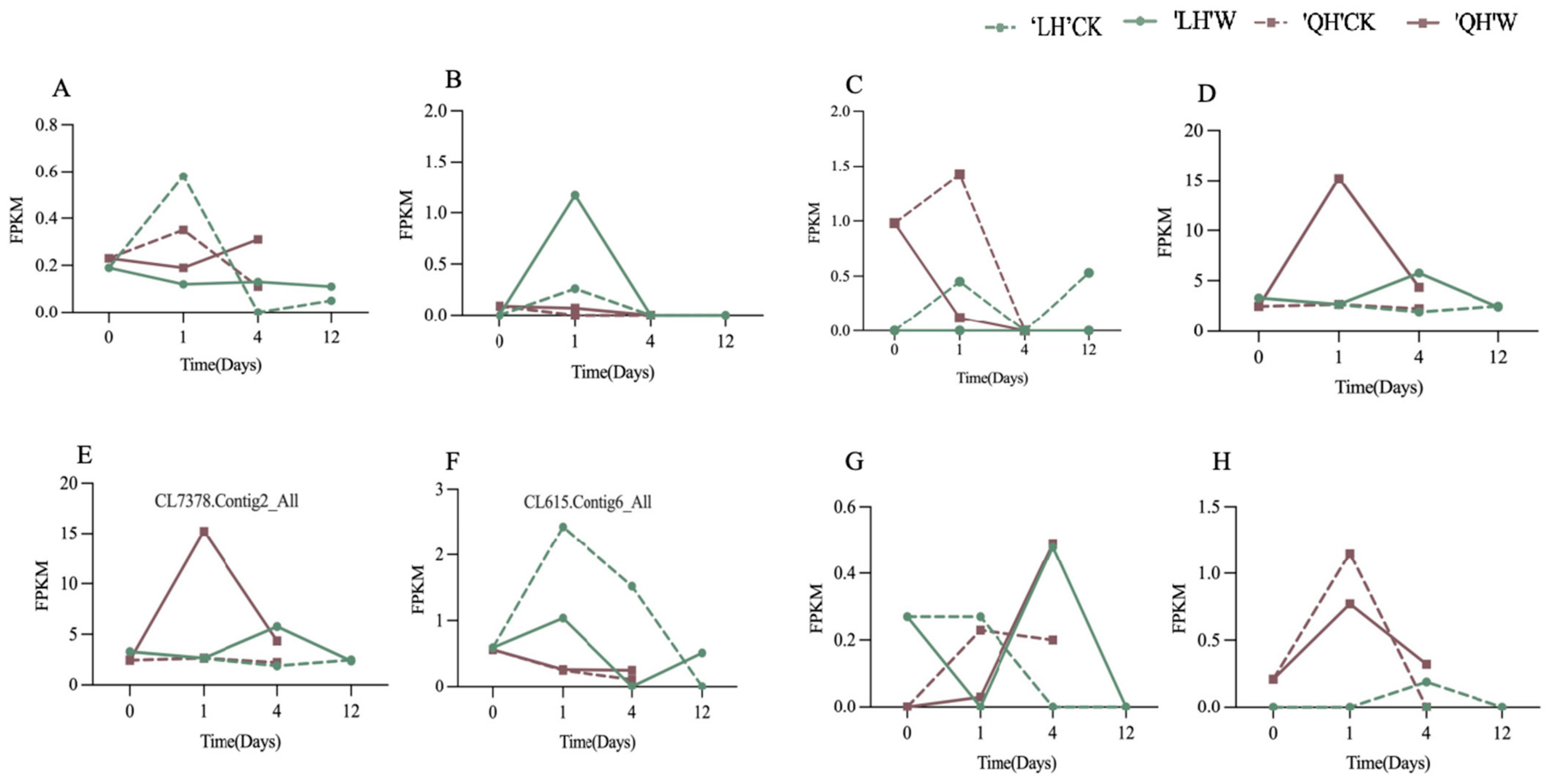
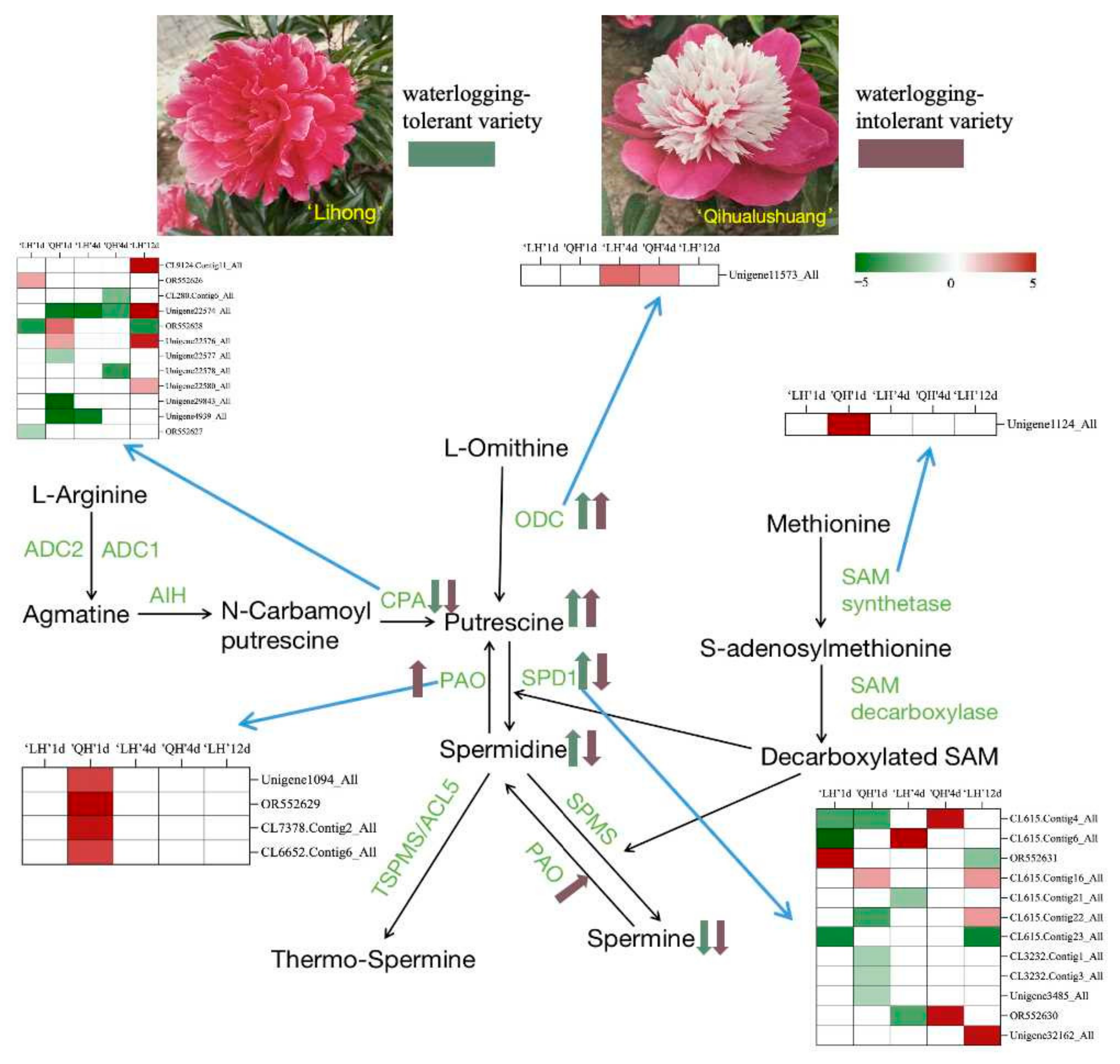
3.2.5. Analysis of Key Differential Genes of the Polyamine Signaling Pathway in Herbaceous Peony
Expression Analysis of Key Differential Genes at Put Synthesis Stage and Quantitative Fluorescence Validation
Expression Analysis of Key Differential Genes in the Synthesis Stage of Spd and Spm and Quantitative Fluorescence Verification
3.2.6. qRT-PCR Validation of Differentially Expressed Genes of the Polyamine Synthesis Pathway
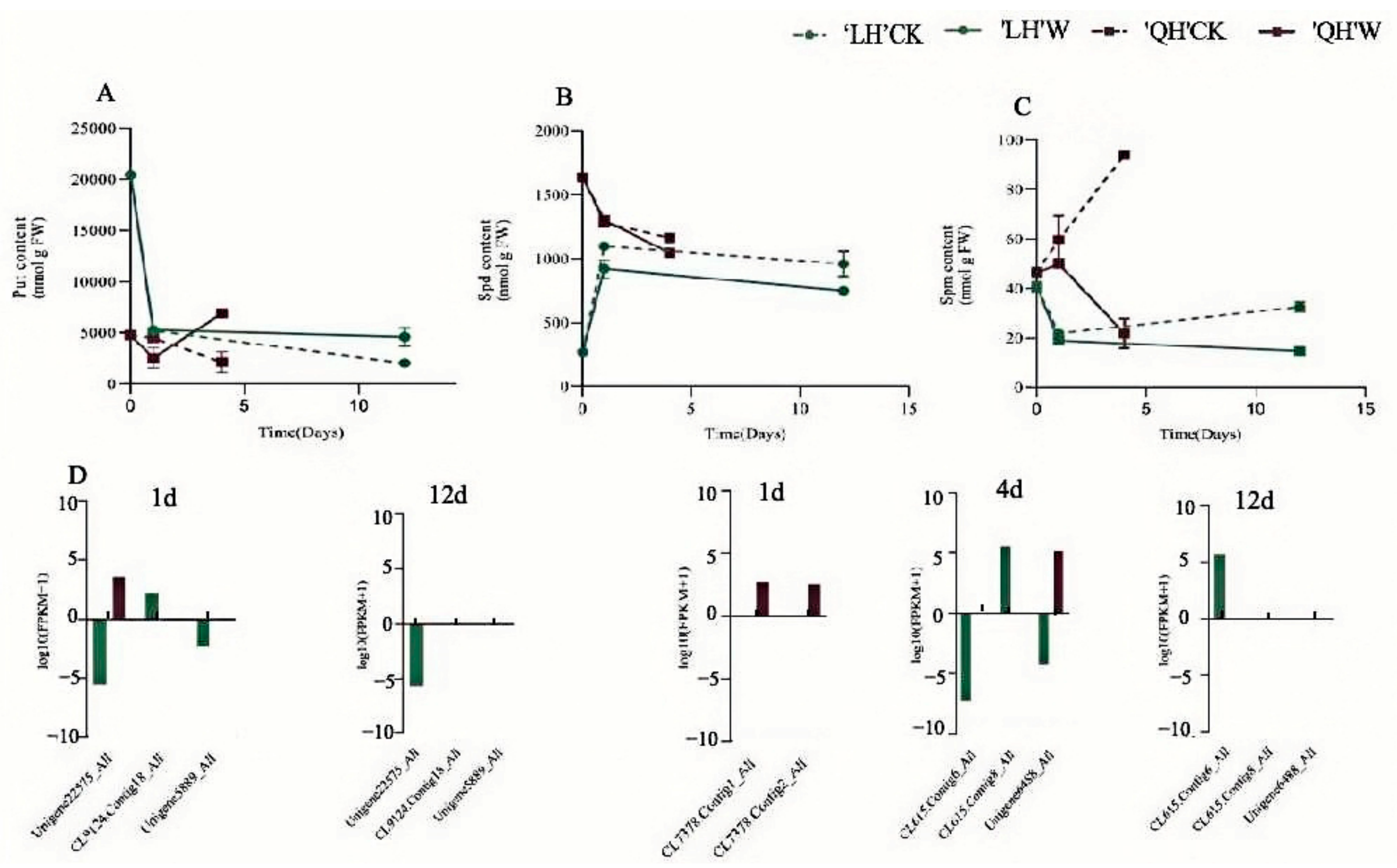
3.2.7. Changes in Endogenous Polyamine Content of Herbaceous Peony Leaves under Waterlogging Stress
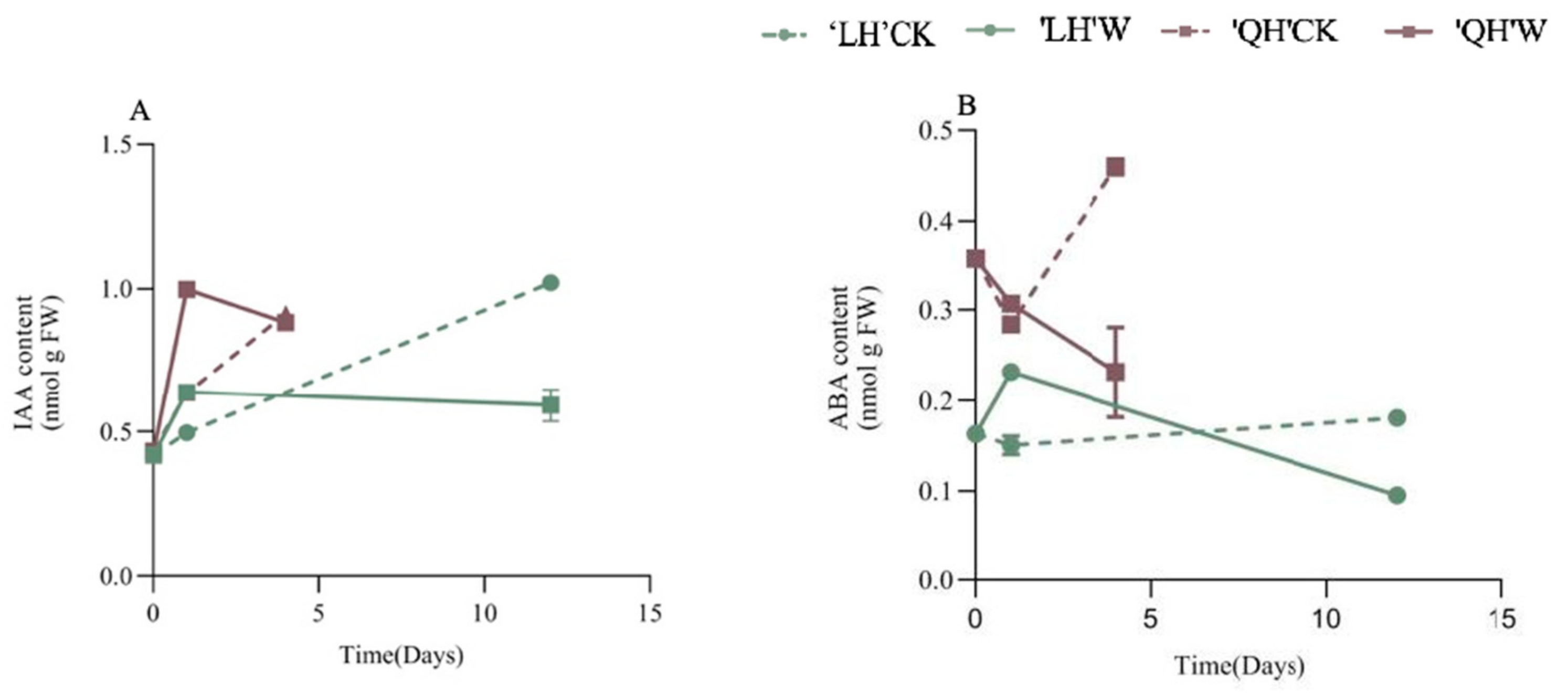
3.2.8. Changes in Endogenous Hormone Content of Peony Leaves under Waterlogging Stress
4. Discussion
4.1. Effects of Waterlogging Stress on the Morphology of Herbaceous Peony
4.2. Analysis of Key Genes for Polyamine Signaling in Response to Waterlogging Stress in Herbaceous Peony
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Synthesis Stage | Protease Name | GeneID | Description | ‘Lihong’ | ‘Qihualushuang’ | |||
|---|---|---|---|---|---|---|---|---|
| 1-CK vs. 1-W | 4-CK vs. 4-W | 12-CK vs. 12-W | 1-CK vs. 1-W | 4-CK vs. 4-W | ||||
| Putrescine synthesis | Ornithine decarboxylase | Unigene11573_All | Ornithine decarboxylase | 1.578 | 3.524 | —— | —— | 2.691 |
| Arginine decarboxylase | Unigene17303_All | Vitis riparia arginine decarboxylase | —— | —— | —— | —— | 1.001 | |
| N-carbamoyl-secantopamine amide hydrolase | CL9124.Contig11_All | Hypothetical predicted protein | —— | —— | 5.858 | —— | —— | |
| CL9124.Contig12_All | −1.071 | —— | —— | —— | —— | |||
| OR552626 | 2.170 | —— | —— | —— | —— | |||
| CL95.Contig1_All | Bromodomain-containing protein 4 | —— | 1.229 | —— | —— | —— | ||
| CL95.Contig22_All | —— | —— | 1.689 | —— | —— | |||
| CL1627.Contig8_All | Uncharacterized protein | —— | —— | —— | 1.384 | —— | ||
| CL280.Contig4_All | —— | —— | —— | −1.194 | —— | |||
| CL280.Contig6_All | —— | —— | —— | −1.980 | −3.700 | |||
| CL9124.Contig29_All | —— | —— | —— | —— | −1.321 | |||
| CL95.Contig18_All | —— | —— | —— | —— | 1.121 | |||
| Unigene22574_All | 1.478 | −7.098 | 6.129 | −6.919 | −5.170 | |||
| OR552628 | −5.492 | −5.728 | 3.564 | —— | ||||
| Unigene22576_All | —— | —— | 5.369 | 2.221 | —— | |||
| Unigene22577_All | —— | —— | —— | −2.647 | —— | |||
| Unigene22578_All | —— | —— | —— | —— | −5.358 | |||
| Unigene22579_All | —— | —— | −1.311 | —— | —— | |||
| Unigene22580_All | —— | —— | 2.150 | —— | —— | |||
| Unigene29843_All | —— | —— | —— | −8.139 | —— | |||
| Unigene3960_All | Synaptonemal complex protein | −1.266 | −1.000 | —— | —— | —— | ||
| Unigene4939_All | Hypothetical protein | —— | −6.954 | —— | −7.516 | —— | ||
| OR552627 | Transposon protein | −2.273 | —— | —— | —— | —— | ||
| Spermidine, spermine synthesis | S-adenosylmethionine decarboxylase | CL2592.Contig2_All | S-adenosylmethionine Decarboxylase proenzyme | 1.513 | ||||
| CL2592.Contig3_All | 1.181 | |||||||
| Unigene1124_All | 2.329 | |||||||
| Spermidine synthase | CL615.Contig2_All | Pistacia vera spermidine synthase 1 | —— | —— | 1.466 | —— | —— | |
| CL615.Contig4_All | −4.322 | —— | —— | −4.392 | 5.209 | |||
| CL615.Contig6_All | −1.224 | −7.248 | 5.672 | —— | —— | |||
| CL615.Contig8_All | —— | 5.585 | —— | −2.939 | —— | |||
| CL615.Contig15_All | —— | −1.722 | —— | —— | —— | |||
| CL615.Contig16_All | —— | —— | —— | 2.295 | 2.147 | |||
| CL615.Contig21_All | —— | —— | −2.778 | —— | —— | |||
| CL615.Contig22_All | 4.524 | —— | —— | 2.283 | −4.392 | |||
| CL615.Contig23_All | —— | −5.523 | —— | −5.644 | —— | |||
| CL615.Contig25_All | −1.295 | 1.924 | —— | —— | —— | |||
| CL3232.Contig1_All | Vitis vinifera spermine synthase | —— | −1.150 | —— | −2.296 | —— | ||
| CL3232.Contig2_All | —— | —— | —— | −1.683 | —— | |||
| CL3232.Contig3_All | Runus pseudocerasus Spermine synthase gene | —— | —— | —— | −2.145 | —— | ||
| OR552630 | Vitis riparia spermidine synthase 2 | −1.523 | −1.322 | —— | —— | −1.170 | ||
| Unigene3485_All | Ziziphus jujuba spermine synthase | —— | —— | —— | −2.076 | −0.263 | ||
| Unigene6488_All | Spermidine synthase 1 | —— | −4.248 | —— | —— | 5.209 | ||
| Unigene23250_All | —— | —— | —— | −1.391 | —— | |||
| Unigene26129_All | Durio zibethinus Spermidine synthase-like | 1.021 | —— | —— | —— | —— | ||
| Unigene32162_All | —— | —— | 5.209 | —— | —— | |||
| Polyamine catabolism | Polyamine oxidase | Unigene1094_All | Probable polyamine oxidase 5 | —— | —— | —— | 2.044 | —— |
| OR552629 | Polyamine oxidase 5 | —— | —— | —— | 2.770 | —— | ||
| CL7378.Contig2_All | Polyamine oxidase 4 | —— | —— | —— | 2.517 | —— | ||
| CL6652.Contig6_All | Primary amine oxidase 2 | —— | —— | —— | 2.056 | —— | ||
References
- Qin, K.J. The Herbaceous Peony; Forestry Press: Beijing, China, 2004; pp. 1–41. [Google Scholar]
- Sun, X. Appreciation of Chinese Peony Varieties; Forestry Press: Beijing, China, 2019. [Google Scholar]
- Zhang, Q.X.; Xu, J.G.; Bao, M.Y.; Liu, M.M.; Xie, A.Q.; Zhang, D.L.; Sun, X. Effect of waterlogging stress on root morphology and polyamine content of herbaceous herbaceous peony (Paeonia lactiflora). Plant Physiol. J. 2020, 56, 1445–1457. [Google Scholar]
- Chen, Y.M. Physiological Research on the Response of KiwifruitTolerant-Rootstock to Hypoxia Stress; Zhejiang A&F University: Hangzhou, China, 2018. [Google Scholar]
- Lv, M.W.; Xu, J.G.; Du, J.; Gao, C.R.; Lu, J.; Zhang, Q.X.; Wang, T.L.; Sun, X. Effects of exogenous gibberellin A3 and paclobutrazol on development of herbaceous peony (Paeonia lactiflora) bulbils. Plant Physiol. 2018, 54, 790–802. [Google Scholar]
- Lu, F.Q.; Wang, X.; Shen, T.; Zhou, F. Polyamine metabolism and plant environmental stress. Tianjin Agric. Sci. 2014, 15–17. [Google Scholar]
- Groppa, M.D.; Benavides, M.P. Polyamines and abiotic stress: Recent advances. Amino Acids 2008, 34, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Alcázar, R.; Cuevas, J.C.; Patron, M.; Altabella, T.; Tiburcio, A.F. Abscisic acid modulates polyamine metabolism under water stress in Arabidopsis thaliana. Physiol. Plant. 2006, 128, 448–455. [Google Scholar] [CrossRef]
- Urano, K.; Yoshiba, Y.; Nanjo, T.; Igarashi, Y.; Seki, M.; Sekiguchi, F.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Characterization of Arabidopsis genes involved in biosynthesis of polyamines in abiotic stress responses and developmental stages. Plant Cell Environ. 2003, 26, 1917–1926. [Google Scholar] [CrossRef]
- Zhang, L.; Tao, Y.J.; Fang, L.; Fan, F.J.; Li, W.Q.; Wang, F.Q.; Xu, Y.; Chen, Z.H.; Jiang, Y.J.; Yang, J.; et al. Metabolism and physiological function of polyamine in plants. Plant Physiol. J. 2020, 56, 2029–2039. [Google Scholar]
- Liu, C.; Li, J.Y.; Feng, G.J.; Liu, D.J.; Yan, Z.S.; Yang, X.X. Effects of low temperature acclimation on endogenous polyamine Contentand expression of biosynthesis and decomposition genes in Phaseolus vulgaris L. North. Hortic. 2021, 23, 30–37. [Google Scholar]
- Zhang, J.P.; Li, D.Q.; Nie, J.J.; Xia, Y.P. Physiological and biochemical responses to the high temperature stress and heat resistance evaluation of Paeonia lactiflora pall. cultivars. J. Nucl. Agric. Sci. 2016, 30, 1848–1856. [Google Scholar]
- Guo, H. Effects of Soil Drought Stress on Physiological and Biochemical Responses of Four Peony Varieties. Ph.D. Thesis, Hunan Agricultural University, Hunan, China, 2009. [Google Scholar]
- Chen, Y.; Ma, Y.; Guo, J.; Guo, X. Cloning and expression analysis of the dehydrin gene PlDHN1 in peony (Paeonia lactiflora). J. Hortic. Sci. Biotechnol. 2018, 93, 557–565. [Google Scholar] [CrossRef]
- Zhao, D.; Tao, J.; Han, C.; Ge, H. An actin gene as the internal control for gene expression analysis in herbaceous peony (Paeonia lactiflora Pall.). Afr. J. Agric. Res. 2012, 7, 2153–2159. [Google Scholar]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ji, X.J.; Liu, Y.L. High performance liquid chromatography method for measuring polyamine content in plant tissue. Plant Physiol. Commun. 2002, 38, 596–598. [Google Scholar]
- Zhang, Y.Q.; Zhong, Y.L.; Gao, C.Y.; Dong, S.R.; Chen, N.; Wang, M.F. Determination of five endogenous hormones in wheat by high performance liquid chromatography. Chin. J. Chromatogr. 2013, 31, 800–803. [Google Scholar] [CrossRef]
- Wang, P.; Hu, Y.H.; Wang, L.M.; Liu, Q.H. Kinds of identification index and evaluation means of plant submergence endurance. North. Hortic. 2007, 11, 78–81. [Google Scholar]
- Zhi, L.Y.; Hu, S.Z.; Yu, L.; You, Q. Effects of waterlogging stress on growth and leaf physiological of Euscaphis konishii Hayata seedlngs. Acta Agric. Univ. Jiangxiensis 2008, 30, 279–282. [Google Scholar]
- Mi, B.B.; Wu, F.F.; Xie, L.L.; Dai, X.Z.; Zhou, H.Q. Morphological structure and physiological response of winter melon seedlings under waterlogging stress. South. J. Agric. 2018, 49, 2419–2424. [Google Scholar]
- Alcázar, R.; Marco, F.; Cuevas, J.C.; Patron, M.; Ferrando, A.; Carrasco, P.; Tiburcio, A.F.; Altabella, T. Involvement of polyamines inplant response to abiotic stress. Biotechnol. Lett. 2006, 28, 1867–1876. [Google Scholar] [CrossRef] [PubMed]
- Borrell, A.; Bloch, D.; Desportes, G. Age trends and reproductive transfer of organochlorine compounds in long-finned pilot whales from the Faroe Islands. Environ. Pollut. 1995, 88, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Pegg, A.E. S-Adenosylmethionine decarboxylase. Essays Biochem. 2009, 46, 25–46. [Google Scholar] [PubMed]
- Kong, L.F.; Zhang, J.Y.; Liu, Z.P.; Wang, Y.R. Cloning of a S-adenosyl methionine synthetase gene from Cleistogenes songorica and its expression under drought stress. Caoye Xuebao 2013, 22, 268–275. [Google Scholar]
- Bai, Z.; Yu, S.; Zhao, N.; Yun, K.; Fan, X.-M. Effects of Water Stress on the Content of Free Polyamine in Cabernet Grape Seedlings (Vitis vinifera L.). Xinjiang Agric. Sci. 2015, 52, 1040. [Google Scholar]
- Wang, S. Effects of Added Spermidine on the Physiological and Biochemical Characteristics of Northeast Mountain Cherry Seedlings Under Waterlogging Stress; Shenyang Agricultural University: Shenyang, China, 2019. [Google Scholar]
- Du, H.Y.; Zhou, X.G.; Liu, G.T.; Liu, H.P.; Kurtenbach, R. Relationship between seed quality and changes in conjugated polyamine in plasma membrane purified from wheat embryos during grain ripening. Russ. J. Plant Physiol. 2017, 64, 325–332. [Google Scholar] [CrossRef]
- Wimalasekera, R.; Tebartz, F.; Scherer, G.F.E. Polyamines, polyamine oxidases and nitric oxide in development, abiotic and biotic stresses. Plant Sci. 2011, 181, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Klingler, J.P.; Batelli, G.; Zhu, J.K. ABA receptors: The START of a new paradigm in phytohormone signalling. J. Exp. Bot. 2010, 61, 3199–3210. [Google Scholar] [CrossRef]
- Toumi, I.; Moschou, P.N.; Paschalidis, K.A.; Bouamama, B.; Salem-fnayou, A.B.; Ghorbel, A.W.; Mliki, A.; Roubelakis-Angelakis, K.A. Abscisic acid signals reorientation of polyamine metabolism to orchestrate stress responses via the polyamine exodus pathway in grapevine. J. Plant Physiol. 2010, 167, 519–525. [Google Scholar] [CrossRef]
- Alcázar, R.; Altabella, T.; Marco, F.; Bortolotti, C.; Reymond, M.; Koncz, C.; Carrasco, P.; Tiburcio, A.F. Polyamines: Molecules with regulatory functions in plant abiotic stress tolerance. Planta 2010, 231, 1237–1249. [Google Scholar] [CrossRef]
- Schafleitner, R.; Rosaes, R.O.G.; Gaudin, A.; Aliaga, C.A.A.; Martinez, G.N.; Marca, L.R.T.; Bolivar, L.A.; Delgado, F.M.; Simon, R.; Bonierbale, M. Capturing candiate drought tolerance traits in two native Andean potato cones by transcription profiling of field grown plants under water stress. Plant Physiol. Biochem. 2007, 45, 673–690. [Google Scholar] [CrossRef]
- Shi, Q.; Zhang, X.; Li, X.; Zhai, L.; Luo, X.; Luo, J.; He, L.; Zhang, Y.; Li, L. Identification of microRNAs and their targets involved in Paeonsr mekii peal variegation using high-throughput sequencing. J. Am. Soc. Hortic. Sci. 2019, 144, 118–129. [Google Scholar] [CrossRef]
- Xie, F.; Wang, Q.; Sun, R.; Zhang, B. Deep sequencing reveals important roles of microRNAs in response to drought and salinity stress in cotton. J. Exp. Bot. 2015, 66, 789–804. [Google Scholar] [CrossRef]
- Fei, C.Y.; Feng, L.S.; Hua, W.J.; Kun, X.C.; Jie, S.; Fa, L.S. Effects of shading on growth and photosynthetic characteristics of oil peony. Acta Bot. Boreali-Occident. Sin. 2016, 36, 1623–1631. [Google Scholar]
- Zhao, D.; Zhang, X.; Wang, R.; Liu, D.; Sun, J.; Tao, J. Herbaccous peony tryptophan decar boxy bise confers drought and salt stresses tolerance. Environ. Exp. Bot. 2019, 162, 345–356. [Google Scholar] [CrossRef]
- Zhu, D.; Chang, Y.; Pei, T.; Zhang, X.; Liu, L.; Li, Y.; Zhuang, J.; Yang, H.; Qin, F.; Song, C.; et al. MAPK-like protein I positively regulates maize seedling drought sensitivity by suppressing ABA biosynthesis. Plant J. 2020, 102, 747–760. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Zhou, Y.; Zhai, H.; He, S.; Zhao, N.; Liu, Q. A Novel Sweetpotato WRKY Transcription Factor, IbWRKY2, Positively Regulates Drought and Salt Tolerance in Transgenic Arabidopsis. Biomolecules 2020, 10, 506. [Google Scholar] [CrossRef]
- Lu, H.F. Effects of Salt Stress and Drought Stress on the Growth, Physiology and Leaf Nitrogen Characteristics of Alfalfa; Yangzhou University: Yangzhou, China, 2023. [Google Scholar]
- Bansal, R.; Srivastava, J.P. Antioxidative defense system in pigeonpea rootsunder waterlogging stress. Acta Physiol. Plant 2012, 34, 515–522. [Google Scholar] [CrossRef]
- Alet, A.I.; Sanchez, D.H.; Cuevas, J.C.; del Valle, S.; Altabella, T.; Tiburcio, A.F.; Marco, F.; Ferrando, A.; Espasandin, S.D.; Gonzalez, M.E.; et al. Putrescine accumulation in Arabidopsis thaliana transgenic lines enhances tolerance to dehydration and freezing stress. Plant Signal. Behav. 2011, 6, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Espasandin, F.D.; Maiale, S.J.; Calzadilla, P.; Ruiz, O.A.; Sansberro, P.A. Transcriptional regulation of 9-cis-epoxycarotenoid dioxygenase (NCED) gene by putrescine accumulation positively modulates ABA synthesis and drought tolerance in Lotus tenuis plants. Plant Physiol. Biochem. 2014, 76, 29–35. [Google Scholar] [CrossRef]
- Cuevas, J.C.; Lópezcobollo, R.; Alcázar, R.; Zarza, X.; Koncz, C.; Altabella, T.; Salinas, J.; Tiburcio, A.F.; Ferrando, A. Putrescine is involved in Arabidopsis freezing tolerance and cold acclimation by regulating abscisic acid levels in response to low temperature. Plant Physiol. 2008, 148, 1094–1105. [Google Scholar] [CrossRef]
- Gui, R. Effects of Polyamine Metabolism on Endogenous Hormone Changes During Flower Formation of Dianthus chinensis L. J. Nanjing For. Univ. 2003, 46, 27. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions, and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of the MDPI and/or editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions, or products referred to in the content. |



| Gene Landing Number | Functional Notes | Primer (5′–3′) |
|---|---|---|
| Actin | Internal reference gene | F: TTATGCCCTTCCTCACGCTATC |
| R: GAGCTGCTTTTGGAAGTCTCCA | ||
| OR552630 | Spermidine synthase | F: GTTCAACATGCTTCTACGCCTACC |
| R: CTCATCACATTCACAGCCACCTC | ||
| OR552629 | Polyamine oxidase | F: GCCTTGCGATTTGATAATGTGTTC |
| R: ACCTTCCAGCAGCCATATAGAC | ||
| OR552626 | Hypothetical predicted protein | F: CGGACGGAAACGGATTCAAAG |
| R: CCATGACATGACACCAATAAGAGG | ||
| OR552627 | Transposon protein | F: TGGAGGTTGACATGGGTATCAC |
| R: TTCAGATGTAGGCTCGGATGG | ||
| OR552628 | Uncharacterized protein | F: TCTTGTGAAACTGGGACCTGATTG |
| R: CAACCATACTTCCTACCGCATCTT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Y.; Lv, M.; Liu, Z.; Yang, X.; Yang, L.; Dong, L.; Lei, F.; Xie, A.; Zhang, D.; Bao, M.; et al. The Polyamine Signaling Pathway in Response to Waterlogging Stress of Paeonia lactiflora. Horticulturae 2024, 10, 928. https://doi.org/10.3390/horticulturae10090928
Shi Y, Lv M, Liu Z, Yang X, Yang L, Dong L, Lei F, Xie A, Zhang D, Bao M, et al. The Polyamine Signaling Pathway in Response to Waterlogging Stress of Paeonia lactiflora. Horticulturae. 2024; 10(9):928. https://doi.org/10.3390/horticulturae10090928
Chicago/Turabian StyleShi, Yajie, Mengwen Lv, Zemiao Liu, Xiao Yang, Lijin Yang, Lingling Dong, Fuling Lei, Anqi Xie, Dongliang Zhang, Mingyue Bao, and et al. 2024. "The Polyamine Signaling Pathway in Response to Waterlogging Stress of Paeonia lactiflora" Horticulturae 10, no. 9: 928. https://doi.org/10.3390/horticulturae10090928





