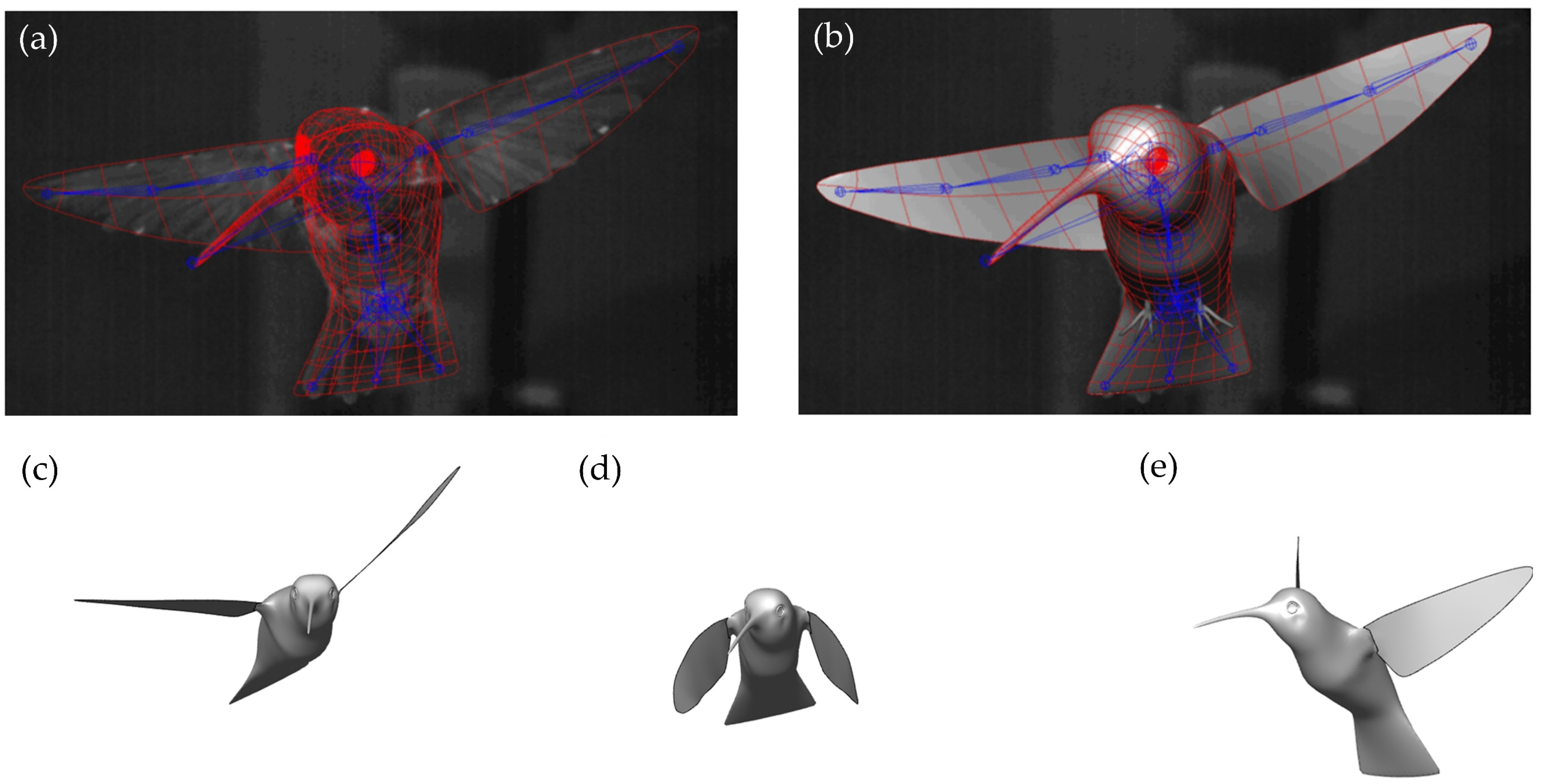
We first report the body and wing kinematics of this flight. This is followed by the results of aerodynamic forces produced by the wings. Next, the three-dimensional flow structures in the far and near wake are explained. By simulating the flow over the six stroke motions performed by the hummingbird, results on the aerodynamic force and wake topology are obtained. Following this, evaluation on the role of wing flexibility in performing the maneuver is conducted.
3.1. Body and Wing Kinematics Measurements
Findings on the body and wing kinematics of the hummingbird performing a yaw turn are presented in this section. First the body kinematics, including the time series of the body yaw, pitch, and roll angles as well as yaw velocity and body position, are displayed in
Figure 4a. During the maneuvering process, the hummingbird performs six flapping motions in total. The downstrokes and upstrokes are differentiated by the coloring of the plot background with grey indicating the downstroke (DS) motion and white indicating the upstroke (US) motion. To better interpret the characteristics of the turn, the process is divided into three phases according to the body yaw angle and yaw velocity.
During phase I, the yaw velocity monotonically increases, and the yaw angle increases slightly (+9°). Phase II is characterized by a sustained increase in yaw angle (+73°) and an oscillating yaw velocity profile. This oscillating profile indicates that the hummingbird is actively controlling its orientation during the turn. Finally, during phase III, the hummingbird yaw angle increases negligibly (0°), and the yaw velocity is close to 0. From the root square error relative to the mean body Euler angle plot, the only body angle that changes considerably throughout the motion is the yawing angle (red), as the pitch and roll (black and blue) vary minimally about each respective mean value. Thus, the motion is termed a pure yawing turn.
In
Figure 4b, top-down views of the hummingbird body at the start (red) and end (blue) of each phases I-III are shown to visualize the progression through the yaw motion. The angular change in body yaw is visualized by lines pointing in the heading direction of the hummingbird at the start (red) and finish (blue) of the phase. The light grey bodies and heading direction lines indicate the body position at the end of each of the middle strokes during phase II. Next, the wing kinematics are presented.
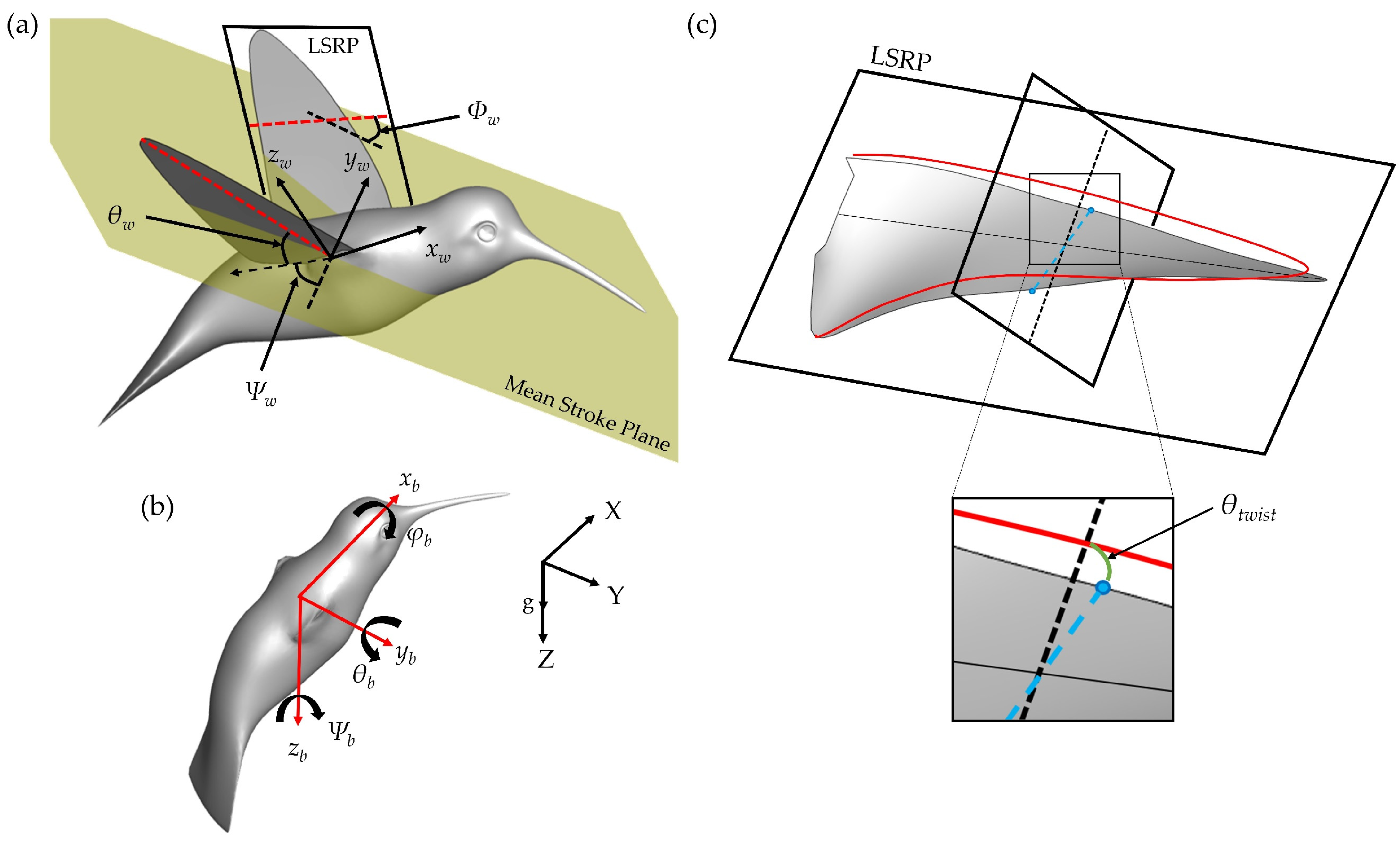
Figure 5 displays time series of the wing kinematics
Ψw,
θw,
Φw, and
θtwist. During the pure yaw maneuver, the hummingbird flapped with considerable angle of incidence, stroke, and twisting angle asymmetries. A quantitative comparison between the wing angle amplitudes is given in
Table 2. This helps to understand how the hummingbird expands or suppresses its wing kinematics to perform the maneuver. To initiate the turn, a suppression of the IW deviation and stroke angle is observed. During the turning phase, the IW maintains an expanded stroke and AOI amplitude compared to the OW, but the ‘figure eight’ shape of the IW is smaller than that of the OW as indicated by the smaller deviation amplitude. During the recovery, in which hovering is resumed, the wing kinematics for the IW and OW are similar with the only considerable asymmetry present for the AOI.
For twisting, chords closest to the wing root experienced the least amount of deformation (within ±5° at 0.25 R). The twist angle amplitude rapidly increases further out on the span with the large deformations occurring at 0.75 R, which is consistent with previous measurements [
14]. However, the IW and OW asymmetries observed in this study are not seen in other measurements. Here, the IW twists nearly perpendicular to the LSRP during stroke I. Subsequently, the deformations stabilize to a more repetitive nature in which the IW typically exceeds the OW twist. Further out along the wing, at 0.75 R, the square-like profile of the IW twisting angle leads to sustained higher twist during the US. Consequently, the average IW twist during the US is higher than the OW by 21.3% at 0.5 R and 46.4% at 0.75 R, suggesting the hummingbird asymmetrically attenuates and expands its wing twisting for the IW and OW to achieve the turn.
Finally, the wing tip velocities are compared. During stroke I, the OW Utip is much higher than that of the IW during the US, which corresponds with the initiation of the yawing motion. During the subsequent DS for strokes II-V, the IW tends to have a higher velocity whereas during the stroke II-V US, neither the IW nor the OW tip velocity is dominant. Interestingly, the OW tip velocity tends to exhibit a plateau during the DS while the IW continues to speed up until reaching a peak velocity at the mid-stroke.
Compared to previous works involving hummingbird turning, the body kinematics identified for the current calliope hummingbird present key differences. Use of revolving feeders limits the turning speed of hummingbirds to around 0.5 Hz to 0.6 Hz at maximum [
26,
27]. The data collected in the current study, in which 82° are traversed in 113 ms, extrapolates to 2.01 Hz, which falls within the range of the free maneuvering turning speed discussed by Altshuler et al., suggesting that the coaxing mechanism constrains the turning performance [
26]. Additionally, the coaxed-turn measurements revealed body banking that is absent in the current free maneuvering hummingbird [
27].
Wing kinematics for the free maneuvering hummingbird also vary from coaxed-turn measurements. Namely, the stoke angle amplitudes observed in the current work are smaller with the primary difference arising due to suppressed maximum US stroke angle. Additionally, larger DS elevation angle amplitude is achieved [
26,
27]. However, these wing kinematic findings do agree with the general trends observed by Read et al., where an increase in yaw rotational speed corresponded with an increase in DS elevation angle. As the turn speed of the hummingbird examined in the current work is nearly four times greater than that in Read et al. [
27], the large DS elevation angle amplitude is within reason. Association of the stroke amplitude is less clear, although hummingbirds often use stroke amplitude changes to manage aerodynamic power, and the shrunken amplitude in the current work may indicate lower power consumption during the turn [
18,
45,
46].
3.2. Wing Aerodynamic Forces
To better understand how the previously discussed wingbeat kinematics are utilized by the hummingbird to perform the yawing maneuver, the results of the aerodynamic forces and power consumption are shown. Forces are obtained by integrating the pressure and shear stress over the surface of the wings. The forces are resolved into two components: along the global-Z direction, and in the global XY plane opposite to the hummingbird heading direction. These forces are termed lift (FL) and drag (FD), respectively. Power is computed by summing the velocity of the surface element (relative to the local fluid velocity) multiplied by the force on that element across all the wing surface elements.
The force and power are plotted as
CD,
CL, and
CPW, which are non-dimensional and normalized values determined by:
where
FD,L indicates drag and lift force, respectively,
Utip represents average tip velocity, and
Sb is the wing surface area. The hummingbird force production during the pure yaw maneuver is shown in
Figure 6 as the time series of
CD and
CL. Under the current convention, a positive drag force generated by the inside wing would point backwards relative to the hummingbird heading and create a clockwise moment about the body.
The balancing of CD generation to achieve the yaw turn is clear. During both the US and DS, the IW achieves about 16% higher magnitude drag force than the OW in total. The direction of the drag force alternates in such a manner that the DS produces positive drag (opposite to the heading direction), and the US produces negative drag (towards the heading direction). This means that the higher magnitude IW drag forces serves to drive the clockwise turn in the DS and damp the turn in the US, which helps to explain the yaw velocity oscillations discussed previously. For the IW, the US contributes about 69% more drag generation than the DS. For the OW, the US accounts for 80% more drag generation than the DS. The OW only consistently exceeds the drag production of the IW in the stroke I US where the drag asymmetry favoring the OW aids in initiating the clockwise turn. During stroke VI, the recovery phase, the drag forces are the same for both the IW and OW. This matches exactly with the period in which the yaw turns slow and hovering resumes.
For the lift production, the IW and OW remain relatively similar across the whole motion with no consistent bias towards lifting force on one wing. The mostly positive
CL means that the hummingbird US and DS both generate upwards force opposing gravity which is likely a mechanism adopted by the hummingbird to maintain level vertical displacement during the motion (as observed in the body kinematics analysis). Further examination reveals that the DS produces significantly higher lift than the US. Averaging between the IW and OW, the DS generates 73% more lift than the US throughout the whole maneuver which is consistent with previous studies on hummingbird flight aerodynamic force generation for hovering flight [
15,
16,
22]. Using the hummingbird mass (2.6 ± 0.2 g,
Table 1), and the gravitational acceleration
g = 9.81 m s
−2, the force required to keep the hummingbird aloft is about 2.5 × 10
−2 ± 0.2 × 10
−3 N. The current
CL definition defines lift to be opposite to gravity. Re-dimensionalizing the current results reveals that the average lift force produced during the maneuver is 2.3 × 10
−2 N. This agrees well with the force necessary to keep the hummingbird aloft with minimal elevation change, which was observed in the body kinematics in
Figure 4a.
Discussing power, to initiate the turn in stroke I the hummingbird expends 153% more power flapping the OW compared to the IW. During the turning phase the power consumption for the IW has higher peaks than the OW at the mid-DS/US. During the resumption of hovering flight in phase III, power consumption is significantly reduced for both wings, and no IW/OW discrepancies are seen.
In addition to the time series data presented in
Figure 6, plots depicting the US and DS average forces during phase II are provided in
Figure 7. The forces are plotted on the model whose position allows for clear visibility of both wings. This serves to further clarify the role of the half strokes in force generation and visualize the asymmetric aerodynamic forces. The DS average, shown in
Figure 7a, illustrates that drag generation is primarily concentrated near the wing tips. Further, the IW has more intense drag generation as indicated by the darker red regions. Thus, the yaw turn is enhanced by the DS. For the US average in
Figure 7b the magnitudes of the averaged drag force are larger than the DS. The IW again demonstrates increased drag production compared to the OW as seen by the larger dark blue region near the mid-span. However, the torque generation during the US is opposite that of the DS as the IW biased drag production serves to damp the yaw turn.
3.3. Turning Motion Wake Topology
In this section, the arrangement and timing of vortex production and shedding is examined. The snapshots of wake topology are provided for the third flapping motion during which the hummingbird is performing its turn. This time frame was selected since the hummingbird has settled into the steady yaw angle increase. Additionally, the forces during this stroke closely match the overall trends noticed in the previous section (i.e., the larger IW US/DS CD as well as significant DS CL generation). Therefore, stroke 3 will serve as a good estimate for the average wakes produced during the turning motion.
To understand the organization of the vortex structures, iso-surfaces visualized with a
Q-criterion value of 375 are displayed. The
Q-criterion, which is dimensionless in nature, is calculated according to Equation (3) where
and
are the vorticity and strain rate tensors, respectively. This variable is helpful in identifying rotationally dominant regions of the fluid.
The downstroke motion is analyzed first in
Figure 8a–c. Due to the orientation of the body and position of the wings, the images are shown from above and behind the hummingbird. This perspective allows for clearer differentiation of the downstroke wake topology. Plots for the DS are shown at
t/T* = 0.16,
t/T* = 0.32 and
t/T* = 0.48. The time stamp
t/T* = 0.32 corresponds closely with the time at which maximum DS force is achieved.
T* stands for the time required to complete a stroke motion. Newly formed vortices are highlighted with dotted red arrows while vortex loops generated during the previous half-stroke are highlighted in solid red.
Early in the DS, formation of the leading-edge vortex (LEV1), trailing-edge vortex (TEV), and root vortex (RV) is observed on the OW in
Figure 8a. At this time, a major discrepancy between the IW and OW is present. The OW LEV1 occupies a large portion of the wing leading edge, with more chordwise expansion towards the distal regions, meanwhile the IW LEV1 is comparatively smaller. In addition to the newly created structures, the detached vortex loop (DVL) from the previous upstroke can be seen above and behind both the IW and OW.
At the mid downstroke, shown in
Figure 8b, the outer wing exhibits a dual-vortex loop formation. The inner-most loop, labeled VL1, forms because of early LEV1 lift-off from the wing and is composed of the detached arch-like LEV1, TEV, and RV. Connection between the previously formed RV and TEV is clear. The outer-most loop, labeled VL2, starts to form between the tip vortex (TV) and newly generated LEV2; however, no clear ring-like structure is seen. The dual loop generation is like that observed with AR 2,4 revolving plates in DPIV experiments and simulations [
47,
48,
49]. For the wings of the current computational model, AR ~3. The inner wing exhibits a more uniform shedding pattern than the outside wing with the LEV1, TEV, and RV forming a single loop much like the topology documented in ruby-throated hummingbird hovering flight [
22].
Finally, the end of the downstroke is shown in
Figure 8c. The previously visible VLs (both for the inner and outer wing) have dissipated. Behind the outer wing, the dual loop vortex pattern is more visible as the TV in VL2 has stretched due to the wing flapping motion. Meanwhile, VL1 has mostly separated from the wing and is positioned behind the wing. As for the inside wing, the coherent VL1 is still prevalent with the TV stretching to connect the VL1 to LEV1. This end-stroke vortex organization for the IW is like the wake of a hovering ruby-throated hummingbird. Song et al. observed the TV connecting the TEV to the primary LEV at the end of the hovering DS at
Re = 1500 [
22].
Next, the wake topology of the upstroke phase is examined. Plots for the upstroke are shown at
t/T* = 0.60,
t/T* = 0.72 and
t/T* = 0.88. Peak US force generation is captured at the
t/T* = 0.72 frame. A very similar process to the downstroke is repeated; however, the wing on which the dual vortex loops is formed is now the inside wing (while this process also occurs on the OW, it is later in the US). This is seen in
Figure 8d–f where the pattern of vortex formation follows closely with that described for the downstroke. The LEV1 formed on the IW detaches before the half downstroke causing VL1 and VL2 formation in
Figure 8d. Meanwhile, the OW demonstrates more an expanded LEV1 formation, with
Figure 8e showing significant chordwise expansion, much more than demonstrated by the IW during the DS. This instant captures the onset of the LEV separation. At the conclusion of the US in
Figure 8f, the generated VLs are visible in the wake. Two distinct loops are visible for the OW, with the size of VL2 being small, indicating that LEV splitting occurred later in the US compared to the IW.
It appears that the main mechanism behind the dual-vortex loop vortex formation is destabilization of the primary formed LEV1. VL1 is formed due to the LEV1, TEV, and RV ring detaching from the upper surface during the downstroke and lower surface during the upstroke. When this process occurs early in the half-stroke, a substantially sized VL2 forms due to TV and LEV2 at the distal regions of the wing. As with the OW during the US, a smaller VL2 may form with later LEV1 detaching. The dual LEV generation is documented to occur beyond the midspan for higher aspect ratio flapping plates, with the tendency of the splitting to propagate from tip to root [
48,
50]. During the DS, the OW motion and body turning constructively interact, giving the OW a higher effective speed, while the IW experiences the same in the US. Extrapolating on the discussion by Harbig et al., where a higher advance ratio led to unstable LEVs during the DS (wing flapping in the direction of travel), we find that the wing flapping in the direction of body rotation also experiences LEV instability [
50]. This is reflected by the early half-stroke LEV1 separation during the DS for the OW and US for the IW. In addition to these wing-generated vortex structures, it is important to note that no body vortex structures, such as a head shear layer and thorax vortex, are observed, which contrasts that of hummingbird forward flight [
25]. This can be attributed to the much lower translational speed of the hummingbird body as most of the motion during the maneuver is rotational.
Figure 9a–d illustrate the down jets produced by the wing flapping.
Figure 9a,b correspond to the s1 and s2 locations shown in
Figure 8b, while
Figure 9c,d correspond to the s1 and s2 locations in
Figure 8e. The vorticity contours represent the global coordinate x-directional vorticity, while the arrowheads indicate the global YZ fluid velocity components in the slice plane. The trend in DS-dominated lift and US-dominated drag production is evident in the velocity gradients. The direction of the VL jets can be thought of as a reflection of the direction of the aerodynamic forces.
Figure 9a,b illustrated that the DS-generated vortex loops exhibit nearly vertical downs jets, representative of the wings generating significant lift as discussed in
Section 3.2. In
Figure 9c,d, the more horizontal orientations of the VL jets indicates the increased US drag generation. The general arrangement of the loops and resulting downwash agrees well with DPIV experiments and quasi-steady simulations performed on hovering hummingbirds, with discrepancies in wake direction attributed to the asymmetric flapping/body motion of the turning hummingbird [
16,
19,
22].
3.4. Near-Body Vortex Structures
To better understand the LEV1,2 generation and destabilization dynamics, this section examines the flow field immediately surrounding the wings. To calculate spanwise vorticity of these vortexes, virtual cameras are positioned to view directly parallel to the root-tip vector of each of the wings during the flapping motion. A schematic of this is shown in
Figure 10a. Slice-cuts are made perpendicular to these vectors and variables such as velocity, vorticity, and pressure in these 2D planes can be computed. This allows for calculation of circulation according to:
where
S represents the boundary of the vortex in the 2D plane (defined by a vorticity threshold of ~15% of the maximum spanwise vorticity) and
represents spanwise vorticity. To minimize the erroneous inclusion of shear layers or other minor vortexes in the calculation, a search range is prescribed to the calculation so that only the region containing the LEV attached to the wing is searched. This method has been used in previous studies for measuring and comparing vortex circulation of various flying insects [
9,
10,
11]. Stroke 3 will be examined in close detail, as with the previous section. In
Figure 10b,d, slice cut vorticity along the span in increments of 0.10 R is shown at peak force production instances for the DS(b) and US(d). Additionally,
Figure 10c,e compares the spanwise circulation calculation along the wing at the same instances.
Due to the early OW LEV1 separation, LEV1 is hardly visible in the slice frame. In contrast, the IW LEV is large and has more coherent dark red vorticity regions in
Figure 10b. No secondary structure, either behind LEV or shed into the wake, is visible. The vorticity regions are much stronger than for the IW, too, suggesting greater LEV strength. This is supported by the
calculations as the peak IW
(at 0.70 R) is 59% greater than the peak OW
Additionally, from
Figure 10c, the IW
is much higher than that of the OW from 0.40 R outwards. For the US, the IW and OW are slightly more balanced in terms of vorticity contour and circulation strength. The multi-LEV arrangement, a consequence of early US LEV1 separation, can be seen for the IW, whereas the onset of such bifurcation is captured for the OW. The spanwise LEV2
distributions are similar between the IW and OW, which generally agrees with the similar drag and lift force generation observed during the US in
Section 3.2.
No direct comparisons to previous studies on turning hummingbird LEV dynamics can be made; however, works addressing revolving plates and hummingbird hovering serve as good benchmarks to discuss how the observed dynamics fit into the existing literature. From DPIV measurements, Warrick et al. concluded that LEV circulation during hovering hummingbird flight was significantly higher during the DS than US, nearly 2× [
16]. Our calculation indicates the difference is 1.59×; however, the body motion and wing motion for hovering flight is different than in the current free maneuvering case, which may explain the discrepancy. Additionally, in the current study the IW LEV1 in the DS is more steadily developed, which is reflected by the strong
beyond the mid-span. This agrees with LEV calculations made for a revolving wing (
Re = 1500) by Chen et al. [
51]. Due to the instability, LEV2 circulation (as opposed to LEV1) is captured by the
calculations. Compared to the IW
distribution shown in
Figure 10c, which represents the primary LEV1, the secondary LEV2s are weaker. Using DPIV experiments, Car et al. visualized the spanwise vorticity during the LEV1 separation, with clear inwards propagation of the split, and reported similar results. The attached LEV2 exhibited stronger vorticity regions until about 0.75 R, after which the LEV2 cross section was less organized [
47].
Figure 10b,d qualitatively support this, while
Figure 10c,e serve as a further quantitative validation: the LEV2 circulation decreases after about the 0.75 R span for the cases in which LEV1 separates.
To better understand the role of the asymmetric vortex generation in force production, non-dimensional pressure iso-surfaces are plotted in
Figure 1a,c. These surfaces helps to visualize the IW/OW pressure discrepancy that produces enhanced IW drag production. The blue surface indicates a non-dimensional pressure coefficient of −3.0, the white surface indicates a pressure coefficient of −1.0. This is paired with topology schematics in
Figure 11b,d to help associate the pressure regions with vortex structures.
For the DS in
Figure 11a,b, the IW exhibits a much stronger region of low pressure behind the wing with noticeable concentration of low pressure at the distal region of the wing due to a steadily formed LEV1. The concentration of low pressure at these positions aligns with observations of the average DS drag generation occurring mostly at the wing tips from
Figure 7a. The OW has comparatively weaker regions of low pressure due to the more unsteady LEV formation. Additionally, a distinguishable VL1 that already detached from the wing is seen behind the OW. Thus, the more steadily developed and stronger LEV1 produces a more enhanced low-pressure region behind the wing leading to the higher IW drag production during the DS.
During the US in
Figure 11c,d, the pressure iso-surfaces are similar between the IW and OW although the IW low-pressure region appears slightly larger than that of the OW, which leads to the observed IW drag force being larger than the OW. For the IW, this low-pressure region is associated with the LEV2 formed after LEV1 lift-off. As for the OW, the steadily developed LEV1 is responsible for the induced low-pressure region. Finally, the low-pressure region is robust even at the wing mid-span, which contrasts the DS. This matches well with the average drag force calculations illustrated in
Figure 7b, where much of the US drag force is shown to be generated near the mid-span.
The dual-vortex loop formation and shedding can be summarized as the following. During the early DS, the LEV1 formed by the OW separates due to the inwards propagating ‘burst’, resulting in the formation of LEV2. Meanwhile, the IW develops a stable and strong LEV1, evident in the vorticity slice cuts in
Figure 10b and circulation distribution in
Figure 10c. Correspondingly, stronger low pressure is induced behind the wing, according to
Figure 11a, through which higher drag is produced. The DS drag asymmetry between the IW and OW sustains the yaw turn. During the US the LEV2s, present on the IW and OW around when maximum force is generated, are similar in strength to the LEV2 on the OW during the DS (
Figure 10c,d). The US low-pressure concentrations are similar too, from
Figure 11c, thus there is only a slight IW drag advantage over the OW during the US, which serves to damp the body turning speed. The observed dual-ring pattern results in vortex-induced force asymmetry that contributes to the hummingbird’s ability to execute the desired yaw turn. The wake pattern led to the IW maintaining higher drag production compared to the OW, which could be due to the expanded stroke amplitudes and more suitable wing twisting for drag generation. Additionally, rotational motion of the body may contribute to the pattern [
52,
53].
3.5. Wing Flexibility
Additionally, it is important to understand the role of wing flexibility in the maneuver. To achieve this, the nominal case (D) (as analyzed in
Section 3.1,
Section 3.2,
Section 3.3 and
Section 3.4) is compared to a non-deforming case (R). To achieve the R case motion, the skeletal joints for the wing model (see
Section 2.1) are altered such that the local joint rotations in the direction of the span and chord are 0, which removes the twisting and bending of the wing. We preserve the rigid pitching and rolling motion of the wing due to the root joint rotations. The difference in the kinematics is qualitatively and quantitatively compared in
Figure 12.
In
Figure 12a, wing chord lines are shown in dotted lines for the deformed wing (D) and solid lines for the rigid wings (R), which helps to identify discrepancies in twisting of the wings. Twisting is seen for the D case but not for R.
Figure 12b compares the time series history of the wing
θtwist at 0.75 R of the IW and OW for the R and D model. This quantitatively shows that where twist deformation for the D model is quite high, the R model has essentially zero deformation. In addition, plots of the average wing kinematics are compared for the cases to demonstrate the effect that the rigid condition has on the stroke, deviation, and angle of incidence. Some differences in kinematics are expected; after all, the tip trajectory is impacted by the lack of bending and twisting (as seen in
Figure 12a).
Figure 12c compares the wing kinematics for the R and D model, with the solid lines representing the whole stroke mean for the six flapping motions, and the shaded regions representing ± 1 s.d. from the mean. The stroke and deviation remain relatively similar, with the largest stroke difference occurring at the stroke reversal. Force generation at the stroke reversal is low, as observed in
Figure 6, so we do not anticipate this leading to an extreme impact on the lift or drag forces. The AOI is quite different, as expected since the twisting angle of the wing directly impacts the computation of the LSRP on which the AOI calculation relies.
The R model force production is shown in
Figure 13. Major differences between R and the D model are clear. Referring to the lift, an extended period of lower lift during the upstroke for both wings is produced by the IW and OW for the R model, a trend that is not observed for the D model. The ∆
CL, shown in orange and green, show this difference as the D-R lift difference is shown to be quite large in the US (with the positive sign indicting D model lift is greater). This indicates that the wing deformations lead to more suitable generation of upwards lift throughout the turning motion to maintain a steady vertical elevation as observed in
Section 3.1. Additionally, the drag force produced by the IW and OW is more symmetric for throughout the six strokes in the maneuver. This contrasts the highly asymmetrical drag generation for phase I and II that is exhibited by the D model. Particularly during the US periods we observe that the D-R drag difference for the OW is quite large, with the negative sign indicating that the R model drag is greater. Where the OW drag generation is less on the D model, permitting the drag asymmetry-induced torque, the R model OW generates nearly the same amount of force as the IW. This reveals that flexibility helps the hummingbird to achieve the drag force asymmetry necessary to complete the desired free yaw turn.
Examining the surface pressure contour and pressure field at the time of maximum DS force production in stroke 3, along with the instantaneous drag force contours, some insight into the cause of the force symmetry is gained. The pressure field is visualized by iso-surfaces that indicate the non-dimensional pressure coefficient of −1.0.
Figure 14a,b depict this information for the R model while
Figure 14c,d are for the D model.
Firstly, it should be noted that the vortex formation, namely to dual-loop structures, is similar between both the rigid and flexible wing models. This is reflected by the generally close geometric organization of the pressure iso-surfaces for the R model in
Figure 14a and D model in
Figure 14c. As with
Figure 8 and
Figure 11, the VLs are labeled. Due to the lack of twist deformation, the IW for the R model exhibits a smaller region of low surface pressure in comparison to the D model. From the R and D model IW drag production in
Figure 14b,d, it is observed that the R model generates correspondingly lower drag.
The OW for both models demonstrate very similar surfaces pressure contours. Despite this, the R model drag production on the OW is considerably larger than that of the F model. Comparing the drag production contours for the OW in
Figure 14b,d, the R model has a concentration of drag production near the tip and relatively consistent, but lower, drag production across the rest of the span. The D model does not demonstrate this same trend; rather, the OW generates nearly no drag force at this particular time. The combination of weakened IW and increased OW drag production explains the general trend of the R model having more symmetric drag forces than the D model. Vortex formation between the rigid and twisting models is quite similar; however, wing flexibility helps to enhance the drag force asymmetry necessary to complete the maneuver in just six wingbeats (as with the D case) while also producing sufficient lift.