1. Introduction
American beech (
Fagus grandifolia Ehrh.) is at risk of beech bark disease (BBD) in the majority of its northern range; that is, from Ontario, Canada, from the northernmost points of Maine, south to Pennsylvania, central West Virginia, northeast Ohio, in both peninsulas of Michigan, and eastern Wisconsin [
1]. Although BBD has been present in North America for over 100 years, it was first reported in Michigan in 2001 and continues to spread throughout the western and southern range of beech [
1,
2]. In this disease complex, invasive beech scale (
Cryptococcus fagisuga L.) infests the bole of American beech trees creating infection courts for a fungus of the genus
Neonectria to infect and weaken the tree, eventually causing mortality [
3]. American beech is important as a wildlife mast species, a foundational species for cavity nesting birds, and conservation efforts for the species have focused on the introduction of seed from scale-resistant genotypes grown in seed orchards [
4].
Grafting as a method of resistant beech propagation has been carried out in a number of facilities by various working groups [
4,
5,
6,
7]. A major challenge in the production of resistant trees through grafting is rootstock sourcing. Currently, the recommended process is to purchase commercially available bare root seedlings or to grow the necessary rootstock from seed. Multiple agencies operating in a region may rapidly deplete the commercial rootstock in an area, and American beech seedlings of a suitable size for grafting take upwards of 2 years to grow from seed in a nursery setting. This creates a bottleneck in sourcing rootstocks. The development of effective transplanting methods will allow for the supplementation of rootstock from wildling stock.
In silviculture, transplanting whole plants is a common method of propagation, as a successful transplant results in a whole, functioning plant immediately upon recovery. In
The Dictionary of Forestry, transplants refer to seedlings “after they have been lifted and replanted, i.e., moved one or occasionally more times in the nursery, in contract to a seedling planted out directly to the seed bed” where the seed bed is the “soil or forest floor on which seed falls, or a prepared area where seed is sown” [
8]. The methods to appropriately store transplant stock or containerized seedlings of any species need to be identified and refined individually to maximize the production yield. In the production of transplants, the root system of the transplanted plant is often damaged prior to replanting in the process of lifting the plant. In one transplanting method, for bare root seedlings, the entire plant is lifted, the root system is cleaned of dirt, and it is kept moist until planting, at which time only the plant is transferred into new growing media or soil. In the planting of containerized seedlings, the entire root system and growth medium are transferred, resulting in no damage to the roots. Entire plants can be transplanted into a container, to create a new containerized seedling. While seedlings of wild or known parentage can be reared and combined with any of these techniques, the viability of the different combinations are not yet published for American beech,
Fagus grandifolia.
When grafting resistant beech trees, the crown portion of a non-resistant seedling is replaced with donor scion material from a resistant tree in the form of a twig with terminal buds present, resulting in a tree that retains the characteristics of the root portion in the rootstock, and the characteristics of the scion donor tree in the grafted portion [
6]. Once the graft union has healed, the two portions of the tree generally retain the characteristics of the donor tree but function as one living plant. Grafted beech trees are therefore maintained in a greenhouse until the tree is fully healed, and are then typically planted into a seed orchard and are maintained while resistant seed is collected and used for restoration projects. This process takes at least 3 to 5 years before it can begin bearing crops of BBD-resistant seed.
More recently, trees growing in seed orchards are likely to have been produced through hot callus grafting; however, this particular method is expensive, time-consuming, technically demanding, and requires specialized, bulky hot-callus apparatuses [
6]. Because private landowners collectively own 45% of the forests in Michigan [
9], costly, technically demanding methods make BBD-resistant American beech largely inaccessible for the private restoration of many forests affected by BBD. The creation of more accessible, quick, and cost-effective processes to propagate resistant beech would benefit forest restoration efforts by making BBD-resistant beech more widely available. The inclusion of wild-type materials as grafting rootstocks stands to reduce the cost of this process of growing bareroot stock from seed in a nursery is timely and expensive.
American beech displays a vigorous root-sprouting response to stress, including that inflicted by BBD on individual trees [
10]. Although the excavation of American beech seedlings and suckers has been utilized to determine origin [
11], we are unaware of attempts to propagate excavated beech trees of either a sucker or seedling origin. In fact, much historical literature about beech propagation specifies methods only to the genus level, and much of this refers to the propagation of European beech (
Fagus sylvatica) and on the production and sowing of beechnuts [
12]. Root suckers of other species have been successfully transplanted for restoration purposes. Jujube trees (
Ziziphus jujube; Rhamnaceae) have been transplanted in New Mexico, with success rates varying by height class [
13]. Small serviceberries (
Amelanchier spp.; Rosaceae) can be transplanted as root suckers [
14]. Pongame oiltree (
Pongamia pinnata; Fabaceae) has also been propagated through suckers [
15].
The ability to propagate resistant American beech by transplanting could save land managers time and money by supplementing costly grafting procedures. The origin of the individual (seed vs. root sucker) may be determined by the presence or absence of a taproot, allowing for the field identification of the genetic origin of small trees [
11]. Transplanting offspring of a resistant parent tree would result in the entire tree displaying resistance to the disease (as opposed to only the apical portion of a scion graft), which would be immediately ready for planting upon hardening.
The relative amount of seedlings or clonal root sprouts in forests is variable and is affected by many factors. Root suckers occur more readily where beech has established lateral roots close to the soil surface, such as in downslope hillsides [
16]. Warmer conditions may lead to an increase in suckering [
17]. Survival and growth rates are higher in naturally established root suckers than seedlings, leading to an increased dominance of clones in the larger sapling size classes [
11,
18]. Beech root sprouts can disperse to a maximum of up to 6 m from the parent tree, but a mean distance closer to 3 m [
18,
19]. These methods have not been explored for restoration, but establishment of a reliable process will begin dialogue about the potential of this route for restoration. The potential for resistant wild rootstock for use in grafting has yet to be explored.
In this study, transplanted wildling American beech seedlings were compared with commercially available bare root seedlings to quantify the viability of the two sources. The development of methods for the successful transplanting of American beech will inform future work regarding transplanting both seedlings and root suckers, with the ultimate goal of transplanting resistant root suckers to reduce the cost of restoration and increase the survival of planted BBD-resistant beech trees.
2. Materials and Methods
Bare root seedlings were purchased from a commercial nursey in northern Michigan, USA, in the indicated size class of 45 to 60 cm tall. Wildling plant material was collected from three field sites in the Hiawatha National Forest near Munising, MI, US. All of the sites were located along road edges to allow access by vehicle. Patches were identified by US Forest Service employees as containing a large number of American beech, 45 to 60 cm in height. Collection occurred in November 2019, when the trees had fully entered winter dormancy.
American beech regeneration was identified by twigs with cigar-shaped buds, visible above the snow. The snow layer was removed with a shovel and the leaf litter layer was removed by hand. A gentle levering motion was used to free the entire dirt clod, 20 to 50 cm from the root collar of the seedling. Dirt clods and root balls were manipulated by hand to remove as much soil while retaining as much fine root mass as possible. The roots were not rinsed so as to retain the local mycorrhizal community.
Excavated seedlings were packed in damp sphagnum peat moss inside heavy duty black contractor bags, which were twisted shut to retain moisture. At the end of the day, the bags were packed with additional damp peat moss sufficient to entirely enclose the entire root mass and were buried under an insulating layer of snow to prevent freezing or overheating.
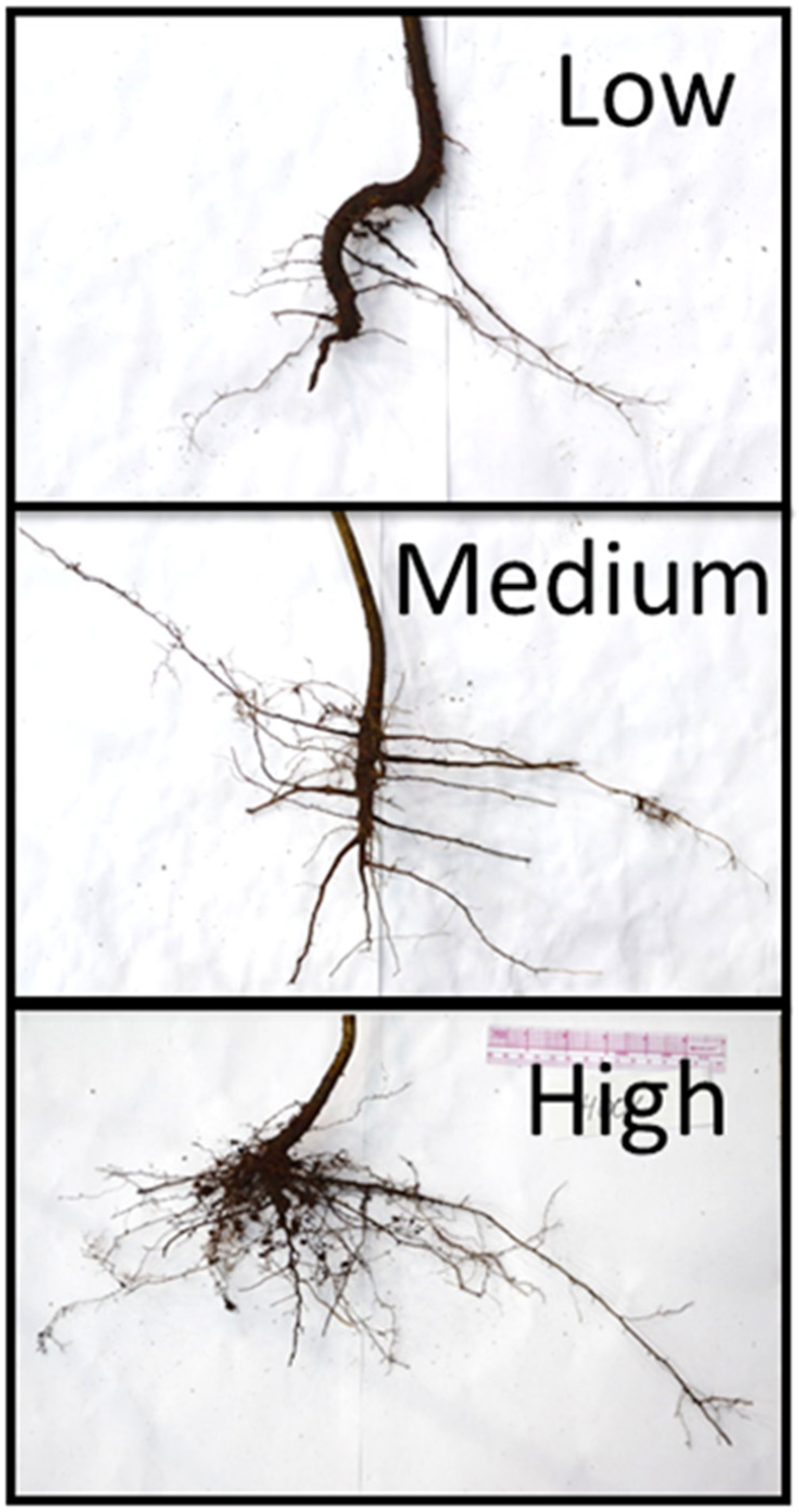
Dormant individuals were then stored in heavy plastic wrap and damp sphagnum moss at 4 °C to keep the entire tree dormant prior to potting. Seedlings were kept in cold storage for no longer than 2 weeks. At the time of potting, the root collar diameter and height were recorded. Damage to the above or below-ground biomass were recorded separately. Root systems were quickly sorted and photographed (
Figure 1), and later categorized into low, medium, or high fine root mass categories based on those images (
Table 1). Volumetric water displacement measures would have required rinsing root systems, and because maintaining local provenance mycorrhizal community was desirable, this method was not appropriate. Trees were potted in 11.4 L round pots with all purpose soil growing media. Potted trees were supplied with water for 5 min through a drip irrigation system three times a week, and were allowed to grow at ambient temperatures in a greenhouse setting. Survival was defined as trees that displayed green leaves through until the end of September 2020.
Overall, 145 excavated seedlings and 88 bare root seedlings were compared. Although 200 bare root seedlings were ordered, many were not included in the analysis because of contamination by scale insects (Hemiptera) for the purchased seedlings, potentially including beech scale. Potentially contaminated trees were not included in the analysis to avoid comparing diseased purchased trees to healthy wildling seedlings.
Chi-square analyses were performed for the entire cohort and among root mass classes to confirm the differences between groups. Regression analysis was performed to test simple variables of the aboveground height (unstretched height from soil surface to top of woody growth) and root mass class (
Figure 1) as predictors of survival, as these metrics were measurable at the time of excavation in a field setting and were potentially useful in the selection of seedlings for transplanting. All of the statistical analyses were performed in statistical software R 4.0.3 [
20].
3. Results
The excavated seedlings survived at higher rates than the bare root seedlings (χ2, 0.05, df1: 3.84, 4.098). The excavated seedlings outperformed the bare root seedlings in all root mass classes, but the differences in survival were significant only in the high root mass class (high: χ2, 0.05, df1: 3.84, 6.41; medium: χ2, 0.05, df1: 3.84, 2.33; low: χ2, 0.05, df1: 3.84, 1.69). In addition, survival was not different among root mass classes in the bare root seedlings, but was significantly different among the root classes for wildling seedlings (bare root: χ2, 0.05, df2: 5.99, 2.06; excavated: χ2, 0.05, df2: 5.99, 6.08). The root collar and height were not significant predictors of wilding survival in seedlings in binomial regression (survival = 0.098 + 0.335 × RootCollar + −0.023 × height; pseudo R2 = 0.054; p = 0.959, 0.16, 0.49; AIC 49.106).
4. Discussion
The increased survival of wildling trees could be the result of the increased individual handling time of wildling seedlings during excavation, resulting in more intact fine roots. The performance between root classes was not significantly different in the wildling seedlings, possibly because the differences were distinctive but small between the low and high root class of the wildling seedlings. This may also be the result of the increased age of the wildling seedlings in the same size class as the bare root seedlings, as American beech is highly shade tolerant and seedlings may persist for many years as small trees before release events cause them to recruit into the canopy [
21,
22]. As hardwood seedlings were purchased based on a height class rating, stock type was not provided for these materials and destructive sampling of wildling seedlings would be necessary to accurately age the wildlings; so, the age was unknown for all of the trees involved in the study. Future work could explore the influence of age and stock type of the purchased seedlings in viability.
There are many factors that may affect the viability of transplanted seedlings, which could serve as future directions for exploration once transplanting methods for American beech are well documented. In European beech (
Fagus sylvatica), transporting seedlings long distances can cause stress in the planted seedlings [
23]. We attempted to minimize this effect by sourcing all of our materials from within the same climactic region and reducing transport and storage time out of soil as much as possible. Taprooted species (including members of the Fagaceae family such as American beech) survive better as transplants in response to containerized growing, which minimizes root disturbance events [
24]. The mechanism driving this survival is not well understood, but may be in response to the root damage that occurs during the lifting of transplants.
Although we are pursuing alternate methods of propagation for American beech, the low viability of bare root seedlings may impact other propagation methods. It is also difficult to obtain bare root seedling when commercial stock is depleted by multiple agencies, and when it is further reduced by scale contamination and the low quality of seedlings. The ability to successfully transplant wildling seedlings can eliminate bottlenecks caused by the relatively low commercial availability of local provenance bare root seedlings.