An Evaluation Modeling Study of Thermal Runaway in Li-Ion Batteries Based on Operation Environments in an Energy Storage System
Abstract
:1. Introduction
2. Mathematical Equations of Thermal Runaway in Li-Ion Batteries for ESSs
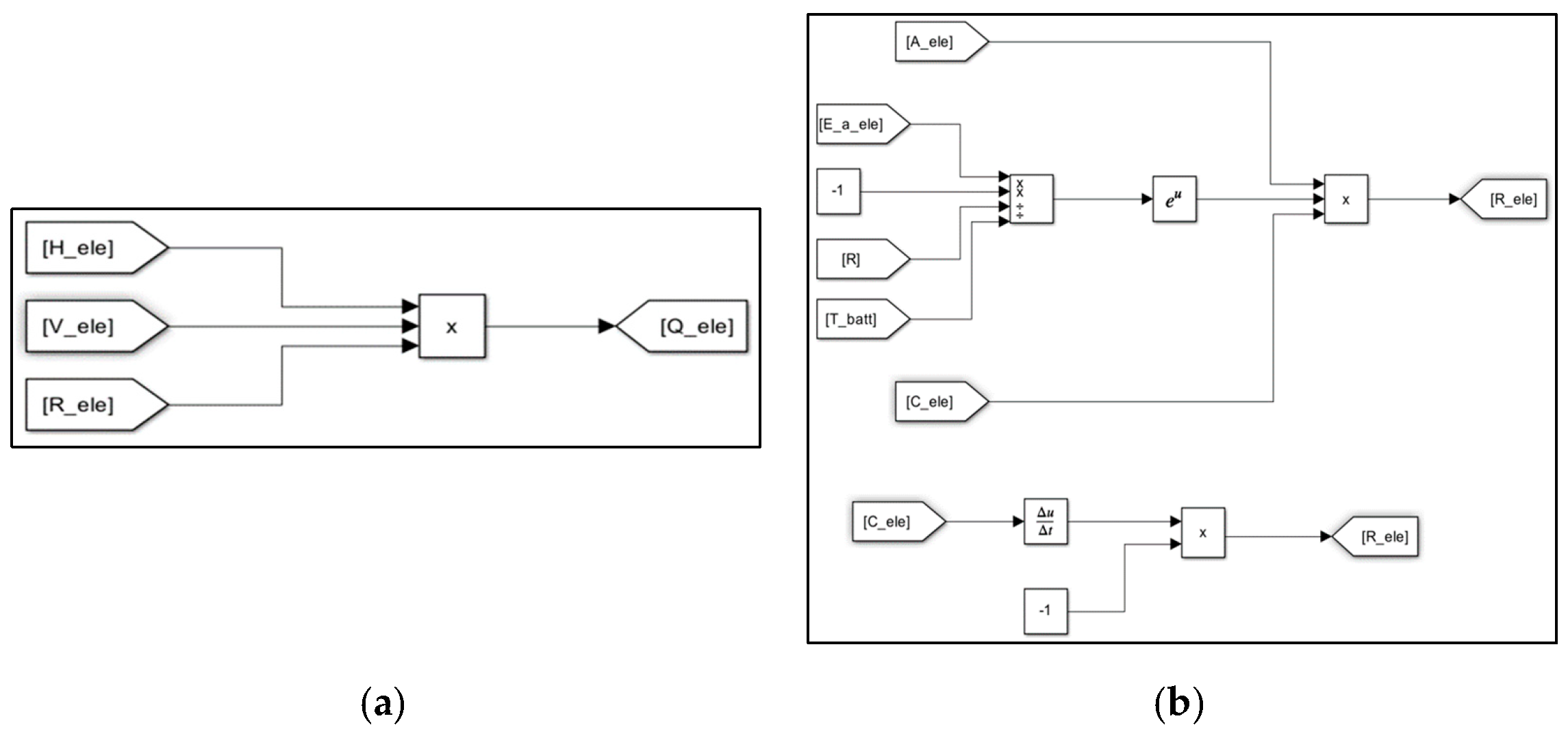
3. Evaluation Modeling of Thermal Runaway Using MATLAB/SIMULINK S/W
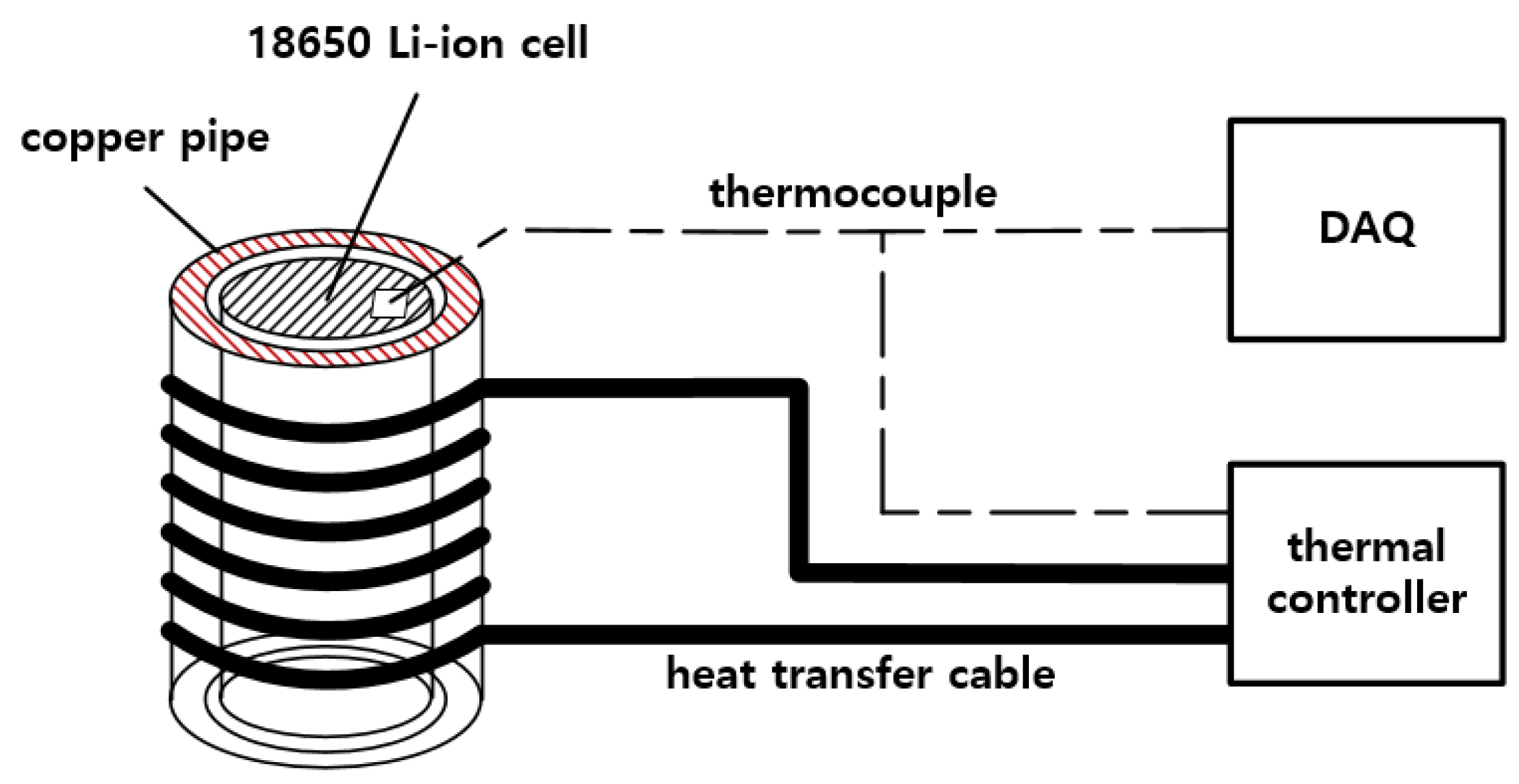
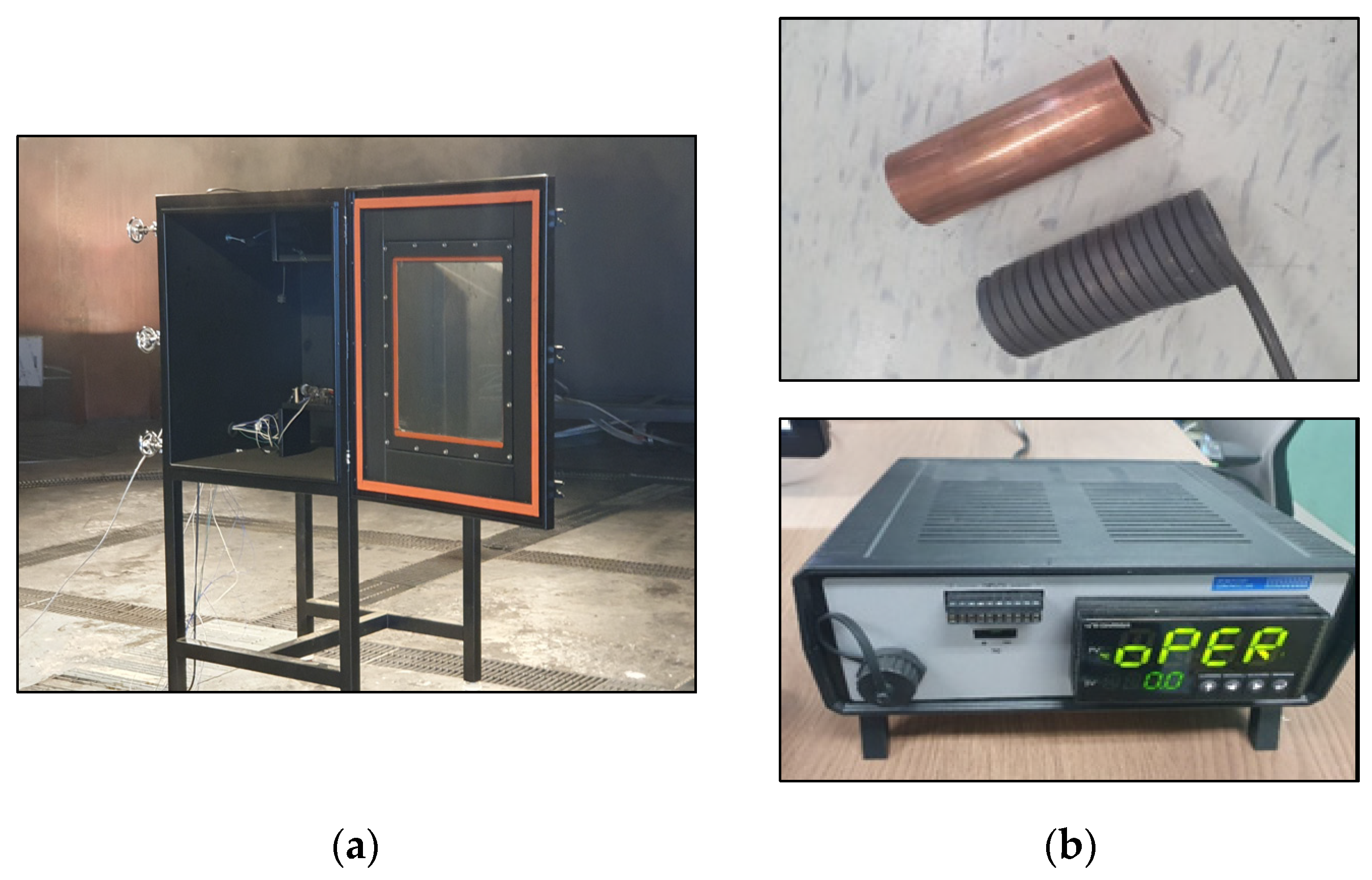
4. Implementation of a Test Device for Thermal Runaway in a Li-Ion Battery
5. Case Studies
5.1. Simulation Conditions
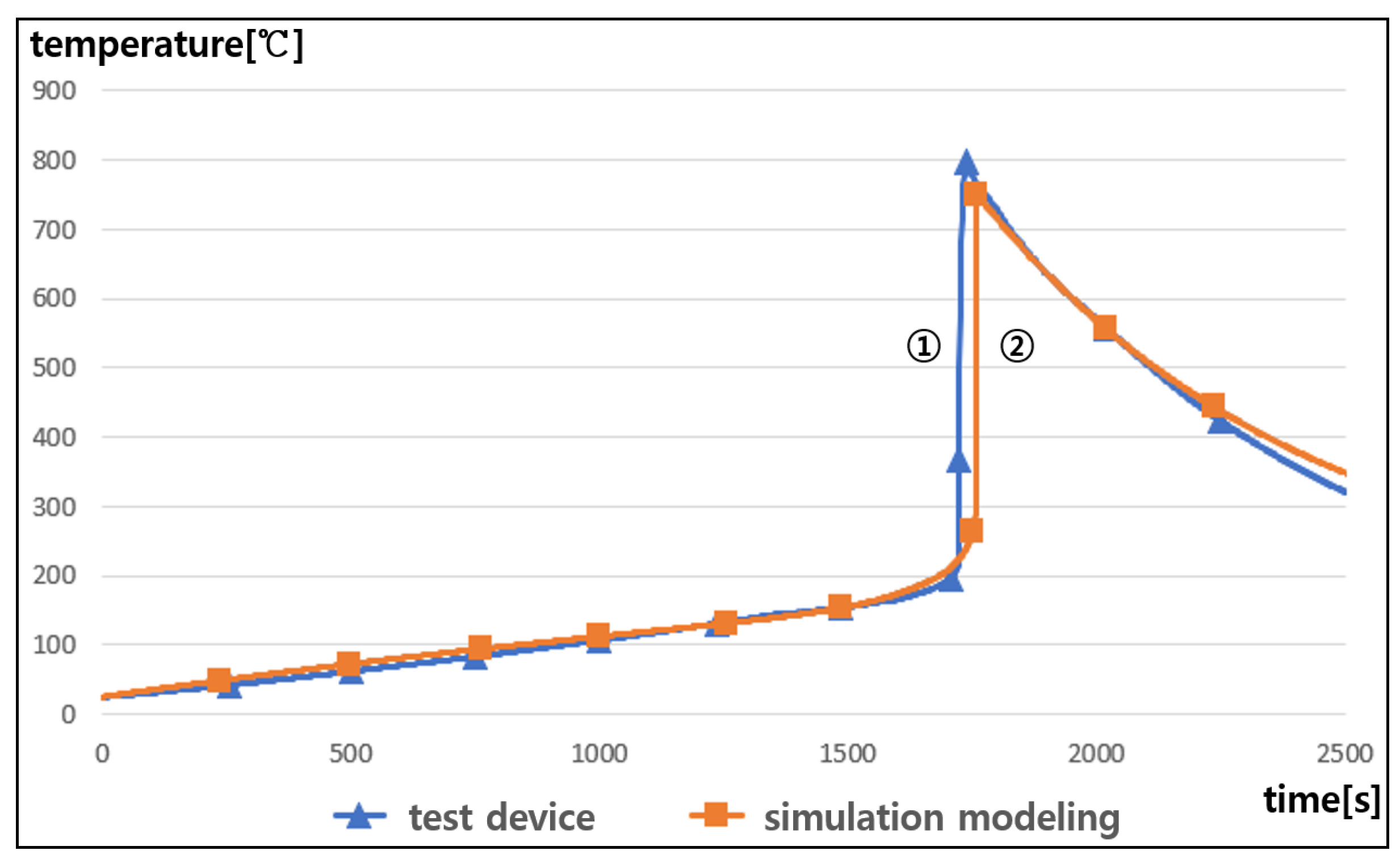
5.2. Validation of the Proposed Modeling with Rising Temperature Rate
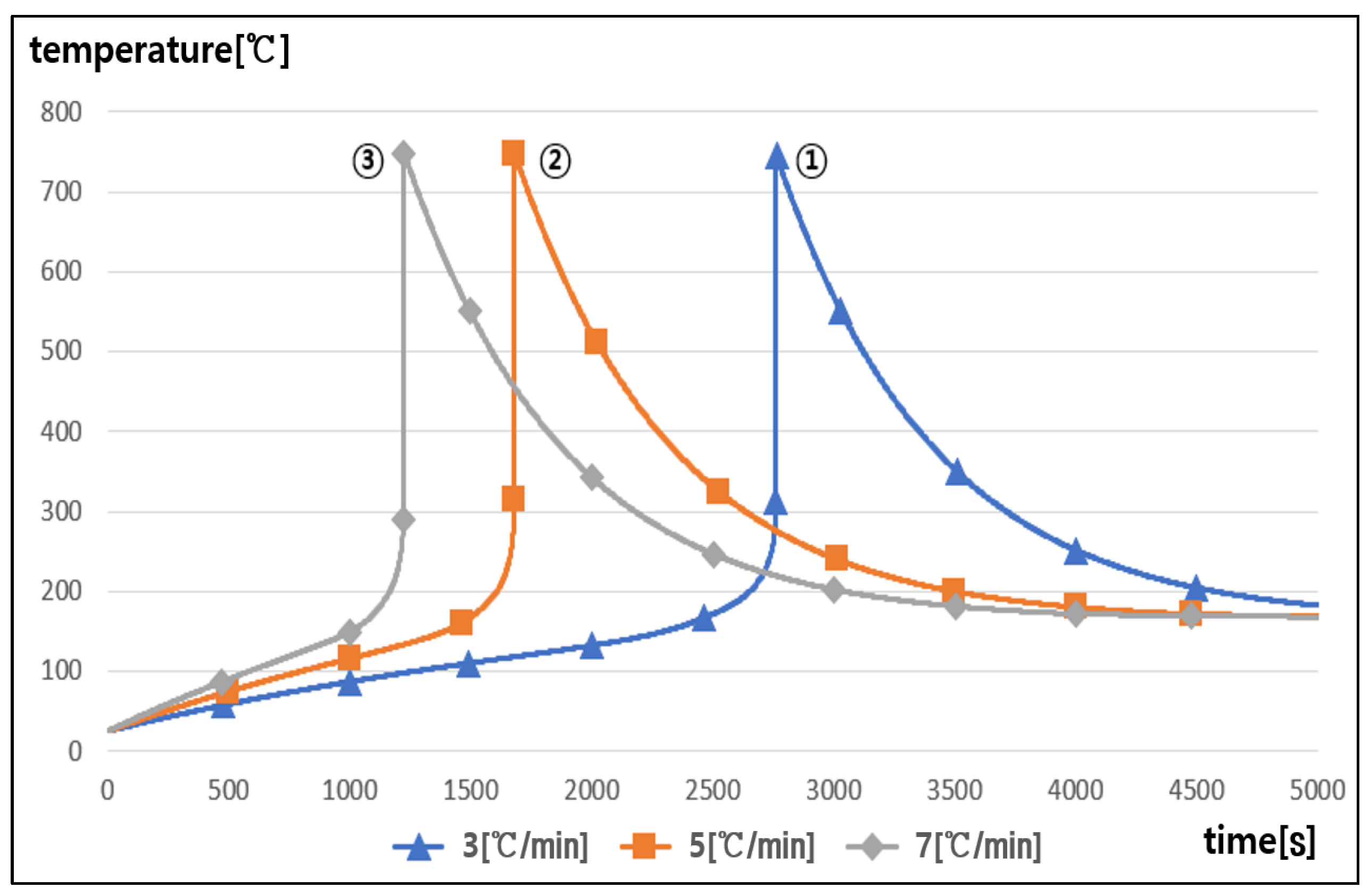
5.3. Thermal Runaway Characteristics with Rising Temperature Rate
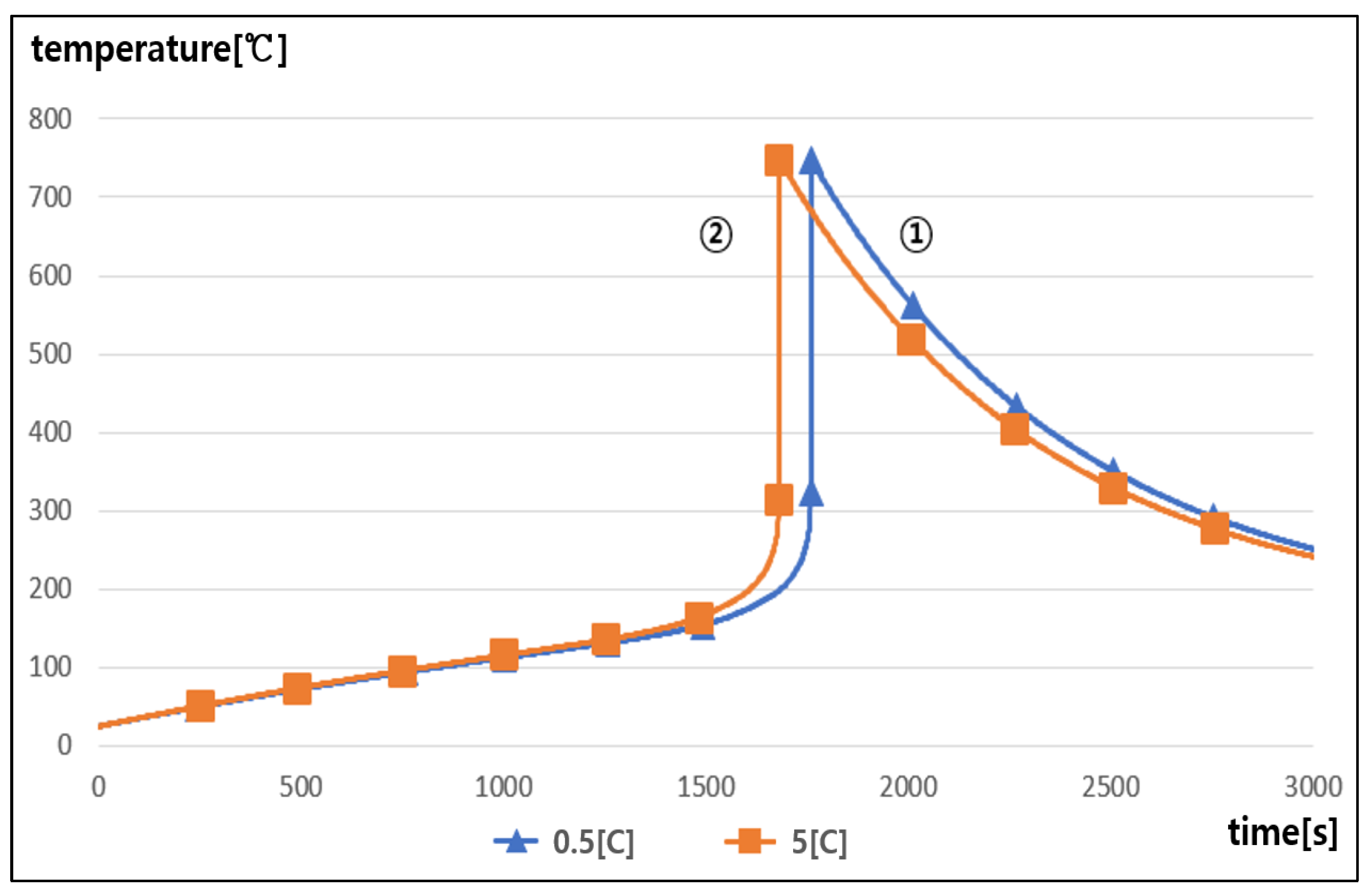
5.4. Thermal Runaway Characteristics with the C-Rate of Charging and Discharging
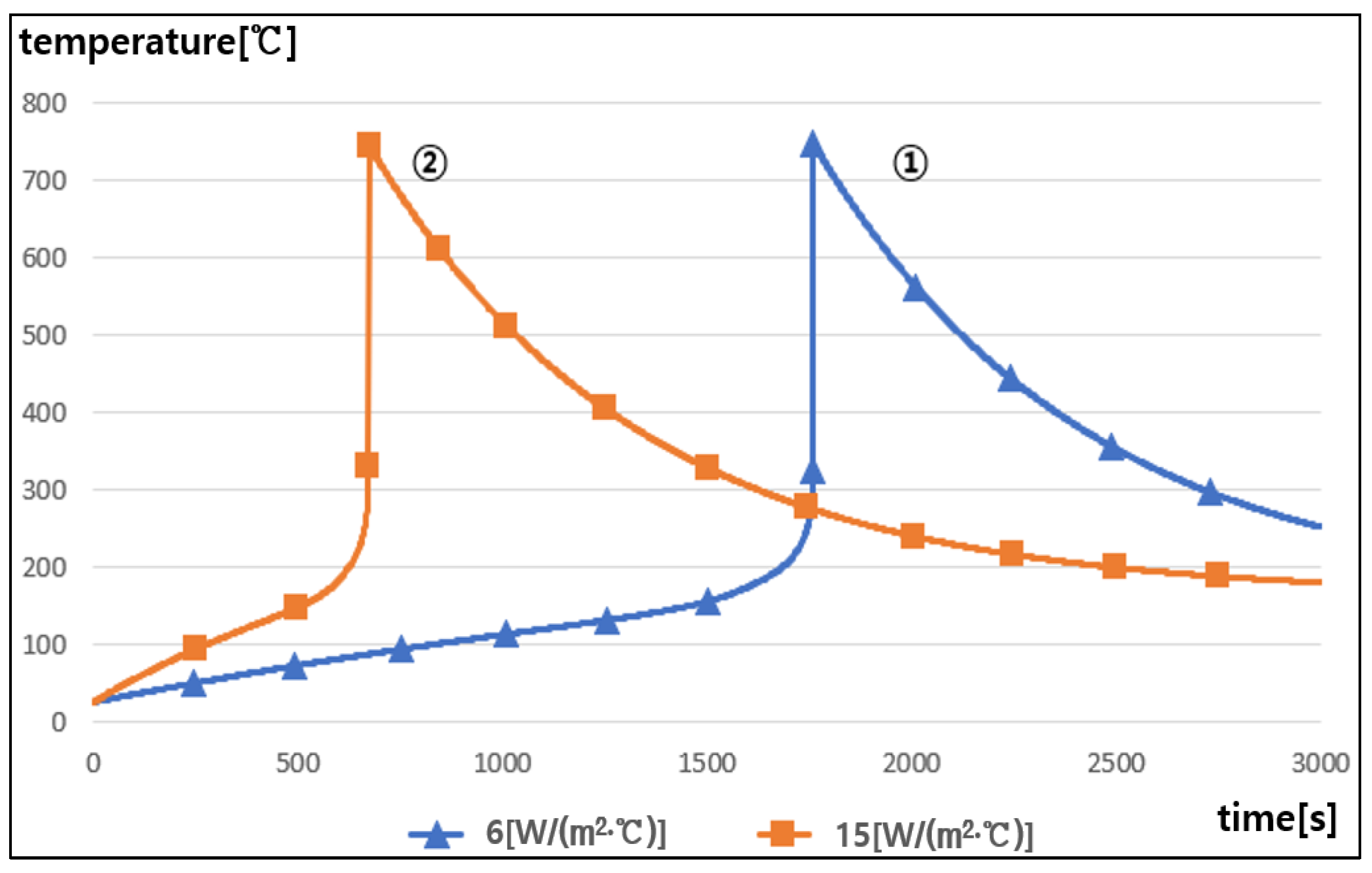
5.5. Thermal Runaway Characteristics with the Convective Heat Transfer Coefficient
5.6. Comprehensive Analysis
6. Conclusions
- (1)
- It is confirmed that the proposed modeling is an effective and reliable tool to evaluate the processing characteristics of thermal runaway because the occurrence time intervals and maximum temperatures had almost the same values in both the test device and simulation modeling.
- (2)
- It was found that as the rising temperature rate became higher, the chemical reaction inside the battery was accelerated and then the occurrence time interval of thermal runaway was shortened, and also, as the C-rate of charging and discharging became higher, the occurrence time interval of thermal runaway was slightly shortened due to the heat release from the internal resistance in the battery.
- (3)
- It is confirmed that as the convective heat transfer coefficient became higher due to the humidity and ventilation condition inside the battery module, thermal runaway occurred within a short time interval, and then thermal runaway from a single cell can easily propagate to adjacent cells depending on the convective heat transfer coefficients.
- (4)
- Based on the operation conditions in ESSs, it was found that the rising temperature rate and the convective heat transfer coefficient were more critical in thermal runaway than the C-rate of charging and discharging.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nam, T.S. The here and the hereafter of Energy Storage System. Trans. Korean Inst. Electr. Eng. 2020, 69, 15–23. [Google Scholar]
- Lim, S.Y.; Park, S.Y.; Yoo, S.H. The Economic Effects of the New and Renewable Energies Sector. J. Energy Eng. 2014, 23, 31–40. [Google Scholar] [CrossRef]
- Park, K.M.; Kim, J.H.; Park, J.Y.; Bang, S.B. A Study on the Fire Risk of ESS through Fire Status and Field Investigation. Fire Sci. Eng. 2018, 32, 91–99. [Google Scholar] [CrossRef]
- Lee, Y.B.; Kim, J.M.; Lee, M.H.; Rho, S.E.; Kim, S.J.; Rho, D.S. Safety Evaluation Method Considering Operation Environments and Applications in ESS. Trans. Korean Inst. Electr. Eng. 2024, 73, 773–783. [Google Scholar] [CrossRef]
- Park, S.H.; Kang, J.G.; Kim, W.U.; Lim, H.W. A Study on Thermal Runaway Suppression Technology in Abnormal State for Energy Storage System(ESS) Using Lithium Secondary Battery. J. Korean Inst. Illum. Electr. Install. Eng. 2022, 36, 26–35. [Google Scholar]
- Jang, H.J.; Song, T.S.; Kim, J.Y.; Kim, S.J.; Jang, T.H. Study on Analysis of Fire Factor and Development Direction of Standard/safety Requirement to Keep Safety for Energy Storage System (ESS). Soc. Stand. Certif. Saf. 2019, 3, 25–49. [Google Scholar] [CrossRef]
- Jung, J.B.; Lim, M.G.; Kim, J.Y.; Rho, D.S. Characteristics of External Short-Circuit in Li-ion Battery Considering Operation and Environment Factors. J. Korea Acad.-Ind. Coop. Soc. 2021, 22, 663–672. [Google Scholar]
- Lim, B.J.; Cho, S.H.; Lee, G.R.; Choi, S.M.; Park, C.D. Characteristics Analysis of Measurement Variables for Detecting Anomaly Signs of Thermal Runaway in Lithium-Ion Batteries. Trans. Korean Hydrog. New Energy Soc. 2022, 33, 85–94. [Google Scholar] [CrossRef]
- ANSI/CAN/UL 9540A(USA); Test Method for Evaluating Thermal Runaway Fire Propagation in Battery Energy Storage Systems. Sustainable Energy Action Committee: New York, NY, USA, 2019.
- Peng, P.; Jiang, F. Thermal Safety of Lithium-ion Batteries with Various Cathode Materials: A Numerical Study. Int. J. Heat Mass Transf. 2016, 103, 1008–1016. [Google Scholar] [CrossRef]
- Jiang, K.; Wang, T.; Li, X.; Duan, B.; Zhang, C. Simulation of Thermal Runaway Prediction Model for Nickel-rich Lithium ion Batteries. In Proceedings of the Chinese Automation Congress, Jinan, China, 20–22 October 2017; pp. 1293–1297. [Google Scholar]
- An, Z.; Shah, K.; Jia, L.; Ma, Y. Modeling and Analysis of Thermal Runaway in Li-ion Cell. Appl. Therm. Eng. 2019, 160, 113960. [Google Scholar] [CrossRef]
- Ren, D.; Feng, X.; Lu, L.; Ouyang, M.; Zheng, S.; Li, J.; He, X. An Electrochemical-thermal Coupled Overcharge-to-thermal-runaway Model for Lithium Ion Battery. J. Power Sources 2017, 364, 328–340. [Google Scholar] [CrossRef]
- Liu, H.; Wei, Z.; He, W.; Zhao, J. Thermal issues about Li-ion batteries and recent progress in battery thermal management systems: A review. Energy Convers. Manag. 2017, 150, 304–330. [Google Scholar] [CrossRef]
- Coman, P.T.; Darcy, E.C.; Veje, C.T.; White, R.E. Modelling Li-Ion Cell Thermal Runaway Triggered by an Internal Short Circuit Device Using an Efficiency Factor and Arrhenius Formulations. J. Electrochem. Soc. 2017, 164, 587–593. [Google Scholar] [CrossRef]
- MacNeil, D.D.; Dahn, J.R. Test of Reaction Kinetics Using Both Differential Scanning and Accelerating Rate Calorimetries As Applied to the Reaction of LixCoO2 in Non-aqueous Electrolyte. Am. Chem. Soc. 2001, 105, 4430–4439. [Google Scholar] [CrossRef]
- Abada, S.; Marlair, G.; Lecocq, A.; Petit, M.; Sauvant-Moynot, V.; Huet, F. Safety focused modeling of lithium-ion batteries: A review. J. Power Sources 2016, 306, 178–192. [Google Scholar] [CrossRef]
- Li, J.; Lotfi, N.; Landers, R.G.; Park, J. A Single Particle Model for Lithium-Ion Batteries with Electrolyte and Stress-Enhanced Diffusion Physics. J. Electrochem. Soc. 2017, 164, 874–883. [Google Scholar] [CrossRef]
- Guduru, A.; Northrop, P.W.; Jain, S.; Crothers, A.C.; Marchant, T.R.; Subramanian, V.R. Analytical solution for electrolyte concentration distribution in lithium-ion batteries. J. Appl. Electrochem. 2012, 42, 189–199. [Google Scholar] [CrossRef]
- Baba, N.; Yoshida, H.; Nagaoka, M.; Okuda, C.; Kawauchi, S. Numerical simulation of thermal behavior of lithium-ion secondary batteries using the enhanced single particle model. J. Power Sources 2014, 252, 214–228. [Google Scholar] [CrossRef]
- An, Z.; Jia, L.; Wei, L.; Dang, C.; Peng, Q. Investigation on Lithium-ion Battery Electrochemical and Thermal Characteristic Based on Electrochemical-thermal Coupled Model. Appl. Therm. Eng. 2018, 137, 792–807. [Google Scholar] [CrossRef]
- Hwang, S.Y.; Choi, S.M.; Jian, S.; Choi, H.S.; Rh, D.S. A Study on the Detection Algorithm of Off-gas to Prevent Thermal runaway of Li-ion Battery for ESS. Trans. Korean Inst. Electr. Eng. 2022, 71, 1787–1795. [Google Scholar] [CrossRef]
- Kim, G.H.; Pesaran, A.; Spotnitz, R. A Three-dimensional Thermal Abuse Model for Lithium-ion Cells. J. Power Sources 2007, 170, 476–489. [Google Scholar] [CrossRef]
- Wang, H.; Du, Z.; Rui, X.; Wang, S.; Jin, C.; He, L.; Zhang, F.; Wang, Q.; Feng, X. A Comparative Analysis on Thermal Runaway Behavior of Li (NixCoyMnz) O2 Battery with Different Nickel Contents at Cell and Module Level. J. Hazard. Mater. 2020, 393, 122361. [Google Scholar] [CrossRef] [PubMed]
- IEC 62619:2022; Secondary Cells and Batteries Containing Alkaline or Other Non-Acid Electrolytes—Safety Requirements for Secondary Lithium Cells and Batteries, for Use in Industrial Applications. International Electrotechnical Commission: Geneva, Switzerland, 2022.
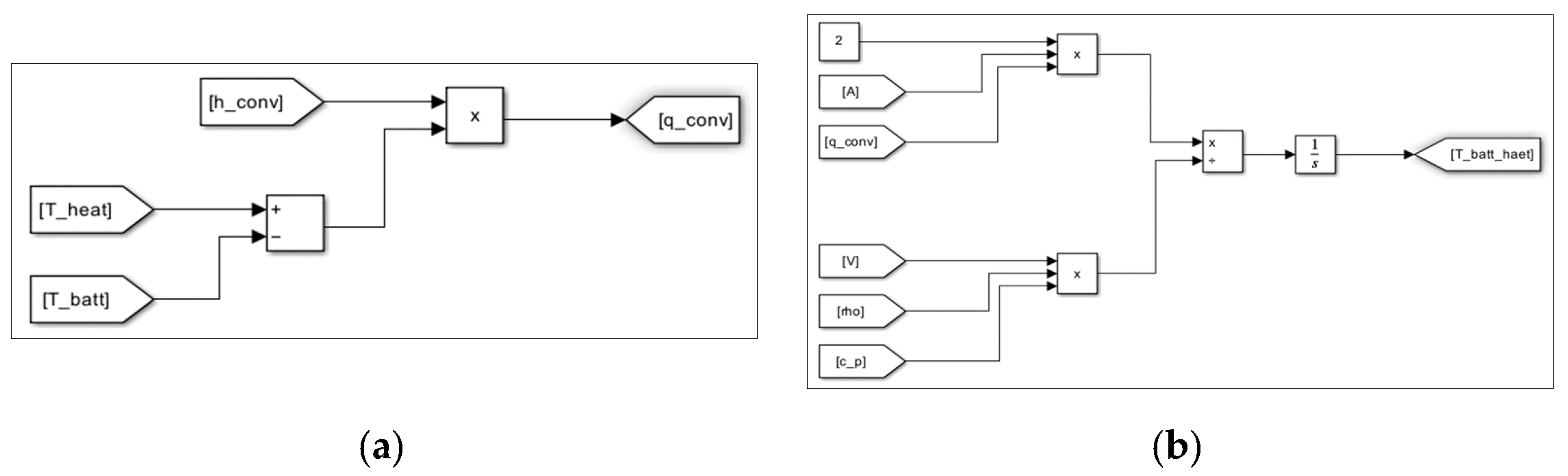
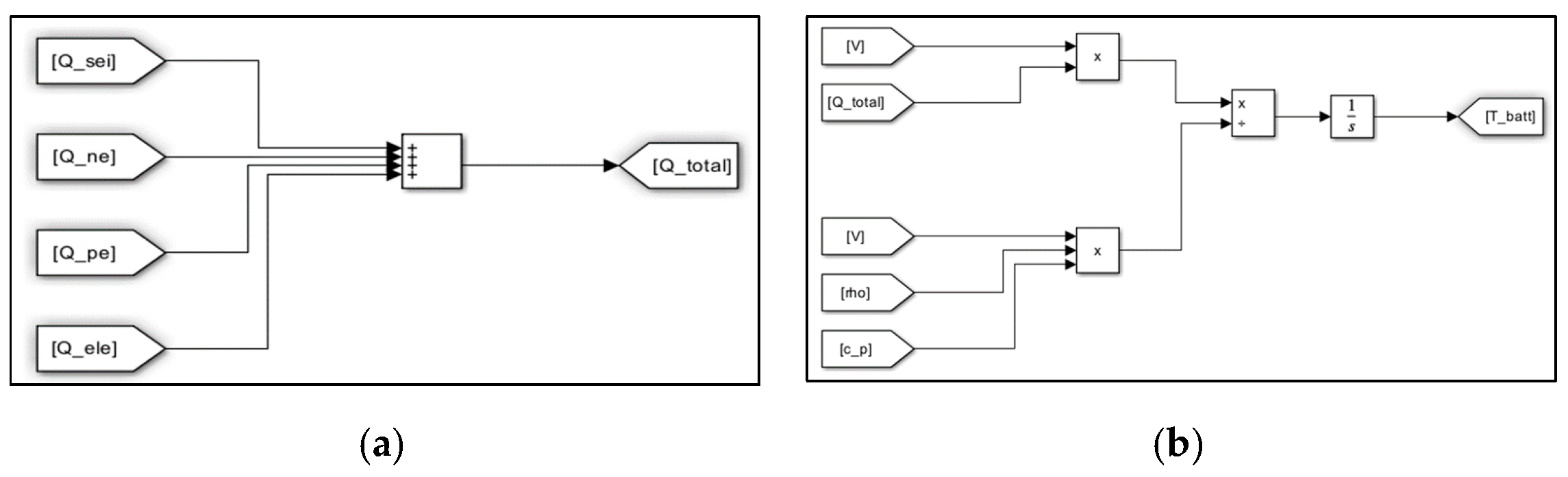
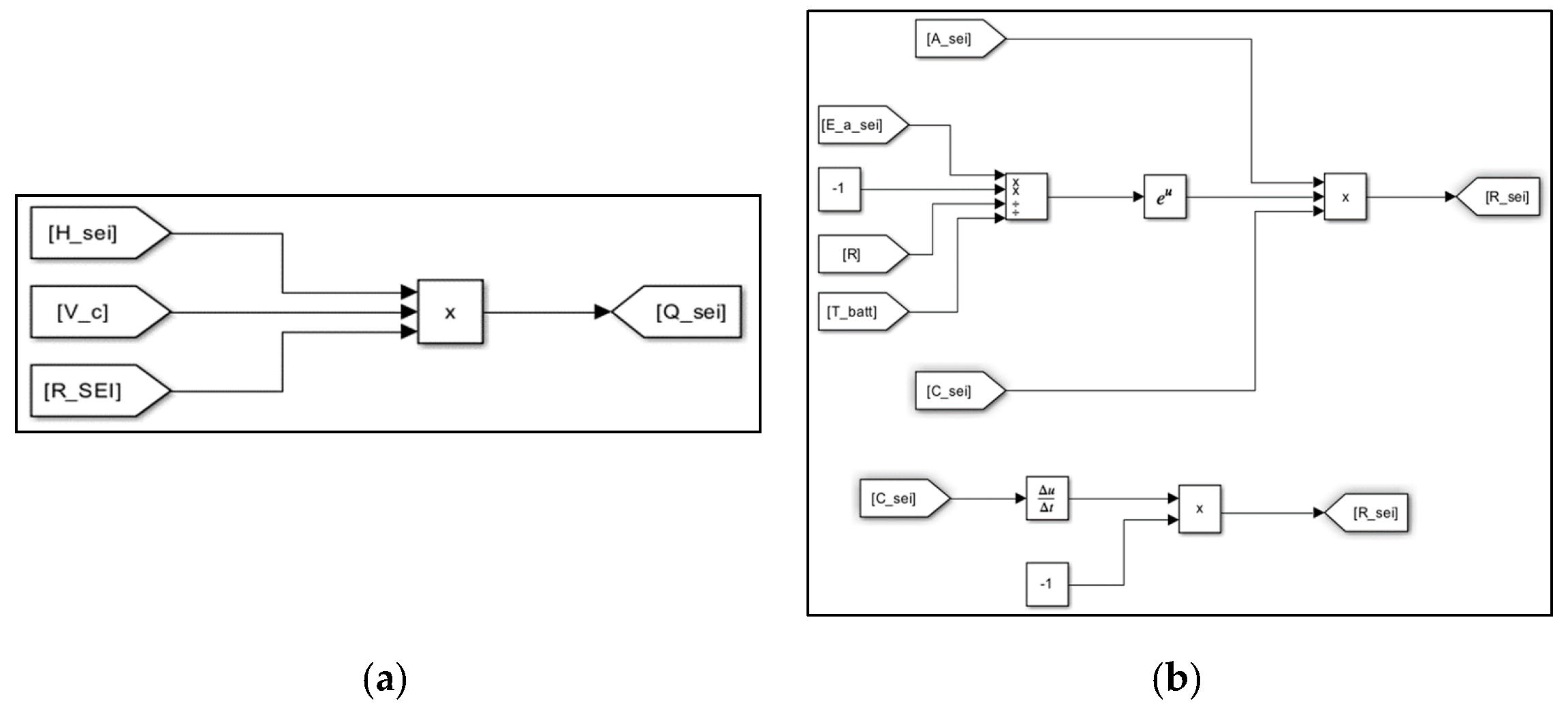
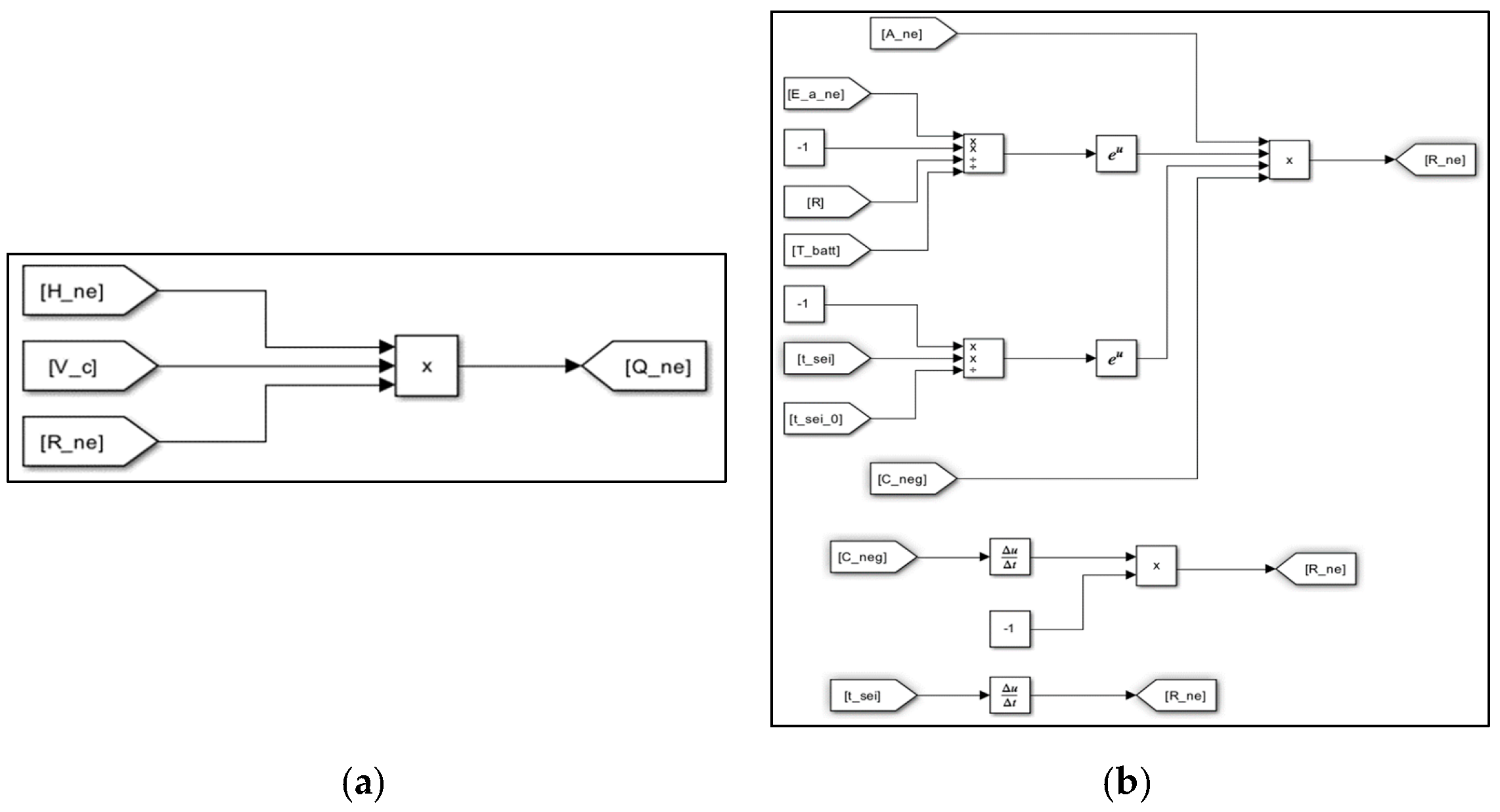
| Items | Contents |
|---|---|
| Rising temperature rate [°C/min] | 3, 5, 7 |
| C-rate of charging and discharging [C] | 0.5, 5 |
| Convective heat transfer coefficient [W/(m2·°C)] | 6, 15 |
| Symbols | Items | Values |
|---|---|---|
| universal gas constant | 8.31 | |
| Faraday constant | 96.485 | |
| reaction heat by decomposition of the SEI layer | 2.6 × 105 | |
| reaction heat between the negative electrode and solvent | 1.7 × 106 | |
| reaction heat between the positive electrode and solvent | 7.9 × 105 | |
| reaction heat by the decomposition of the electrolyte | 1.6 × 105 | |
| volume-specific carbon content in the SEI layer | 1.7 × 103 | |
| volume-specific positive active content | 1.3 × 103 | |
| volume-specific electrolyte active content | 5 × 102 | |
| frequency factor of the decomposition of the SEI layer | 1.7 × 1014 | |
| frequency factor between the negative electrode and solvent | 5.0 × 1012 | |
| frequency factor between the positive electrode and solvent | 2.3 × 1013 | |
| frequency factor of the decomposition of the electrolyte | 5.1 × 1024 | |
| activation energy of the decomposition of the SEI layer | 1.4 × 105 | |
| activation energy between the negative electrode and solvent | 1.4 × 105 | |
| activation energy between the positive electrode and solvent | 1.5 × 105 | |
| activation energy of the decomposition of the electrolyte | 2.7 × 105 | |
| dimensionless amount of lithium-containing meta-stable species in the SEI layer | 0.15 | |
| dimensionless amount of lithium within the carbon in a negative electrode | 0.75 | |
| dimensionless concentration of the electrolyte | 1 | |
| conversion rate | 0.04 | |
| initial value of | 0.033 |
| Operation Conditions | Occurrence Time Interval of Thermal Runaway [min] | |
|---|---|---|
| Rising temperature rate [°C/min] | 3 | 46 |
| 5 | 28 | |
| 7 | 20 | |
| Convective heat transfer coefficient [W/(m2·°C)] | 6 | 29 |
| 15 | 11 | |
| C-rate of charging and discharging [C] | 0.5 | 29.33 |
| 5 | 28 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, M.-H.; Choi, S.-M.; Kim, K.-H.; You, H.-S.; Kim, S.-J.; Rho, D.-S. An Evaluation Modeling Study of Thermal Runaway in Li-Ion Batteries Based on Operation Environments in an Energy Storage System. Batteries 2024, 10, 332. https://doi.org/10.3390/batteries10090332
Lee M-H, Choi S-M, Kim K-H, You H-S, Kim S-J, Rho D-S. An Evaluation Modeling Study of Thermal Runaway in Li-Ion Batteries Based on Operation Environments in an Energy Storage System. Batteries. 2024; 10(9):332. https://doi.org/10.3390/batteries10090332
Chicago/Turabian StyleLee, Min-Haeng, Sung-Moon Choi, Kyung-Hwa Kim, Hyun-Sang You, Se-Jin Kim, and Dae-Seok Rho. 2024. "An Evaluation Modeling Study of Thermal Runaway in Li-Ion Batteries Based on Operation Environments in an Energy Storage System" Batteries 10, no. 9: 332. https://doi.org/10.3390/batteries10090332














