Multipurpose Impacts of Silver Nitrate on Direct Organogenesis of Begonia rex cv. DS-EYWA via Transverse Thin Cell Layering (tTCL) Technique
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Establishment of Transverse Thin Cell Layer Explants (tTCLs) for Controlling In Vitro Contaminations
2.3. Direct Organogenesis of Transverse Thin Cell Layer Explants (tTCLs) and Culture Conditions
2.4. Experimental Design and Statistical Analysis
3. Results
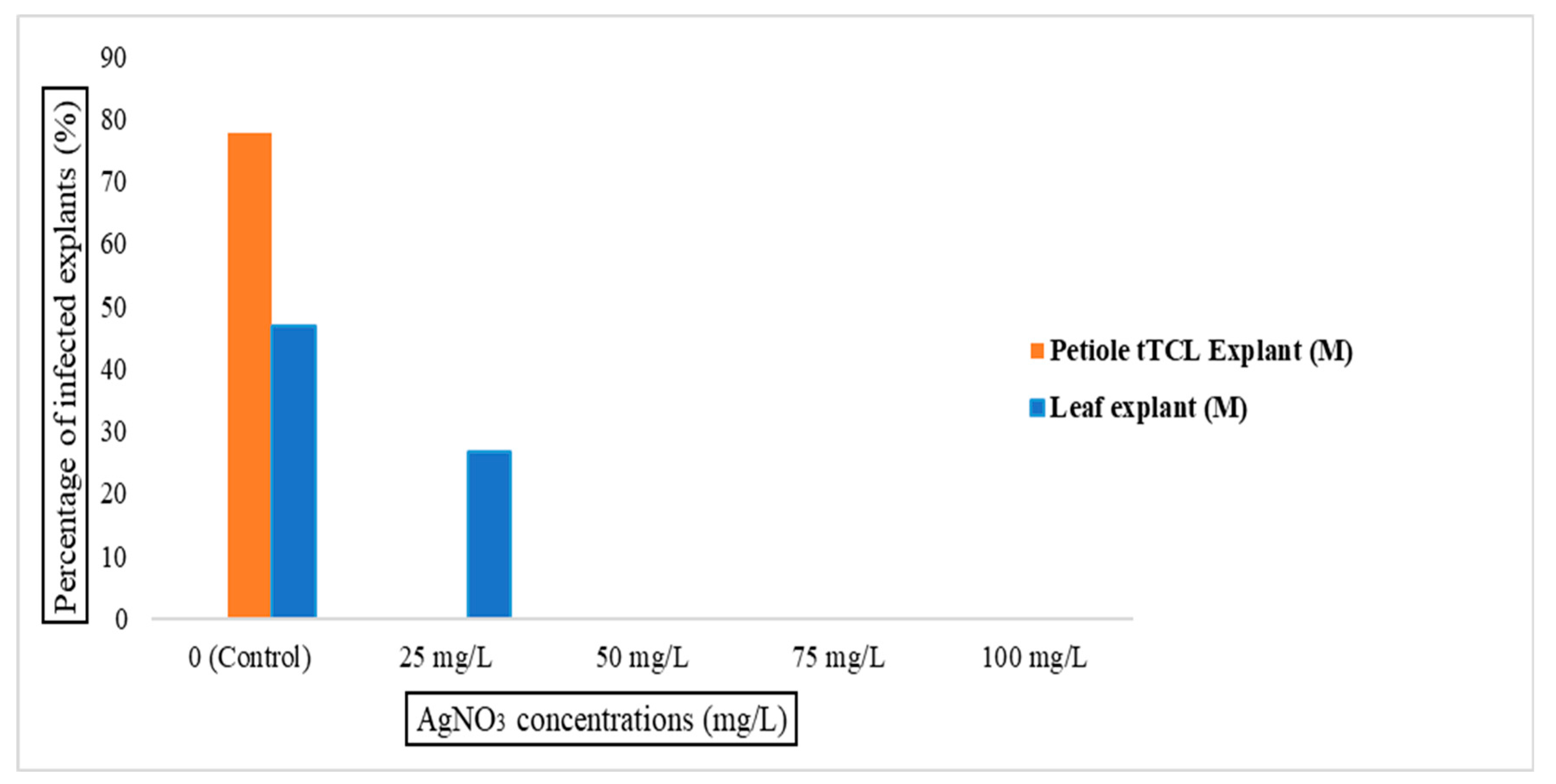
3.1. The Effect of Silver Nitrate on In Vitro Bacterial Infection in tTCL Petiole Begonia Explants
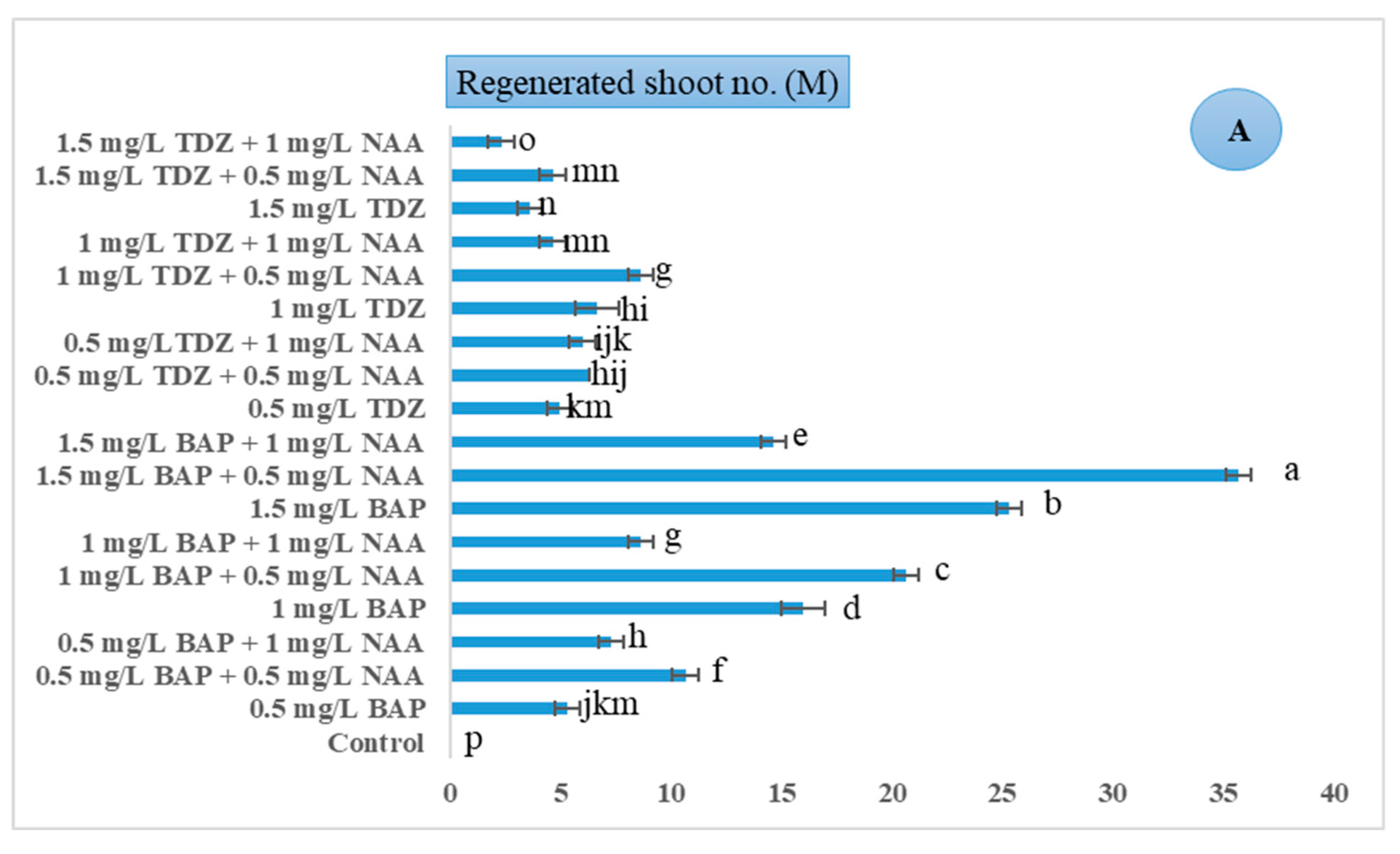
3.2. The Combined Impacts of PGRs and AgNO3 on Direct Organogenesis of tTCL Petiole Begonia Explants
3.3. Enhancing the Length of Regenerated Shoots Can Be in Parallel with New Leaf Production in the Same Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Available online: https://www.etsy.com/at/listing/1105190163/begonia-rex-ds-eywa-ukrainian-variety?show_sold_out_detail=1&ref=nla_listing_details (accessed on 20 April 2023).
- Hosseinabadi, S.; Khaleghi, A.; Akramian, M.; Khadivi, A. A highly efficient plant regeneration of Begonia rex Putz. by direct organogenesis of leaf explants. J. Hortic. Sci. Biotechnol. 2022, 97, 496–502. [Google Scholar] [CrossRef]
- Zarei, S.; Ehsanpour, A.A. Ethylene inhibition with silver nitrate (AgNO3) and pyrazinamide (PZA) ameliorates in vitro salt tolerance of tomato (Lycopersicon esculentum L.) plantlets. Plant Cell Tiss. Organ. Cult. 2023, 154, 239–247. [Google Scholar] [CrossRef]
- Kazemi, D.; Dehestani-Ardakani, M.; Hatami, M.; Ghorbanpour, M. Research on the Differences in Phenotypic and Photosynthetic Biophysical Parameters of Begonias (Begonia rex) Cultivars Under Various Light Spectral Compositions. J. Plant Growth Regul. 2023, 20, 106–121. [Google Scholar] [CrossRef]
- Nhut, D.T.; Hai, N.T.; Phan, M.X. A Highly Efficient Protocol for Micropropagation of Begonia tuberous. In Protocols for In Vitro Propagation of Ornamental Plants; Jain, S., Ochatt, S., Eds.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2010; Volume 589. [Google Scholar] [CrossRef]
- Guo, B.; Xiong, Y.; Ren, H.; Wu, T.; SILVA, J.A.; Zeng, S.; Ma, G. Shoot organogenesis and plant regeneration in Begonia coptidifolia. Turk. J. Agric. For. 2022, 45, 381–388. [Google Scholar] [CrossRef]
- Hosseini, F.; Moshtaghi, N.; Sharifi, A.; Bagheri, A.; Marashi, H.; Keykha Akhar, F. Effect of Kind and Plant Growth Regulator Composition on Micropropagation of Three Begonia Species. J. Plant Prod. 2021, 44, 25–36. [Google Scholar] [CrossRef]
- Li, H.; Liu, F.; Song, S.; Wang, C.; Sun, H. Highly effective organogenesis and somatic embryogenesis of Clivia. Sci. Hortic. 2022, 306, 111443. [Google Scholar] [CrossRef]
- Ho, T.; Pak, H.; Ri, S.; Kim, K.; Mun, N. Improvement of Water Stress Tolerance of Tuberous Begonia (Begonia × tuberhybrida) by OsmiR393a Gene Transformation. J. Plant Sci. 2021, 9, 257–265. [Google Scholar] [CrossRef]
- Hirutani, S.; Shimomae, K.; Yaguchi, A.; Chin, D.P.; Mii, M.; Igawa, T. Efficient plant regeneration and Agrobacterium-mediated transformation of Begonia semperflorens-cultorum. Plant Cell Tissue Organ Cult. 2020, 142, 435–440. [Google Scholar] [CrossRef]
- Iqbal, A.; Khan, R.S.; Khan, M.A.; Gul, K.; Aizaz, M.; Usman, M.; Arif, M. Efficient Regeneration in Sugarcane Using Thin Cell Layer (TCL) Culture System. Sugar Tech. 2023, 25, 168–176. [Google Scholar] [CrossRef]
- Da Silva, J.A.T. Thin cell layer technology in ornamental plant micropropagation and biotechnology. Afr. J. Biotechnol. 2003, 2, 683–691. [Google Scholar] [CrossRef]
- Da Silva, J.A.T. Thin Cell Layers: Power-Tool for Organogenesis of Floricultural Crops. In Protocols for In Vitro Propagation of Ornamental Plants; Jain, S., Ochatt, S., Eds.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2010; Volume 589. [Google Scholar] [CrossRef]
- Sowmya, M.; Jinu, U.; Sarathikannan, D.; Geetha, N.; Girija, S.; Venkatachalam, P. Effect of silver nitrate and growth regulators on direct shoot organogenesis and in vitro flowering from internodal segment explants of Alternanthera sessilis L. Biocatal. Agric. Biotechnol. 2020, 30, 101855. [Google Scholar] [CrossRef]
- Teixeira da Silva, J.A.; Dobránszki, J. Plant Thin Cell Layers: A 40-Year Celebration. J. Plant Growth Regul. 2013, 32, 922–943. [Google Scholar] [CrossRef]
- Aswathi, N.V.; Thomas, T.D. Transverse thin cell layer (tTCL) technology: A promising tool for micropropagation of Centratherum punctatum Cass. Vitr. Cell Dev. Biol. Plant 2023, 18, 340–353. [Google Scholar] [CrossRef]
- Tung, H.T.; Van, H.T.; Bao, H.G.; Khai, H.D.; Luan, V.Q.; Phong, T.H.; Nhut, D.T. Silver nanoparticles enhanced efficiency of explant surface disinfection and somatic embryogenesis in Begonia tuberous via thin cell layer culture. Vietnam. J. Biotechnol. 2021, 19, 337–347. [Google Scholar] [CrossRef]
- Cuong, D.M.; Mai, N.T.; Tung, H.T.; Khai, H.D.; Luan, V.Q.; Phong, T.H.; Van The Vinh, B.; Phuong, H.T.; Van Binh, N.; Tan Nhut, D. Positive effect of silver nanoparticles in micropropagation of Limonium sinuatum (L.) Mill. ‘White’. Plant Cell Tissue Organ Cult. 2023, 22, 417–432. [Google Scholar] [CrossRef]
- Manokari, M.; Raj, M.C.; Dey, A.; Faisal, M.; Alatar, A.A.; Joshee, N.; Shekhawat, M.S. Silver nanoparticles improved morphogenesis, biochemical profile and micro-morphology of Gaillardia pulchella Foug cv. ‘Torch Yellow’. Plant Cell Tiss Organ Cult. 2023, 6, 433–445. [Google Scholar] [CrossRef]
- Tung, H.T.; Hieu, T.; Phong, T.H.; Khai, H.D.; Hanh, N.T.; Van, K.T.; Nhut, D.T. The Application of Thin Cell Layer Culture Technique in Plant Regeneration and Micropropagation: Latest Achievements. In Plant Tissue Culture: New Techniques and Application in Horticultural Species of Tropical Region; Nhut, D.T., Tung, H.T., Yeung, E.C.T., Eds.; Springer: Singapore, 2023. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Abdi, G.; Salehi, H.; Khosh-Khui, M. Nano silver: A novel nanomaterial for removal of bacterial contaminants in valerian (Valeriana officinalis L.) tissue culture. Acta Physiol. Plant 2008, 30, 709–714. [Google Scholar] [CrossRef]
- Nasir, S.M.; Abdulhussein, M.A.A. Effects of AgNO3 in combination with some plant growth regulators on micropropagation of strawberry (Fragaria ananassa Duch). Kufa J. Agric. Sci. 2022, 14, 33–40. [Google Scholar] [CrossRef]
- Sgamma, T.; Thomas, B.; Muleo, R. Ethylene inhibitor silver nitrate enhances regeneration and genetic transformation of Prunus avium (L.) cv Stella. Plant Cell Tissue Organ Cult. 2015, 120, 79–88. [Google Scholar] [CrossRef]
- Abogarra, L.; Eisa, N.; EL-habbaa, G.; Darwesh, R.S.; EL-habbak, M. Superiority of Nano-Silver Nitrate and Nano-Chitosan in Controlling Bacterial Contamination and Promoting Growth of in vitro Date Palm Cultures. Plant Cell Biotechnol. Mol. Biol. 2022, 23, 85–104. [Google Scholar] [CrossRef]
- Salman, H.D. Evaluation and comparison the antibacterial activity of silver nano particles (AgNPs) and silver nitrate (AgNO3) on some pathogenic bacteria. J. Glob. Pharma Technol 2017, 9, 238–248. [Google Scholar]
- Mahendran, D.; Geetha, N.; Venkatachalam, P. Role of Silver Nitrate and Silver Nanoparticles on Tissue Culture Medium and Enhanced the Plant Growth and Development. In Plant Breeding towards Novel Agronomic Traits; Kumar, M., Muthusamy, A., Kumar, V., Bhalla-Sarin, N., Eds.; Springer: Singapore, 2019; pp. 59–74. [Google Scholar] [CrossRef]
- Sibeko, L.; Johns, T.; Cordeiro, L.S. Traditional plant use during lactation and postpartum recovery: Infant development and maternal health roles. J. Ethnopharmacol. 2021, 279, 114377. [Google Scholar] [CrossRef] [PubMed]
- Paladi, R.K.; Rai, A.N.; Penna, S. Silver nitrate modulates organogenesis in Brassica juncea (L.) through differential antioxidant defense and hormonal gene expression. Sci. Hortic. 2017, 226, 261–267. [Google Scholar] [CrossRef]
- Sarmast, M.; Salehi, H.; Khosh-Khui, M. Nano silver treatment is effective in reducing bacterial contaminations of Araucaria excelsa R. Br. var. glauca explants. Acta Biol. Hung. 2011, 62, 477–484. [Google Scholar] [CrossRef]
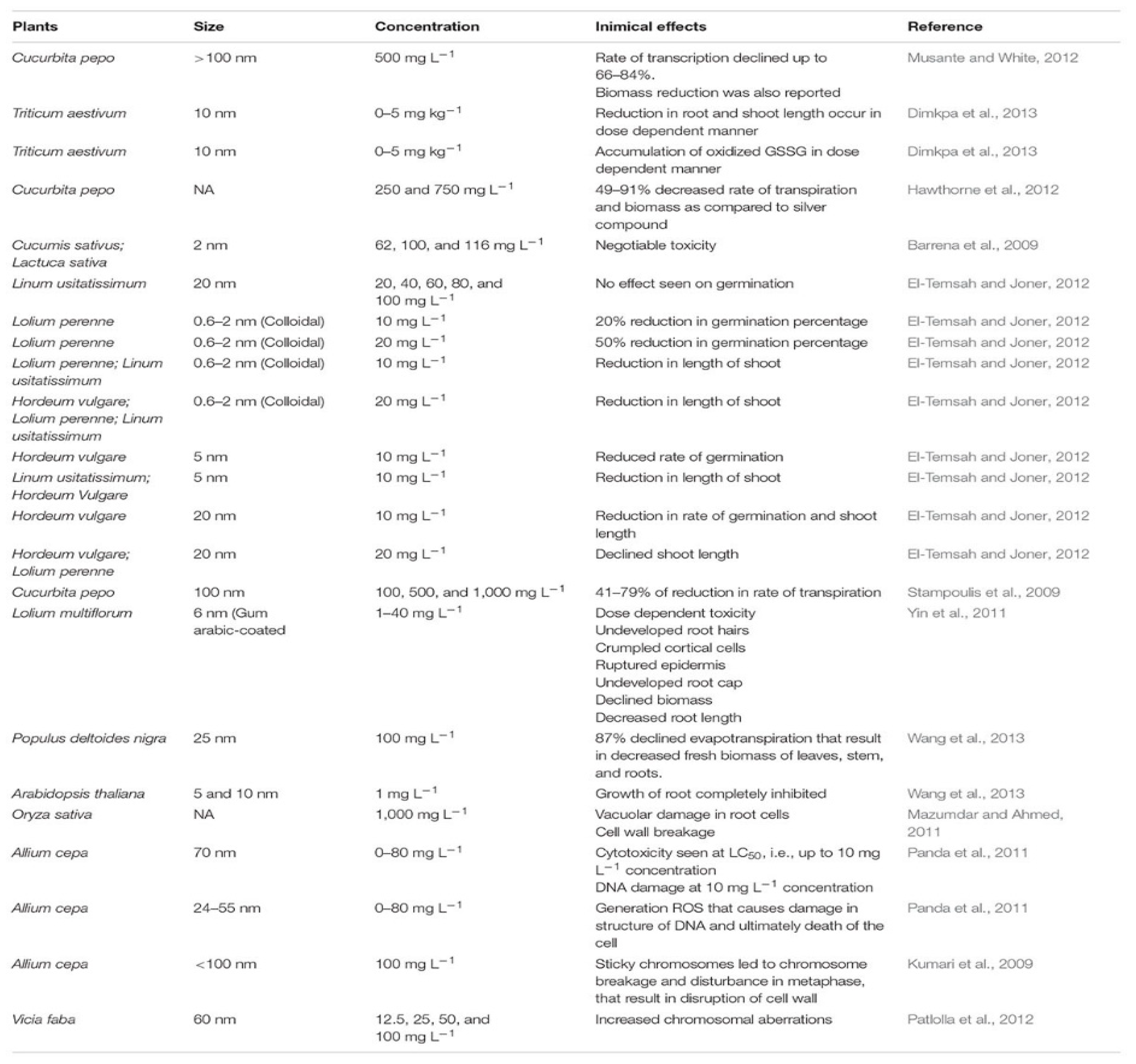
- Tripathi, D.K.; Tripathi, A.; Shweta; Singh, S.; Singh, Y.; Vishwakarma, K.; Yadav, G.; Sharma, S.; Singh, V.K.; Mishra, R.K.; et al. Uptake, accumulation and toxicity of silver nanoparticle in autotrophic plants, and heterotrophic microbes: A concentric review. Front. Microbiol. 2017, 8, 7. [Google Scholar] [CrossRef]
- Musante, C.; White, J.C. Toxicity of silver and copper to Cucurbita pepo: Differential effects of nano and bulk-size particles. Environ. Toxicol. 2012, 27, 510–517. [Google Scholar] [CrossRef]
- Dimkpa, C.O.; McLean, J.E.; Martineau, N.; Britt, D.W.; Haverkamp, R.; Anderson, A.J. Silver nanoparticles disrupt wheat (Triticum aestivum L.) growth in a sand matrix. Environ. Sci. Technol. 2013, 47, 1082–1090. [Google Scholar] [CrossRef]
- Hawthorne, J.; Musante, C.; Sinha, S.K.; White, J.C. Accumulation and phytotoxicity of engineered nanoparticles to Cucurbita pepo. Int. J. Phytoremed. 2012, 14, 429–442. [Google Scholar] [CrossRef]
- Barrena, R.; Casals, E.; Colón, J.; Font, X.; Sánchez, A.; Puntes, V. Evaluation of the ecotoxicity of model nanoparticles. Chemosphere 2009, 75, 850–857. [Google Scholar] [CrossRef]
- El-Temsah, Y.S.; Joner, E.J. Impact of Fe and Ag nanoparticles on seed germination and differences in bioavailability during exposure in aqueous suspension and soil. Environ. Toxicol. 2012, 27, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Stampoulis, D.; Sinha, S.K.; White, J.C. Assay-dependent phytotoxicity of nanoparticles to plants. Environ. Sci. Technol. 2009, 43, 9473–9479. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Koo, Y.; Alexander, A.; Yang, Y.; Westerhof, S.; Zhang, Q.; Schnoor, J.L.; Colvin, V.L.; Braam, J.; Alvarez, P.J. Phytostimulation of poplars and Arabidopsis exposed to silver nanoparticles and Ag+ at sublethal concentrations. Environ. Sci. Technol. 2013, 47, 5442–5449. [Google Scholar] [CrossRef] [PubMed]
- Mazumdar, H.; Ahmed, G.U. Phytotoxicity effect of silver nanoparticles on Oryza sativa. Int. J. ChemTech Res. 2011, 3, 1494–1500. [Google Scholar]
- Panda, K.K.; Achary, V.M.; Krishnaveni, R.; Padhi, B.K.; Sarangi, S.N.; Sahu, S.N.; Panda, B.B. In vitro biosynthesis and genotoxicity bioassay of silver nanoparticles using plants. Toxicol. In Vitro 2011, 25, 1097–1105. [Google Scholar] [CrossRef]
- Kumari, M.; Mukherjee, A.; Chandrasekaran, N. Genotoxicity of silver nanoparticles in Allium cepa. Sci. Total Environ. 2009, 407, 5243–5246. [Google Scholar] [CrossRef]
- Patlolla, A.K.; Berry, A.; May, L.; Tchounwou, P.B. Genotoxicity of silver nanoparticles in Vicia faba: A pilot study on the environmental monitoring of nanoparticles. Int. J. Environ. Res. Public Health 2012, 9, 1649–1662. [Google Scholar] [CrossRef]
- Yin, L.; Cheng, Y.; Espinasse, B.; Colman, B.P.; Auffan, M.; Wiesner, M.; Rose, J.; Liu, J.; Bernhardt, E.S. More than the Ions: The Effects of Silver Nanoparticles on Lolium multiflorum. Environ. Sci. Technol. 2011, 45, 2360–2367. [Google Scholar] [CrossRef]
- Phong, T.H.; Hieu, T.; Tung, H.T.; Mai, N.T.; Khai, H.D.; Cuong, D.M.; Luan, V.Q.; Nam, N.B.; Nhut, D.T. Silver nanoparticles enhance the in vitro plant regeneration via thin cell layer culture system in purple passion fruit. Plant Cell Tissue Organ Cult. 2023, 155, 403–415. [Google Scholar] [CrossRef]
- Kaviani, B.; Hashemabadi, D.; Khodabakhsh, H.; Onsinejad, R.; Ansari, M.H.; Haghighat, N. Micropropagation of Begonia rex Putz. by 6-benzyladenine and α-naphthalene acetic acid. Int. J. Biosci. 2015, 6, 8–15. [Google Scholar] [CrossRef]
- Bao, H.G.; Tung, H.T.; Van, H.T.; Bien, L.T.; Khai, H.D.; Mai, N.T.; Luan, V.Q.; Cuong, D.M.; Nam, N.B.; Van the Vinh, B.; et al. Copper nanoparticles enhanced surface disinfection, induction and maturation of somatic embryos in tuberous begonias (Begonia × tuberhybrida Voss) cultured in vitro. Plant Cell Tissue Organ Cult. 2022, 151, 385–399. [Google Scholar] [CrossRef]
- Lai, I.L.; Lin, C.W.; Chen, T.Y.; Hu, W.H. Micropropagation shortens the time to blooming of Begonia montaniformis × Begonia ningmingensis var. bella F1 Progeny. HortScience 2018, 53, 1855–1861. [Google Scholar] [CrossRef]
- Kulus, D.; Tymoszuk, A. Induction of callogenesis, organogenesis, and embryogenesis in non-meristematic explants of bleeding heart and evaluation of chemical diversity of key metabolites from callus. Int. J. Mol. Sci. 2020, 21, 5826. [Google Scholar] [CrossRef] [PubMed]
- Davoudi Pahnekolayi, M.; Tehranifar, A.; Samiei, L.; Shoor, M. Optimizing culture medium ingredients and micrografting devices can promote in vitro micrografting of cut roses on different rootstocks. Plant Cell Tissue Organ Cult. 2019, 137, 265–274. [Google Scholar] [CrossRef]
- Nhut, D.T.; Khai, H.D.; Hung, N.V.; Vinh, N.Q.; Dung, D.M.; Tung, H.T.; Mai, N.T.N.; Luan, V.Q.; Cuong, D.M. Effect of explant age on phytochemicals and morphogenesis in begonia. Plant Cell Tissue Organ Cult. 2023, 155, 267–282. [Google Scholar] [CrossRef]
- Pawar, B.D.; Markad, N.R.; Wagh, R.S.; Neumann, M.; Kale, A.A.; Chimote, V.P. Proline and Silver Nitrate Promotes Multiple Shoot Induction from Mature Embryo and Shoot Tip Explants of Sorghum. Sugar Tech. 2023, 25, 1187–1195. [Google Scholar] [CrossRef]



| Treatments | Investigated Characteristic |
|---|---|
| Control (MS basal medium without any AgNO3) | The percentage of infected explants |
| 25 mgL−1 AgNO3 | |
| 50 mgL−1 AgNO3 | |
| 75 mgL−1 AgNO3 | |
| 100 mgL−1 AgNO3 |
| Treatments | Investigated Characteristic |
|---|---|
| Control (MS basal medium without any PGRs) | Regenerated shoot number Regenerated shoot length Number of new leaves per shoot |
| 0.5 mgL−1 NAA | |
| 1 mgL−1 NAA | |
| 0.5 mgL−1 BAP | |
| 0.5 mgL−1 BAP + 0.5 mgL−1 NAA | |
| 0.5 mgL−1 BAP + 1 mgL−1 NAA | |
| 1 mgL−1 BAP | |
| 1 mgL−1 BAP + 0.5 mgL−1 NAA | |
| 1 mgL−1 BAP + 1 mgL−1 NAA | |
| 1.5 mgL−1 BAP | |
| 1.5 mgL−1 BAP + 1 mgL−1 NAA | |
| 1.5 mgL−1 BAP + 0.5 mgL−1 NAA | |
| 0.5 mgL−1 TDZ | |
| 0.5 mgL−1 TDZ + 0.5 mgL−1 NAA | |
| 0.5 mgL−1 TDZ + 1 mgL−1 NAA | |
| 1 mgL−1 TDZ | |
| 1 mgL−1 TDZ + 0.5 mgL−1 NAA | |
| 1 mgL−1 TDZ + 1 mgL−1 NAA | |
| 1.5 mgL−1 TDZ | |
| 1.5 mgL−1 TDZ + 1 mgL−1 NAA | |
| 1.5 mgL−1 TDZ + 0.5 mgL−1 NAA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davoudipahnekolayi, M.; Nezamdoost Darestani, D.; Mirshahi, H. Multipurpose Impacts of Silver Nitrate on Direct Organogenesis of Begonia rex cv. DS-EYWA via Transverse Thin Cell Layering (tTCL) Technique. Horticulturae 2024, 10, 986. https://doi.org/10.3390/horticulturae10090986
Davoudipahnekolayi M, Nezamdoost Darestani D, Mirshahi H. Multipurpose Impacts of Silver Nitrate on Direct Organogenesis of Begonia rex cv. DS-EYWA via Transverse Thin Cell Layering (tTCL) Technique. Horticulturae. 2024; 10(9):986. https://doi.org/10.3390/horticulturae10090986
Chicago/Turabian StyleDavoudipahnekolayi, Mahboubeh, Delaram Nezamdoost Darestani, and Homa Mirshahi. 2024. "Multipurpose Impacts of Silver Nitrate on Direct Organogenesis of Begonia rex cv. DS-EYWA via Transverse Thin Cell Layering (tTCL) Technique" Horticulturae 10, no. 9: 986. https://doi.org/10.3390/horticulturae10090986





