Impact of Irrigation with Contaminated Water on Heavy Metal Bioaccumulation in Water Chestnut (Trapa natans L.)
Abstract
:1. Introduction
2. Materials and Methods
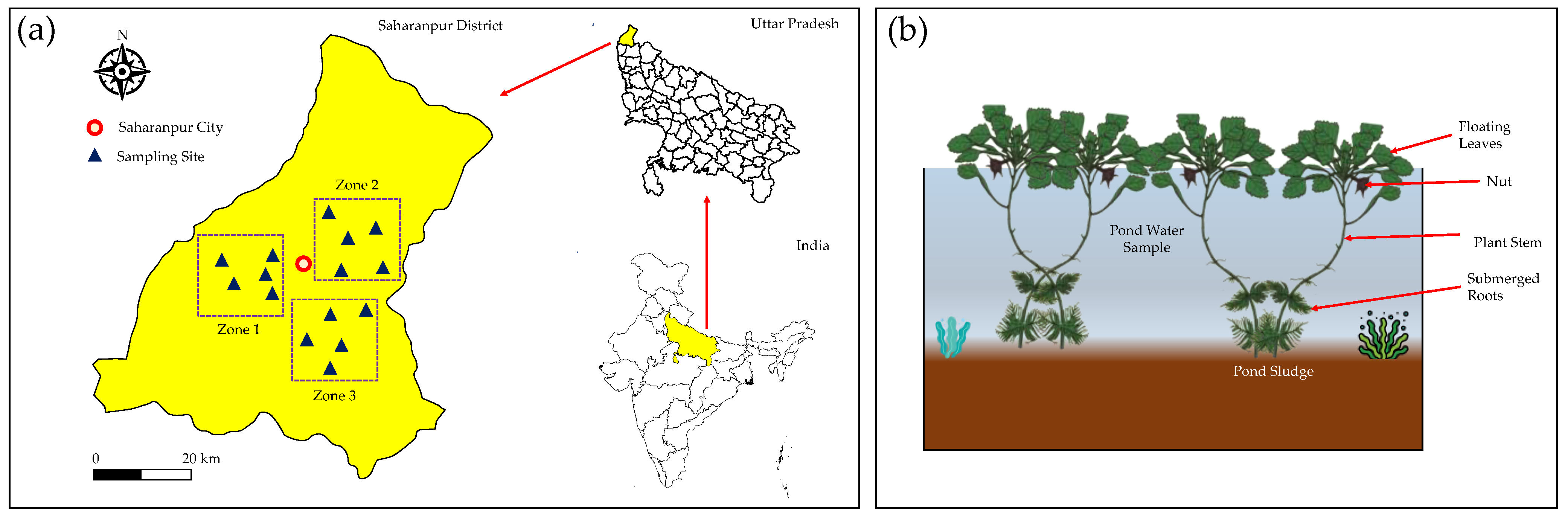
2.1. Description of the Study Area
2.2. Sample Collection
2.3. Chemical Analyses
2.4. Data Analysis and Software
3. Results and Discussion
3.1. Status of Water Quality and Heavy Metals Pollution in Pond Water
3.2. Status of Heavy Metals in Pond Sludge
3.3. Concentration and Bioaccumulation of Heavy Metals in Water Chestnut
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hamulczuk, M.; Pawlak, K. Determinants for International Competitiveness of the Food Industry in 43 Countries Worldwide: Evidence from Panel Models. Equilib. Q. J. Econ. Econ. Policy 2022, 17, 635–667. [Google Scholar] [CrossRef]
- Kumar, P.; Eid, E.M.; Al-Huqail, A.A.; Širić, I.; Adelodun, B.; Abou Fayssal, S.; Valadez-Blanco, R.; Goala, M.; Ajibade, F.O.; Choi, K.S.; et al. Kinetic Studies on Delignification and Heavy Metals Uptake by Shiitake (Lentinula edodes) Mushroom Cultivated on Agro-Industrial Wastes. Horticulturae 2022, 8, 316. [Google Scholar] [CrossRef]
- Kumar, P.; Eid, E.M.; Taher, M.A.; El-Morsy, M.H.E.; Osman, H.E.M.; Al-Bakre, D.A.; Adelodun, B.; Abou Fayssal, S.; Andabaka, Ž.; Goala, M.; et al. Sustainable Upcycling of Mushroom Farm Wastewater through Cultivation of Two Water Ferns (Azolla spp.) in Stagnant and Flowing Tank Reactors. Horticulturae 2022, 8, 506. [Google Scholar] [CrossRef]
- AL-Huqail, A.A.; Kumar, P.; Eid, E.M.; Adelodun, B.; Abou Fayssal, S.; Singh, J.; Arya, A.K.; Goala, M.; Kumar, V.; Širić, I. Risk Assessment of Heavy Metals Contamination in Soil and Two Rice (Oryza sativa L.) Varieties Irrigated with Paper Mill Effluent. Agriculture 2022, 12, 1864. [Google Scholar] [CrossRef]
- Beeregowda, K.N.; Mathew, B.B.; Jaishankar, M.; Anbalagan, N.; Tseten, T. Toxicity, Mechanism and Health Effects of Some Heavy Metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar]
- Adelodun, B.; Kumar, P.; Odey, G.; Ajibade, F.O.; Ibrahim, R.G.; Alamri, S.A.M.; Alrumman, S.A.; Eid, E.M.; Kumar, V.; Adeyemi, K.A.; et al. A Safe Haven of SARS-CoV-2 in the Environment: Prevalence and Potential Transmission Risks in the Effluent, Sludge, and Biosolids. Geosci. Front. 2022, 13, 101373. [Google Scholar] [CrossRef]
- Castine, S.A.; Bogard, J.R.; Barman, B.K.; Karim, M.; Mokarrom Hossain, M.; Kunda, M.; Mahfuzul Haque, A.B.M.; Phillips, M.J.; Thilsted, S.H. Homestead Pond Polyculture Can Improve Access to Nutritious Small Fish. Food Secur. 2017, 9, 785–801. [Google Scholar] [CrossRef]
- EPA. EPA Preliminary Data Summary of Urban Storm Water Best Management Practices; EPA: Washington, DC, USA, 1999. [Google Scholar]
- Gołub, A.; Piekutin, J. Pollution of Sedimentary Ponds at an Industrial Plant in Janikowo (Poland). Water 2020, 12, 536. [Google Scholar] [CrossRef]
- Marsalek, J.; Marsalek, P.M. Characteristics of Sediments from a Stormwater Management Pond. Water Sci. Technol. 1997, 36, 117–122. [Google Scholar] [CrossRef]
- Strzelecka, J.; Dąbrowski, M.; Hulisz, P.; Piernik, A. Changes in Soil Properties and Plant Biomass under the Influence of Soda Waste Ponds in Inowrocław, Poland. Ecol. Quest. 2011, 14, 69–71. [Google Scholar] [CrossRef]
- Ramachandra, T.V.; Sudarshan, P.B.; Mahesh, M.K.; Vinay, S. Spatial Patterns of Heavy Metal Accumulation in Sediments and Macrophytes of Bellandur Wetland, Bangalore. J Env. Manag. 2018, 206, 1204–1210. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Natural Resources. Ontario Ministry of Natural Resources Field Guide to Aquatic Invasive Species: Identification, Collection, and Reporting of Aquatic Invasive Species in Ontari o Waters; Queen’s Printer for Ontario: Quebec, ON, Canda, 2010; pp. 1–207. [Google Scholar]
- Les, D.H. Aquatic Dicotyledons of North America: Ecology, Life History, and Systematics; CRC Press: Boca Raton, FL, USA, 2017; ISBN 9781482225037. [Google Scholar]
- O’Neill, C.R.; Eill, J.S.S., Jr. Water Chestnut (Trapa natans) in the Northeast NYSG Invasive Species Factsheet Series: 06-1. In New York Sea Grant; Sea Grant Brockport: New York, NY, USA, 2006; p. 4. [Google Scholar]
- Anderson, J.W.; Baird, P.; Davis, R.H.; Ferreri, S.; Knudtson, M.; Koraym, A.; Waters, V.; Williams, C.L. Health Benefits of Dietary Fiber. Nutr. Rev. 2009, 67, 188–205. [Google Scholar] [CrossRef] [PubMed]
- Sahu, B.L.; Ramteke, S.; Rajhans, K.P.; Patel, K.S.; Wysocka, I.; Jaron, I. Contamination of Pond with Fluoride and Heavy Metals in the Central India. Water Resour. 2018, 45, 992–1001. [Google Scholar] [CrossRef]
- Goyal, V.C.; Singh, O.; Singh, R.; Chhoden, K.; Malyan, S.K. Appraisal of Heavy Metal Pollution in the Water Resources of Western Uttar Pradesh, India and Associated Risks. Environ. Adv. 2022, 8, 230. [Google Scholar] [CrossRef]
- Yadav, S.; Goyal, V.C. Current Status of Ponds in India: A Framework for Restoration, Policies and Circular Economy. Wetlands 2022, 42, 107. [Google Scholar] [CrossRef]
- Kumar, P.; Kumar, V.; Eid, E.M.; Al-Huqail, A.A.; Adelodun, B.; Abou Fayssal, S.; Goala, M.; Arya, A.K.; Bachheti, A.; Andabaka, Ž.; et al. Spatial Assessment of Potentially Toxic Elements (PTE) Concentration in Agaricus bisporus Mushroom Collected from Local Vegetable Markets of Uttarakhand State, India. J. Fungi 2022, 8, 452. [Google Scholar] [CrossRef]
- Širić, I.; Eid, E.M.; El-Morsy, M.H.E.; Osman, H.E.M.; Adelodun, B.; Abou Fayssal, S.; Mioč, B.; Goala, M.; Singh, J.; Bachheti, A.; et al. Health Risk Assessment of Hazardous Heavy Metals in Two Varieties of Mango Fruit (Mangifera indica L. Var. Dasheri and Langra). Horticulturae 2022, 8, 832. [Google Scholar] [CrossRef]
- GOI District Aligarh. Government of Uttar Pradesh City of Locks India. Available online: https://aligarh.nic.in/ (accessed on 12 December 2022).
- Saha, S.; Bhadana, D. Rural Agricultural Work Experience: Socio-Economic Survey in Budha Khera Village, Saharanpur, Uttarpradesh, India. Int. J. Sci. Res. 2022, 11, 585–588. [Google Scholar] [CrossRef]
- Apha, A. Standard Methods for the Examination of Water and Wastewater, 22nd ed.; American Public Health Association: Washington, DC, USA, 2012. [Google Scholar]
- Latimer, G.W. Official Methods of Analysis of AOAC International, 21st ed.; AOAC International: Rockville, MD, USA, 2019. [Google Scholar]
- Sáez-Plaza, P.; Navas, M.J.; Wybraniec, S.; Michałowski, T.; Asuero, A.G. An Overview of the Kjeldahl Method of Nitrogen Determination. Part II. Sample Preparation, Working Scale, Instrumental Finish, and Quality Control. Crit. Rev. Anal. Chem. 2013, 43, 224–272. [Google Scholar] [CrossRef]
- Kanwal, A.; Farhan, M.; Sharif, F.; Hayyat, M.U.; Shahzad, L.; Ghafoor, G.Z. Effect of Industrial Wastewater on Wheat Germination, Growth, Yield, Nutrients and Bioaccumulation of Lead. Sci. Rep. 2020, 10, 11361. [Google Scholar] [CrossRef]
- Ginni, G.; Adishkumar, S.; Rajesh Banu, J.; Yogalakshmi, N. Treatment of Pulp and Paper Mill Wastewater by Solar Photo-Fenton Process. Desalination Water Treat. 2014, 52, 2457–2464. [Google Scholar] [CrossRef]
- Azimi, S.C.; Shirini, F.; Pendashteh, A. Evaluation of COD and Turbidity Removal from Woodchips Wastewater Using Biologically Sequenced Batch Reactor. Process. Saf. Environ. Prot. 2019, 128, 211–227. [Google Scholar] [CrossRef]
- Laohaprapanon, S.; Kaczala, F.; Salomon, P.S.; Marques, M.; Hogland, W. Wastewater Generated during Cleaning/Washing Procedures in a Wood-Floor Industry: Toxicity on the Microalgae Desmodesmus subspicatus. Environ. Technol. 2012, 33, 2439–2446. [Google Scholar] [CrossRef] [PubMed]
- Rey-Romero, D.C.; Domínguez, I.; Oviedo-Ocaña, E.R. Effect of Agricultural Activities on Surface Water Quality from Páramo Ecosystems. Environ. Sci. Pollut. Res. 2022, 29, 83169–83190. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Ma, Y.; Fang, J.; Hu, L.; Li, X. Particle Size Distribution and Total Suspended Solid Concentrations in Urban Surface Runoff. Sci. Total Environ. 2022, 815, 2533. [Google Scholar] [CrossRef]
- Aniyikaiye, T.E.; Oluseyi, T.; Odiyo, J.O.; Edokpayi, J.N. Physico-Chemical Analysis of Wastewater Discharge from Selected Paint Industries in Lagos, Nigeria. Int. J. Environ. Res. Public Health 2019, 16, 1235. [Google Scholar] [CrossRef]
- Alvarado, A.; Sanchez, E.; Durazno, G.; Vesvikar, M.; Nopens, I. CFD Analysis of Sludge Accumulation and Hydraulic Performance of a Waste Stabilization Pond. Water Sci. Technol. 2012, 66, 2370–2377. [Google Scholar] [CrossRef]
- van Puijenbroek, P.J.T.M.; Beusen, A.H.W.; Bouwman, A.F. Global Nitrogen and Phosphorus in Urban Waste Water Based on the Shared Socio-Economic Pathways. J. Environ. Manag. 2019, 231, 446–456. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.Q.; Wang, D.F. Immobilization of Heavy Metals in Sewage Sludge during Land Application Process in China: A Review. Sustainability 2017, 9, 2020. [Google Scholar] [CrossRef]
- Tytła, M. Assessment of Heavy Metal Pollution and Potential Ecological Risk in Sewage Sludge from Municipal Wastewater Treatment Plant Located in the Most Industrialized Region in Poland—Case Study. Int. J. Enviorn. Res. Public Health 2019, 16, 2430. [Google Scholar] [CrossRef]
- Keffala, C.; Harerimana, C.; Vasel, J.L. A Review of the Sustainable Value and Disposal Techniques, Wastewater Stabilisation Ponds Sludge Characteristics and Accumulation. Env. Monit. Assess. 2013, 185, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Astatkie, H.; Ambelu, A.; Mengistie, E. Contamination of Stream Sediment with Heavy Metals in the Awetu Watershed of Southwestern Ethiopia. Front. Earth Sci. 2021, 9, 8737. [Google Scholar] [CrossRef]
- Azimi, A.; Azari, A.; Rezakazemi, M.; Ansarpour, M. Removal of Heavy Metals from Industrial Wastewaters: A Review. ChemBioEng Rev. 2017, 4, 37–59. [Google Scholar] [CrossRef]
- Guerra, F.; Trevizam, A.R.; Muraoka, T.; Marcante, N.C.; Canniatti-Brazaca, S.G. Heavy Metals in Vegetables and Potential Risk for Human Health. Sci. Agric. 2012, 69, 54–60. [Google Scholar] [CrossRef]
- FAO; WHO. FAO/WHO Report of the Thirty Eight Session of the Codex Committee on Food Hygiene; FAO: Rome, Italy; WHO: Geneva, Switzerland, 2007. [Google Scholar]
- Awasthi, S.K. Prevention of Food Adulteration Act No. 37 of 1954. In Central and State Rules as Amended for 1999, 3rd ed.; Ashoka Law House: New Delhi, India, 2000. [Google Scholar]
- Jaiswal, A.; Verma, A.; Jaiswal, P. Detrimental Effects of Heavy Metals in Soil, Plants, and Aquatic Ecosystems and in Humans. J. Environ. Pathol. Toxicol. Oncol. 2018, 37, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Krivokapić, M. Study on the Evaluation of (Heavy) Metals in Water and Sediment of Skadar Lake (Montenegro), with Bcf Assessment and Translocation Ability (Ta) by Trapa natans and a Review of Sdgs. Water 2021, 13, 876. [Google Scholar] [CrossRef]
- Babu, M.; Dwivedi, D.H.; Ram, Y.R.B.; Meena, M.L. Bioaccumulation and Distribution of Heavy Metals in Water Chestnut (Trapa natans var. bispinosa Roxb.) in the Lucknow Region. Afr. J. Agric. Res. 2013, 8, 2765–2768. [Google Scholar]


| Parameters | Sampling Zone ^ | Average | Standard Limit * | ||
|---|---|---|---|---|---|
| Zone 1 (Agricultural) | Zone 2 (Urban) | Zone 3 (Rural) | |||
| pH | 7.72 ± 0.05 b | 7.34 ± 0.07 a | 7.89 ± 0.03 c | 7.88 ± 0.54 | 5.50–9.00 |
| Electrical Conductivity (EC: dS/m) | 0.57 ± 0.09 a | 0.93 ± 0.04 b | 0.54 ± 0.05 a | 0.68 ± 0.22 | - |
| Total Dissolved Solids (TDS: mg/L) | 1629.08 ± 297.10 a | 2508.60 ± 68.29 c | 2156.11 ± 79.04 b | 2097.93 ± 442.64 | 1900.00 |
| Total Suspended Solids (TSS: mg/L) | 736.22 ± 10.25 a | 913.04 ± 31.60 c | 825.84 ± 26.92 b | 825.03 ± 88.41 | - |
| Biological Oxygen Demand (BOD: mg/L) | 191.56 ± 8.86 a | 383.45 ± 12.24 c | 269.03 ± 10.05 b | 281.35 ± 96.54 | 100.00 |
| Chemical Oxygen Demand (COD: mg/L) | 677.06 ± 27.01 a | 1108.59 ± 66.17 c | 820.20 ± 42.93 b | 868.62 ± 219.80 | 250.00 |
| Total Nitrogen (TN: mg/L) | 10.88 ± 2.04 a | 24.60 ± 3.81 c | 18.51 ± 1.65 b | 18.00 ± 6.87 | - |
| Total Phosphorus (TP: mg/L) | 4.36 ± 0.53 a | 9.32 ± 0.14 c | 7.02 ± 0.32 b | 6.90 ± 2.48 | - |
| Cadmium (Cd: mg/L) | 0.014 ± 0.003 a | 0.032 ± 0.010 b | 0.023 ± 0.006 b | 0.023 ± 0.009 | 2.000 |
| Chromium (Cr: mg/L) | 0.011 ± 0.005 a | 0.026 ± 0.009 b | 0.020 ± 0.007 b | 0.019 ± 0.008 | 2.000 |
| Copper (Cu: mg/L) | 0.106 ± 0.017 a | 0.157 ± 0.011 b | 0.119 ± 0.043 b | 0.127 ± 0.027 | 3.000 |
| Iron (Fe: mg/L) | 0.845 ± 0.090 a | 1.822 ± 0.124 c | 1.250 ± 0.065 b | 1.306 ± 0.491 | 3.000 |
| Manganese (Mn: mg/L) | 0.132 ± 0.007 a | 0.290 ± 0.019 c | 0.226 ± 0.030 b | 0.216 ± 0.079 | 2.000 |
| Zinc (Zn: mg/L) | 0.207 ± 0.060 a | 0.315 ± 0.044 b | 0.280 ± 0.052 a | 0.267 ± 0.055 | 5.000 |
| Parameters | Sampling Zone ^ | Average | ||
|---|---|---|---|---|
| Zone 1 (Agricultural) | Zone 2 (Urban) | Zone 3 (Rural) | ||
| pH | 7.52 ± 0.23 a | 7.46 ± 0.17 a | 7.75 ± 0.06 b | 7.58 ± 0.15 |
| Electrical Conductivity (EC: dS/m) | 3.04 ± 0.09 a | 3.11 ± 0.16 a | 3.09 ± 0.12 a | 3.08 ± 0.04 |
| Organic Matter (OM: g/kg) | 5.72 ± 0.17 a | 6.13 ± 0.31 ab | 6.02 ± 0.24 a | 5.96 ± 0.21 |
| Total Nitrogen (TN: g/kg) | 0.25 ± 0.03 a | 0.37 ± 0.02 c | 0.31 ± 0.01 b | 0.31 ± 0.06 |
| Total Phosphorus (TP: g/kg) | 0.18 ± 0.02 a | 0.25 ± 0.04 b | 0.22 ± 0.01 b | 0.22 ± 0.04 |
| Cadmium (Cd: mg/kg) | 0.064 ± 0.012 a | 0.125 ± 0.016 c | 0.098 ± 0.009 b | 0.096 ± 0.031 |
| Chromium (Cr: mg/kg) | 1.480 ± 0.140 a | 2.443 ± 0.120 c | 1.910 ± 0.276 b | 1.944 ± 0.482 |
| Copper (Cu: mg/kg) | 2.031 ± 0.101 a | 5.854 ± 0.315 c | 3.722 ± 0.149 b | 3.869 ± 1.916 |
| Iron (Fe: mg/kg) | 7.517 ± 0.226 a | 12.406 ± 2.620 bc | 9.416 ± 0.577 b | 9.780 ± 2.465 |
| Manganese (Mn: mg/kg) | 0.804 ± 0.024 a | 1.210 ± 0.105 c | 1.154 ± 0.046 b | 1.056 ± 0.220 |
| Zinc (Zn: mg/kg) | 4.346 ± 0.330 a | 8.621 ± 1.431 c | 5.736 ± 0.529 b | 6.234 ± 2.181 |
| Parameters | Chestnut Plant Part | Sampling Zone ^ | Average | ||
|---|---|---|---|---|---|
| Zone 1 (Agricultural) | Zone 2 (Urban) | Zone 3 (Rural) | |||
| Cadmium (Cd: mg/kg) | Nut (Without Shell) | 0.003 ± 0.002 a | 0.006 ± 0.002 ab | 0.005 ± 0.001 a | 0.005 ± 0.002 |
| Shoots (Above Water) | 0.006 ± 0.001 a | 0.008 ± 0.003 ab | 0.008 ± 0.003 ab | 0.007 ± 0.001 | |
| Roots (Below Water) | 0.008 ± 0.003 a | 0.011 ± 0.001 a | 0.010 ± 0.001 a | 0.010 ± 0.002 | |
| Chromium (Cr: mg/kg) | Nut (Without Shell) | 0.125 ± 0.006 a | 0.142 ± 0.007 bc | 0.136 ± 0.007 b | 0.134 ± 0.009 |
| Shoots (Above Water) | 0.152 ± 0.005 a | 0.160 ± 0.008 a | 0.162 ± 0.007 ab | 0.158 ± 0.005 | |
| Roots (Below Water) | 0.173 ± 0.009 a | 0.209 ± 0.010 c | 0.180 ± 0.005 b | 0.187 ± 0.019 | |
| Copper (Cu: mg/kg) | Nut (Without Shell) | 0.952 ± 0.048 a | 1.157 ± 0.053 c | 1.021 ± 0.041 b | 1.043 ± 0.104 |
| Shoots (Above Water) | 2.705 ± 0.135 a | 3.210 ± 0.160 b | 3.304 ± 0.165 b | 3.073 ± 0.322 | |
| Roots (Below Water) | 3.460 ± 0.073 a | 3.820 ± 0.098 c | 3.615 ± 0.183 b | 3.632 ± 0.181 | |
| Iron (Fe: mg/kg) | Nut (Without Shell) | 8.533 ± 0.720 a | 10.401 ± 1.020 bc | 9.832 ± 0.290 b | 9.589 ± 0.957 |
| Shoots (Above Water) | 46.984 ± 2.349 a | 62.550 ± 3.128 c | 50.127 ± 2.006 ab | 53.220 ± 8.231 | |
| Roots (Below Water) | 75.002 ± 4.250 a | 90.384 ± 3.019 b | 91.936 ± 4.597 b | 89.107 ± 3.639 | |
| Manganese (Mn: mg/kg) | Nut (Without Shell) | 3.530 ± 0.507 a | 5.028 ± 1.251 bc | 4.420 ± 0.321 b | 4.326 ± 0.753 |
| Shoots (Above Water) | 6.149 ± 0.307 a | 10.907 ± 0.545 c | 8.001 ± 0.400 b | 8.352 ± 2.398 | |
| Roots (Below Water) | 8.814 ± 0.441 a | 13.012 ± 2.050 b | 10.547 ± 0.823 b | 10.791 ± 2.110 | |
| Zinc (Zn: mg/kg) | Nut (Without Shell) | 0.952 ± 0.069 a | 2.003 ± 0.446 b | 1.665 ± 0.120 b | 1.540 ± 0.537 |
| Shoots (Above Water) | 6.087 ± 1.304 a | 6.260 ± 0.810 a | 6.431 ± 0.372 a | 6.259 ± 0.172 | |
| Roots (Below Water) | 7.305 ± 0.092 a | 7.432 ± 0.472 a | 7.012 ± 0.210 a | 7.250 ± 0.215 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taher, M.A.; Zouidi, F.; Kumar, P.; Abou Fayssal, S.; Adelodun, B.; Goala, M.; Kumar, V.; Andabaka, Ž.; Širić, I.; Eid, E.M. Impact of Irrigation with Contaminated Water on Heavy Metal Bioaccumulation in Water Chestnut (Trapa natans L.). Horticulturae 2023, 9, 190. https://doi.org/10.3390/horticulturae9020190
Taher MA, Zouidi F, Kumar P, Abou Fayssal S, Adelodun B, Goala M, Kumar V, Andabaka Ž, Širić I, Eid EM. Impact of Irrigation with Contaminated Water on Heavy Metal Bioaccumulation in Water Chestnut (Trapa natans L.). Horticulturae. 2023; 9(2):190. https://doi.org/10.3390/horticulturae9020190
Chicago/Turabian StyleTaher, Mostafa A., Ferjeni Zouidi, Pankaj Kumar, Sami Abou Fayssal, Bashir Adelodun, Madhumita Goala, Vinod Kumar, Željko Andabaka, Ivan Širić, and Ebrahem M. Eid. 2023. "Impact of Irrigation with Contaminated Water on Heavy Metal Bioaccumulation in Water Chestnut (Trapa natans L.)" Horticulturae 9, no. 2: 190. https://doi.org/10.3390/horticulturae9020190
APA StyleTaher, M. A., Zouidi, F., Kumar, P., Abou Fayssal, S., Adelodun, B., Goala, M., Kumar, V., Andabaka, Ž., Širić, I., & Eid, E. M. (2023). Impact of Irrigation with Contaminated Water on Heavy Metal Bioaccumulation in Water Chestnut (Trapa natans L.). Horticulturae, 9(2), 190. https://doi.org/10.3390/horticulturae9020190











