Effects of Dietary Lentinus edodes Fermentation Supplementation on Digestive Enzyme Activity, Antioxidant Capacity and Morphology of the Liver and Intestine in Largemouth Bass (Micropterus salmoides) Fed High Plant Protein Diets
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Experimental Diet
2.2. Experimental Fish and Samples Collection
2.3. Growth Performance
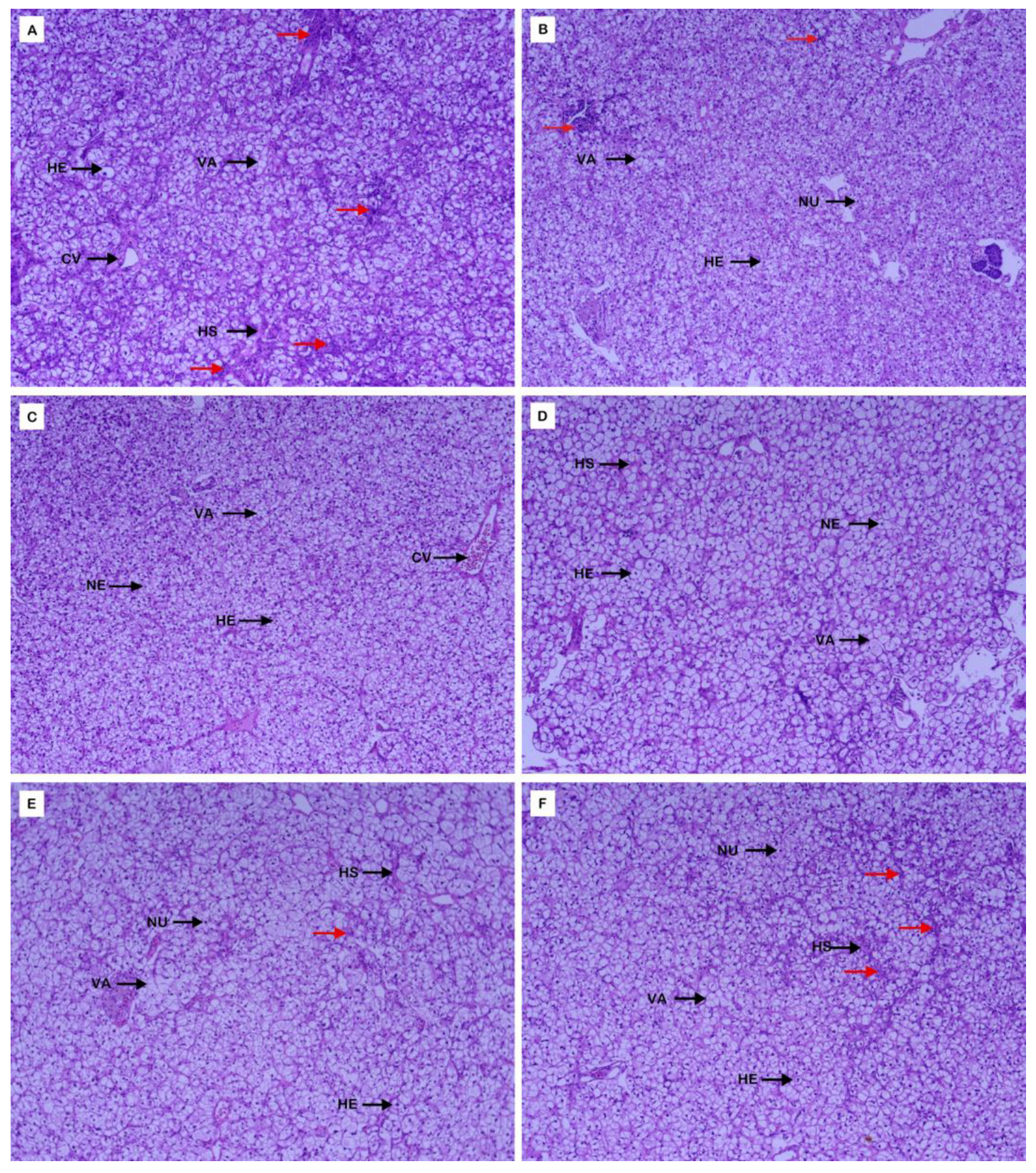
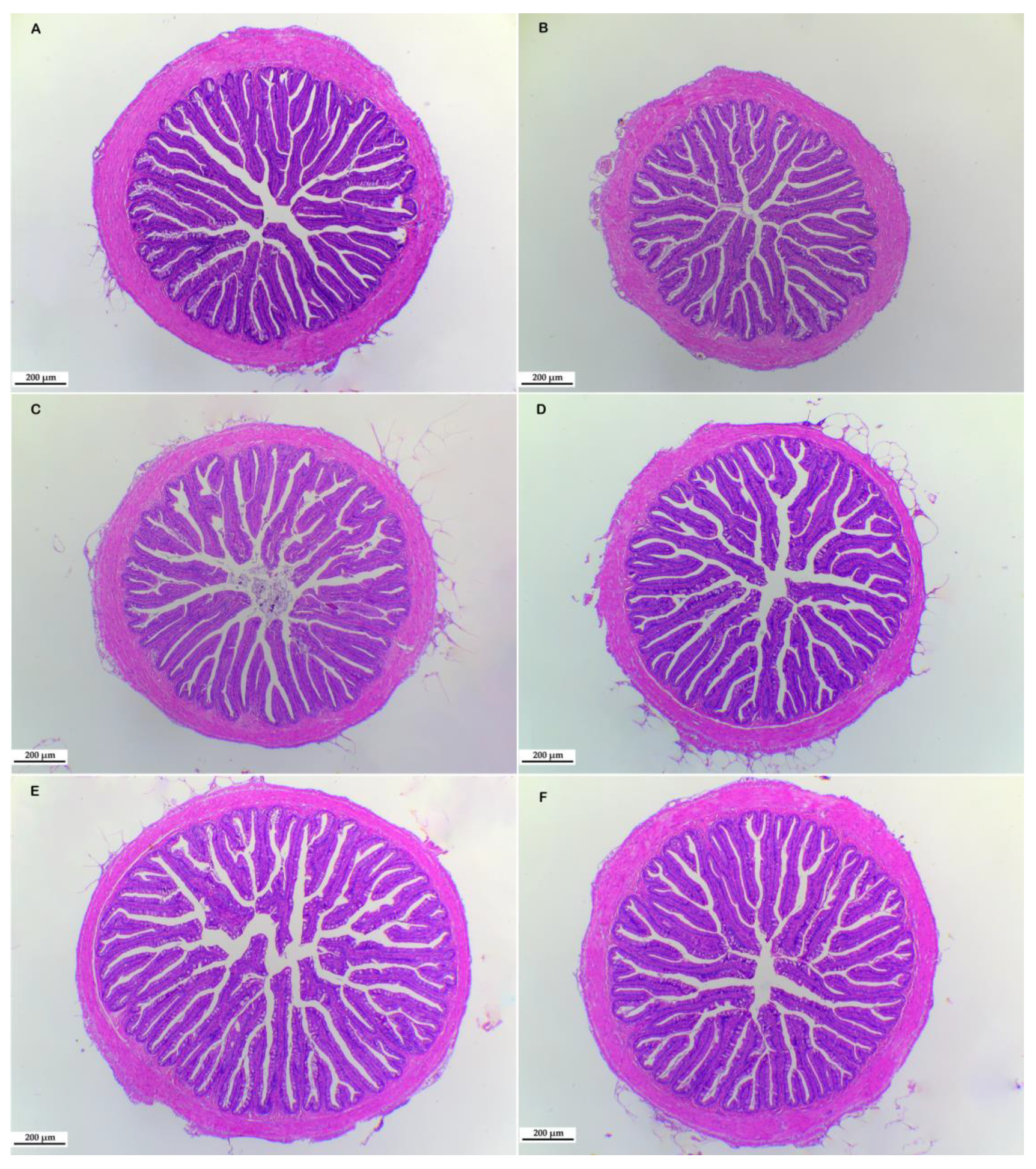
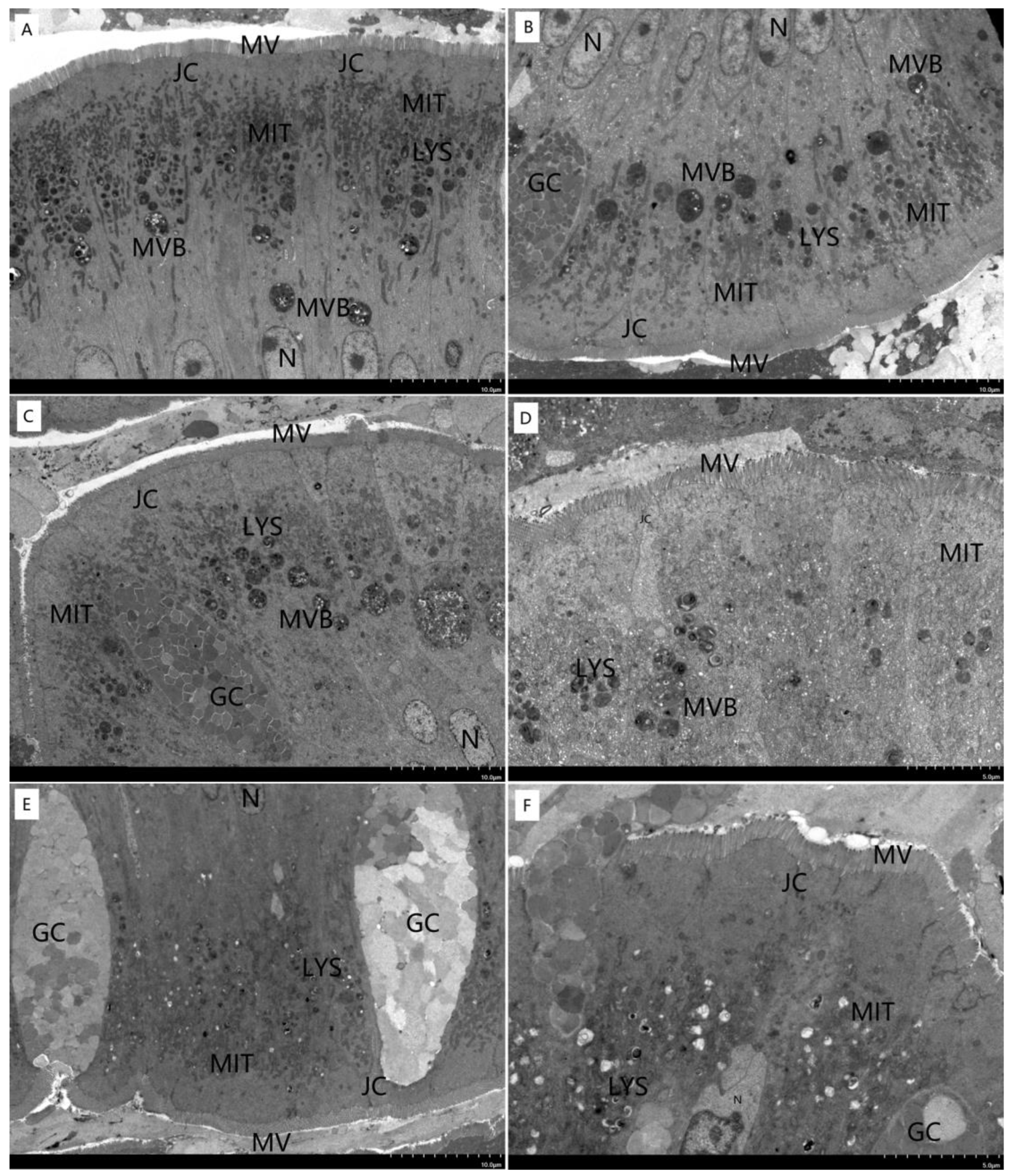
2.4. Morphology of Liver and Intestine
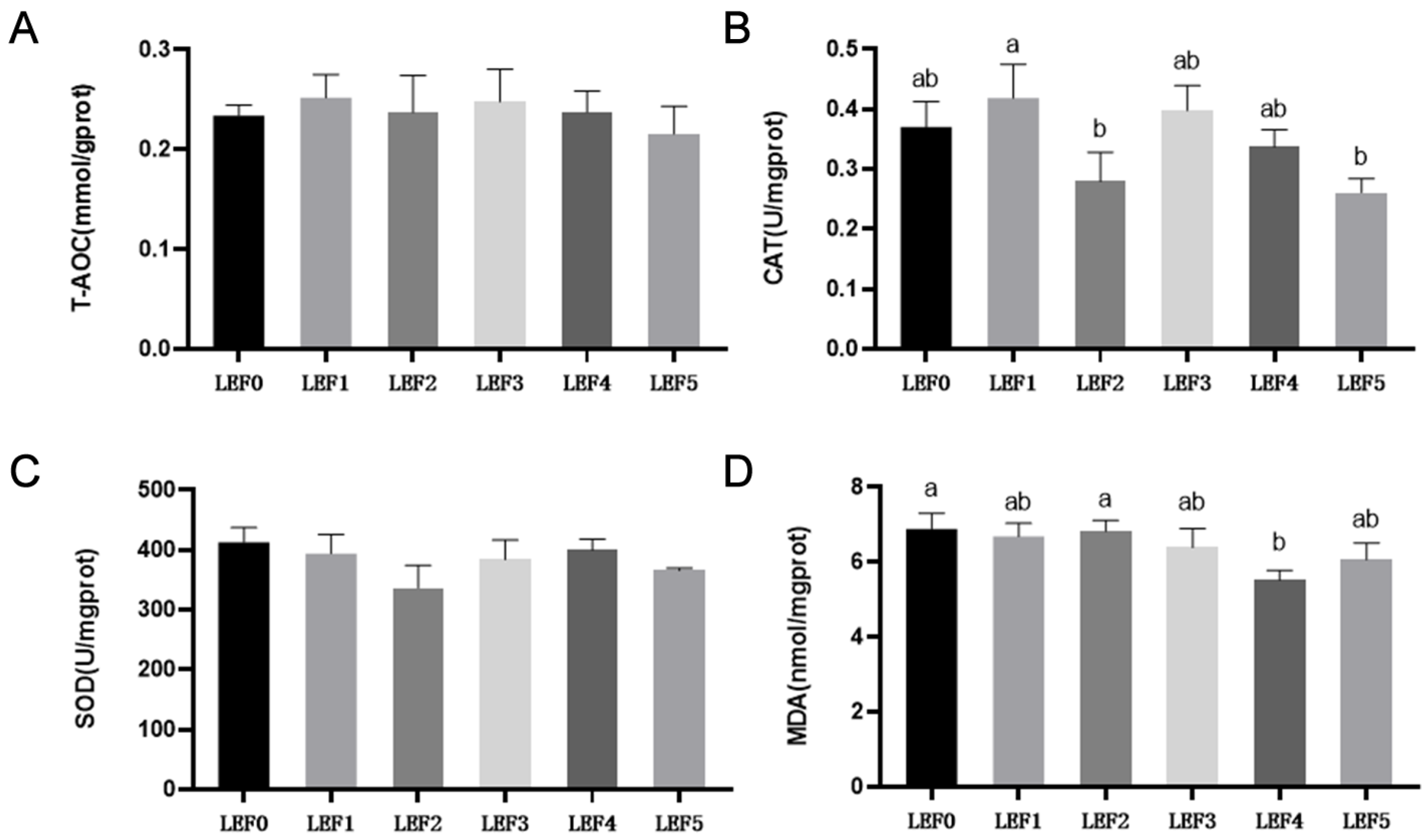
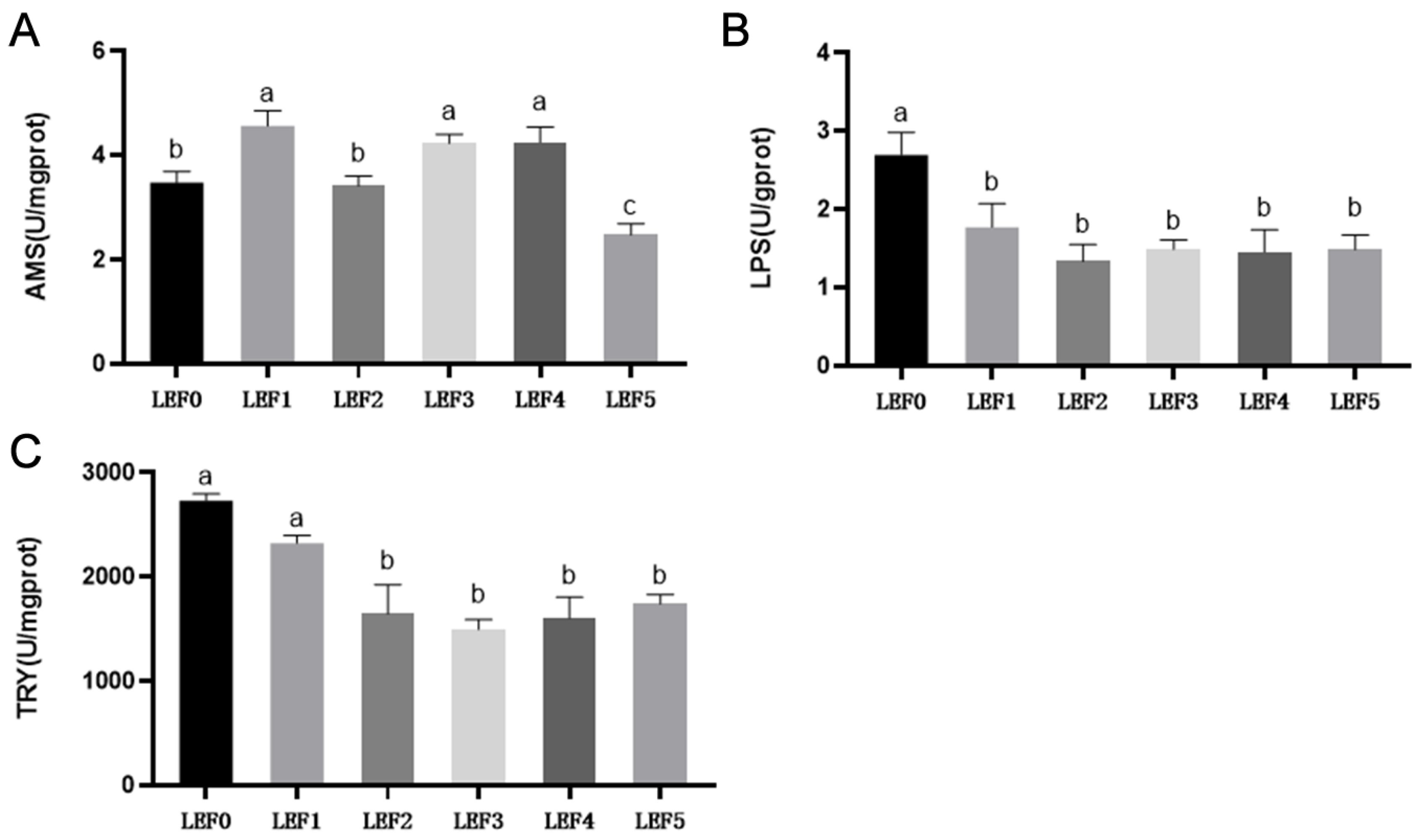
2.5. Activities of Digestive Enzymes and Antioxidant Enzymes
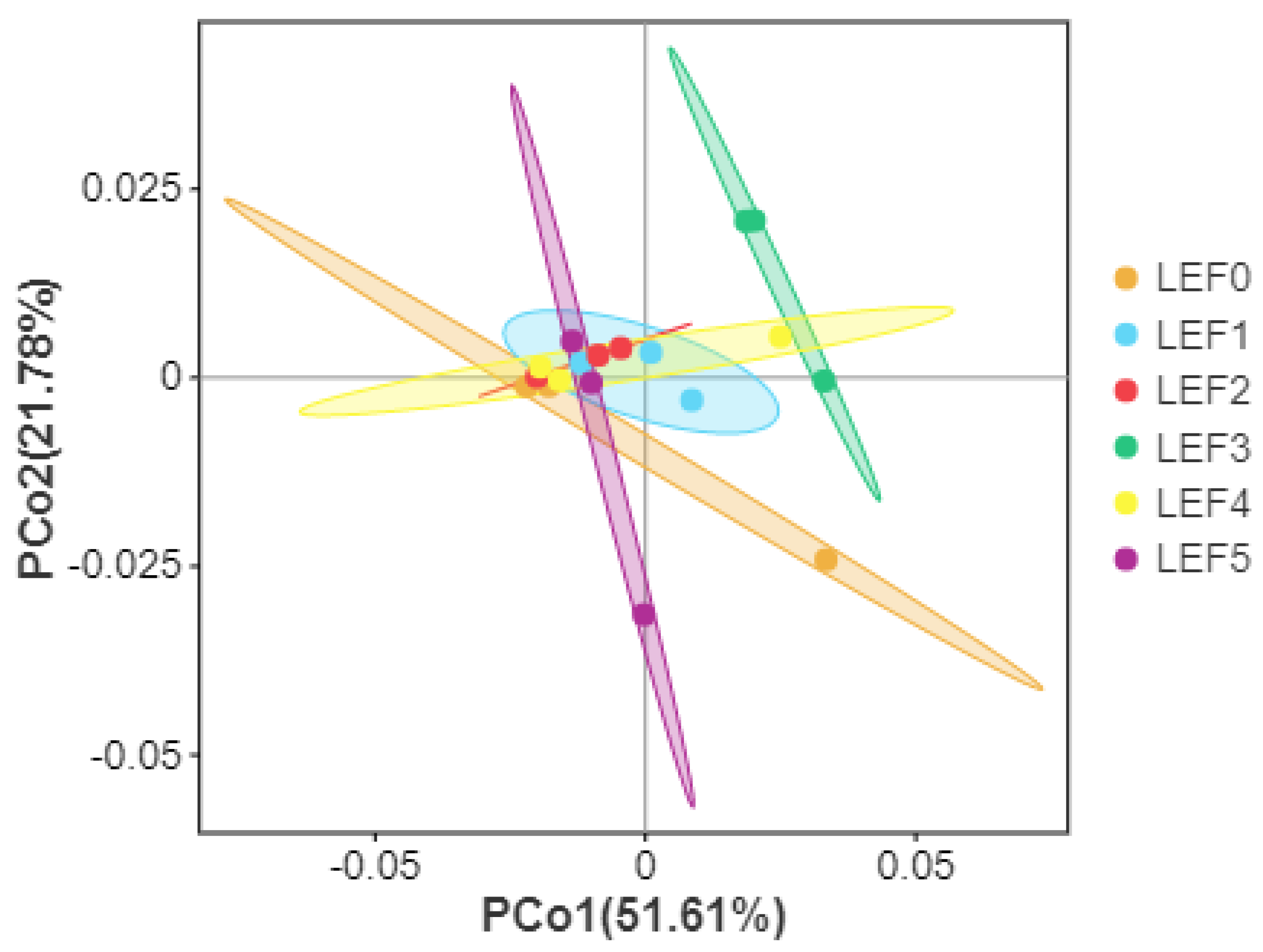
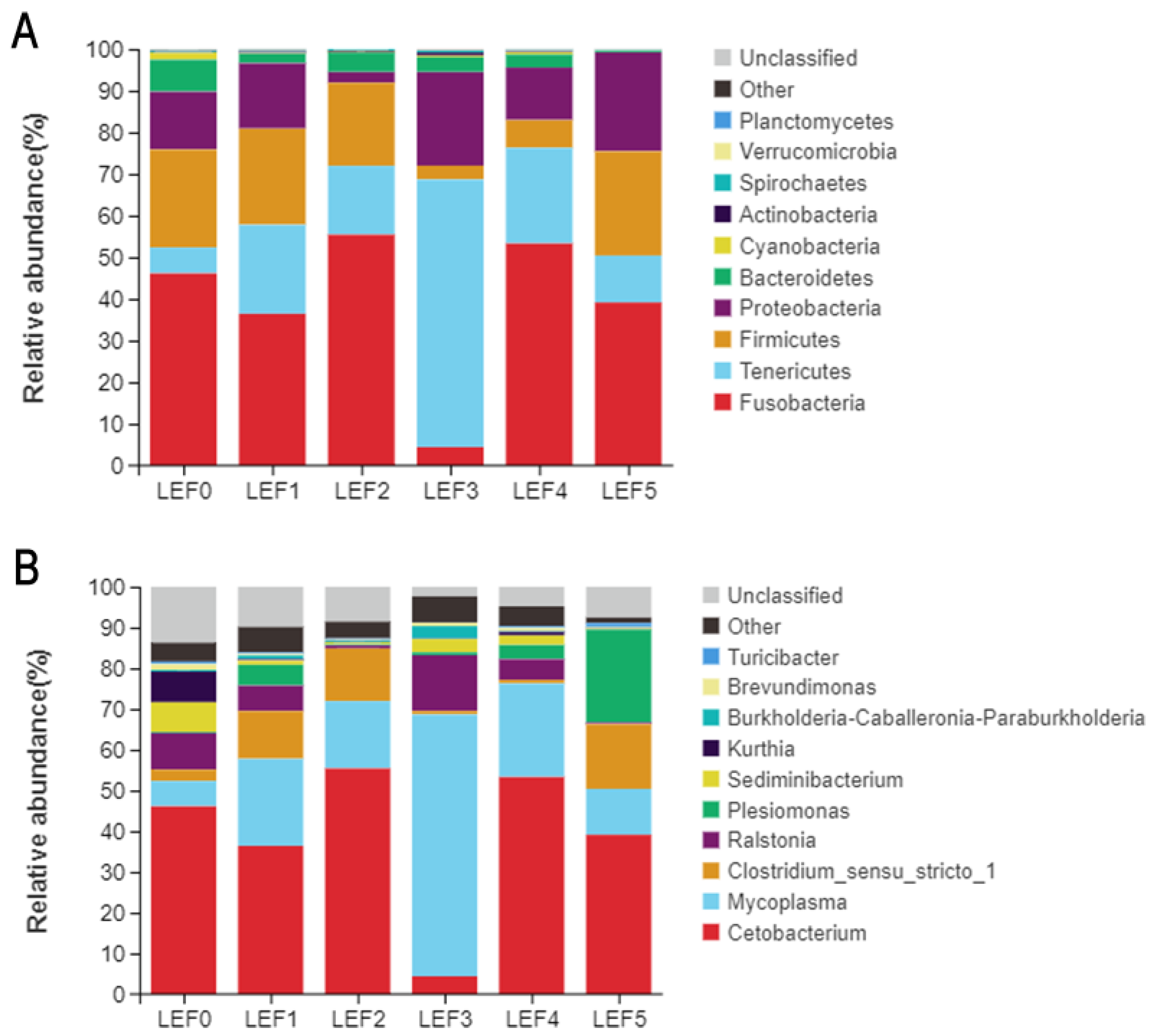
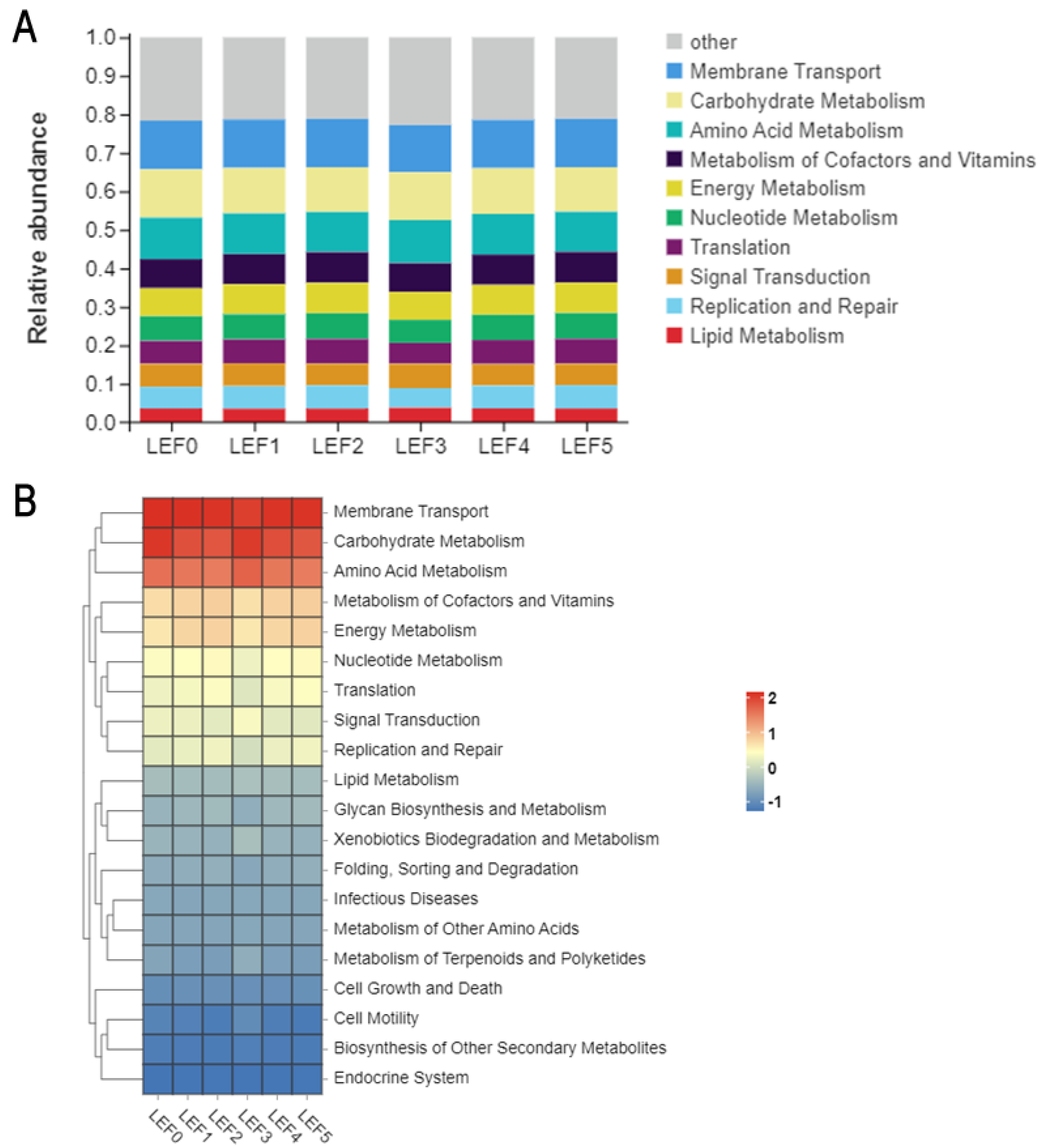
2.6. Intestinal Microbiota Analysis
2.7. Statistical Analysis
3. Results
3.1. Growth Properties
3.2. Antioxidant Capacity
3.3. Digestive Enzyme Activity
3.4. Morphological Structure of Liver and Intestine
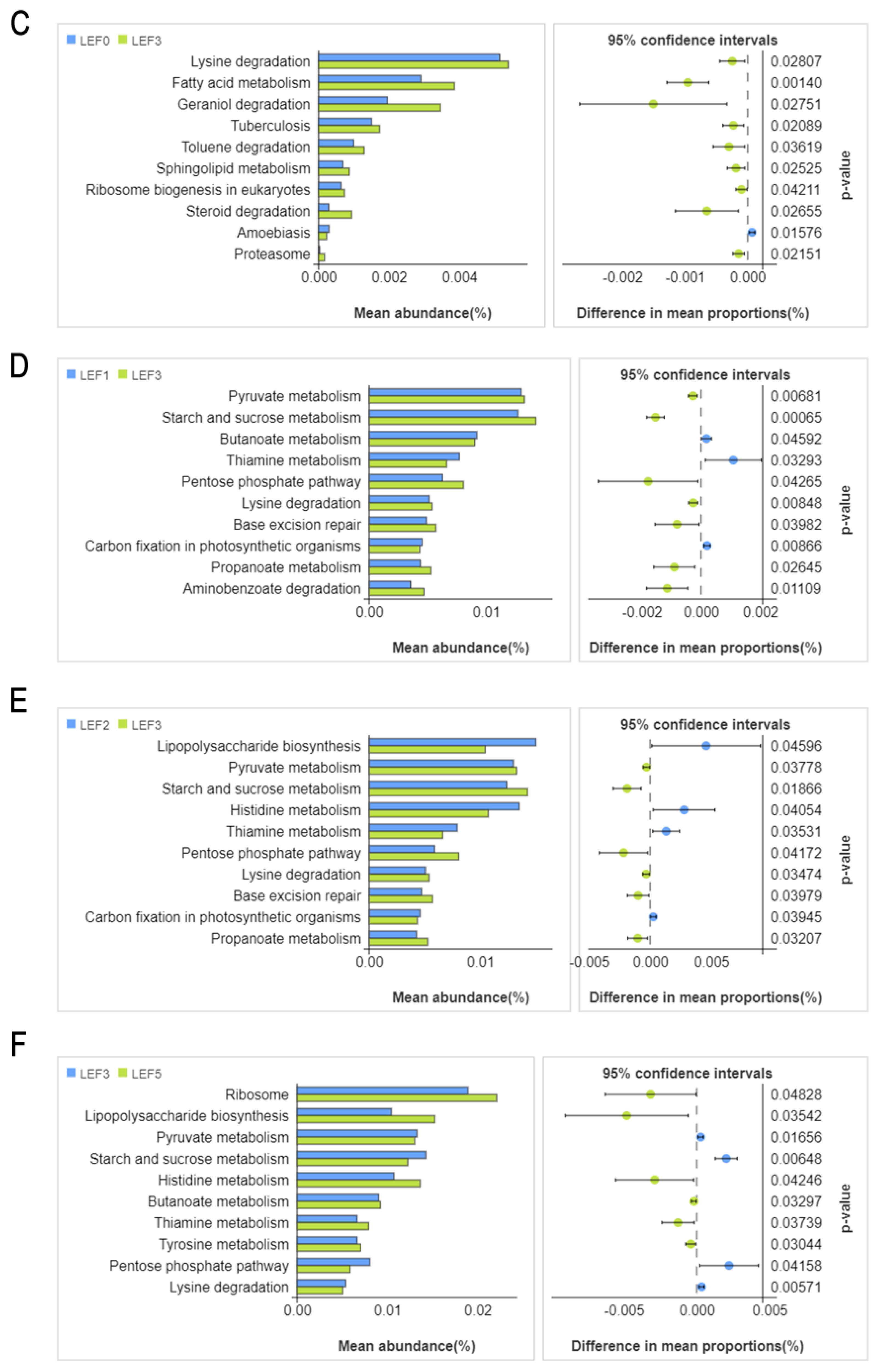
3.5. Intestinal Microbiota
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, M.; Yu, Y.; Li, X.; Poolsawat, L.; Yang, P.; Bian, Y.; Guo, Z.; Leng, X. An evaluation of replacing fish meal with fermented soybean meal in the diets of largemouth bass (Micropterus salmoides): Growth, nutrition utilization and intestinal histology. Aquac. Res. 2020, 51, 4302–4314. [Google Scholar] [CrossRef]
- Ma, Z.; Hassan, M.; Allais, L.; He, T.; Leterme, S.; Ellis, A.V.; McGraw, B.; Qin, J.G. Replacement of fishmeal with commercial soybean meal and EnzoMeal in juvenile barramundi Lates calcarifer. Aquac. Res. 2018, 49, 3258–3269. [Google Scholar] [CrossRef]
- Jannathulla, R.; Rajaram, V.; Kalanjiam, R.; Ambasankar, K.; Muralidhar, M.; Dayal, J.S. Fishmeal availability in the scenarios of climate change: Inevitability of fishmeal replacement in aquafeeds and approaches for the utilization of plant protein sources. Aquac. Res. 2019, 50, 3493–3506. [Google Scholar] [CrossRef]
- Kobayashi, M.; Msangi, S.; Batka, M.; Vannuccini, S.; Dey, M.M.; Anderson, J.L. Fish to 2030: The role and opportunity for aquaculture. Aquac. Econ. Manag. 2015, 19, 282–300. [Google Scholar] [CrossRef] [Green Version]
- Dawood, M.A.O.; Koshio, S. Application of fermentation strategy in aquafeed for sustainable aquaculture. Rev. Aquac. 2020, 12, 987–1002. [Google Scholar] [CrossRef]
- Jha, R.; Fouhse, J.M.; Tiwari, U.P.; Li, L.; Willing, B.P. Dietary Fiber and Intestinal Health of Monogastric Animals. Front. Vet. Sci. 2019, 6, 48. [Google Scholar] [CrossRef] [Green Version]
- Parma, L.; Candela, M.; Soverini, M.; Turroni, S.; Consolandi, C.; Brigidi, P.; Mandrioli, L.; Sirri, R.; Fontanillas, R.; Gatta, P.P.; et al. Next-generation sequencing characterization of the gut bacterial community of gilthead sea bream (Sparus aurata, L.) fed low fishmeal based diets with increasing soybean meal levels. Anim. Feed. Sci. Technol. 2016, 222, 204–216. [Google Scholar] [CrossRef]
- Liang, X.; Han, J.; Xue, M.; Yu, H.; Huang, H.; Wu, X.; Zheng, Y.; Qin, Y.; Liang, X. Growth and feed intake regulation responses to anorexia, adaptation and fasting in Japanese seabss, Lateolabrax japonicas when fishmeal is totally replaced by plant protein. Aquaculture 2019, 498, 528–538. [Google Scholar] [CrossRef]
- Zhu, R.; Duan, J.; Li, L.; Li, M.; Yu, Z.; Wang, H.; Quan, Y.; Wu, L. Effects of Substituting Fish Meal with Two Types of Soybean Meal on Growth, Enzyme Activities, and Intestinal Morphology in Juvenile Rhynchocypris lagowskii. N. Am. J. Aquac. 2021, 83, 267–282. [Google Scholar] [CrossRef]
- Wang, J.; Mai, K.; Ai, Q. Conventional soybean meal as fishmeal alternative in diets of Japanese Seabass (Lateolabrax japonicus): Effects of functional additives on growth, immunity, antioxidant capacity and disease resistance. Antioxidants 2022, 11, 951. [Google Scholar] [CrossRef] [PubMed]
- Novriadi, R.; Rhodes, M.; Powell, M.; Hanson, T.; Davis, D.A. Effects of soybean meal replacement with fermented soybean meal on growth, serum biochemistry and morphological condition of liver and distal intestine of Florida pompano Trachinotus carolinus. Aquac. Nutr. 2018, 24, 1066–1075. [Google Scholar] [CrossRef]
- Liu, H.; Jin, J.; Zhu, X.; Han, D.; Yang, Y.; Xie, S. Effect of substitution of dietary fish meal by soybean meal on different sizes of gibel carp (Carassius auratus gibelio): Digestive enzyme gene expressions and activities, and intestinal and hepatic histology. Aquac. Nutr. 2017, 23, 129–147. [Google Scholar] [CrossRef]
- Huang, D.; Wu, Y.; Lin, Y.; Chen, J.; Karrow, N.; Ren, X.; Wang, Y. Dietary Protein and Lipid Requirements for Juvenile Largemouth Bass, Micropterus salmoides. J. World Aquac. Soc. 2017, 48, 782–790. [Google Scholar] [CrossRef]
- Sampaio-Oliveira, A.; Cyrino, J. Digestibility of plant protein-based diets by largemouth bass Micropterus salmoides. Aquac. Nutr. 2008, 14, 318–323. [Google Scholar] [CrossRef]
- Ma, D.; Fan, J.; Tian, Y.; Jiang, P.; Wang, J.; Zhu, H.; Bai, J. Selection of reference genes for quantitative real-time PCR normalisation in largemouth bass Micropterus salmoides fed on alternative diets. J. Fish Biol. 2019, 95, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Amoah, A.; Coyle, S.D.; Webster, C.D.; Durborow, R.M.; Bright, L.A.; Tidwell, J.H. Effects of Graded Levels of Carbohydrate on Growth and Survival of Largemouth Bass, Micropterus salmoides. J. World Aquac. Soc. 2008, 39, 397–405. [Google Scholar] [CrossRef]
- Li, X.; Zheng, S.; Ma, X.; Cheng, K.; Wu, G. Use of alternative protein sources for fishmeal replacement in the diet of largemouth bass (Micropterus salmoides). Part I: Effects of poultry by-product meal and soybean meal on growth, feed utilization, and health. Amino Acids 2021, 53, 33–47. [Google Scholar] [CrossRef]
- Li, X.; Zheng, S.; Cheng, K.; Ma, X.; Wu, G. Use of alternative protein sources for fishmeal replacement in the diet of largemouth bass (Micropterus salmoides). Part II: Effects of supplementation with methionine or taurine on growth, feed utilization, and health. Amino Acids 2021, 53, 49–62. [Google Scholar] [CrossRef]
- Rossi, W., Jr.; Allen, K.M.; Habte-Tsion, H.-M.; Meesala, K.-M. Supplementation of glycine, prebiotic, and nucleotides in soybean meal-based diets for largemouth bass (Micropterus salmoides): Effects on production performance, whole-body nutrient composition and retention, and intestinal histopathology. Aquaculture 2021, 532, 736031. [Google Scholar] [CrossRef]
- Gaitán-Hernández, R.; López-Peña, D.; Esqueda, M.; Gutiérrez, A. Review of Bioactive Molecules Production, Biomass, and Basidiomata of Shiitake Culinary-Medicinal Mushrooms, Lentinus edodes (Agaricomycetes). Int. J. Med. Mushrooms 2019, 21, 841–850. [Google Scholar] [CrossRef]
- Bisen, P.; Baghel, R.; Sanodiya, B.; Thakur, G.; Prasad, G. Lentinus edodes: A Macrofungus with Pharmacological Activities. Curr. Med. Chem. 2010, 17, 2419–2430. [Google Scholar] [CrossRef] [PubMed]
- Conway, E.; Sweeney, T.; Dowley, A.; Maher, S.; Rajauria, G.; Yadav, S.; Wilson, J.; Gabrielli, W.; O’doherty, J.V. The effects of mushroom powder and vitamin D2-enriched mushroom powder supplementation on the growth performance and health of newly weaned pigs. J. Anim. Physiol. Anim. Nutr. 2022, 106, 517–527. [Google Scholar] [CrossRef]
- Van Nevel, C.J.; Decuypere, J.A.; Dierick, N.; Molly, K. The influence of Lentinus edodes (Shiitake mushroom) preparations on bacteriological and morphological aspects of the small intestine in piglets. Arch. Anim. Nutr. 2003, 57, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Li, D.; Chang, B.; Gong, L.; Piao, X.; Yi, G.; Zhang, J. Effects of lentinan on broiler splenocyte proliferation, interleukin-2 production, and signal transduction. Poult. Sci. 2003, 82, 760–766. [Google Scholar] [CrossRef]
- Dong, L.; Zhang, H.; Gao, Y.; Diao, Q. Dynamic profiles of fermentation characteristics and bacterial community composition of Broussonetia papyrifera ensiled with perennial ryegrass. Bioresour. Technol. 2020, 310, 123396. [Google Scholar] [CrossRef]
- Spim, S.R.V.; de Oliveira, B.G.C.C.; Leite, F.G.; Gerenutti, M.; Grotto, D. Effects of Lentinula edodes consumption on biochemical, hematologic and oxidative stress parameters in rats receiving high-fat diet. Eur. J. Nutr. 2017, 56, 2255–2264. [Google Scholar] [CrossRef] [PubMed]
- Cahu, C.; Rønnestad, I.; Grangier, V.; Infante, J.Z. Expression and activities of pancreatic enzymes in developing sea bass larvae (Dicentrarchus labrax) in relation to intact and hydrolyzed dietary protein; involvement of cholecystokinin. Aquaculture 2004, 238, 295–308. [Google Scholar] [CrossRef] [Green Version]
- Amiri, O.; Miandare, H.K.; Hoseinifar, S.H.; Shabni, A.; Safari, R. Skin mucus protein profile, immune parameters, immune-related gene expression, and growth performance of rainbow rrout (Oncorhynchus mykiss) fed white button mushroom (Agaricus bisporus) powder. Int. J. Med. Mushrooms 2018, 20, 337–347. [Google Scholar] [CrossRef]
- Song, X.; Feng, Z.; Tan, J.; Wang, Z.; Zhu, W. Dietary administration of Pleurotus ostreatus polysaccharides (POPS) modulates the non-specific immune response and gut microbiota diversity of Apostichopus japonicus. Aquac. Rep. 2021, 19, 100578. [Google Scholar] [CrossRef]
- Chang, S.-C.; Lin, M.-J.; Chao, Y.-P.; Chiang, C.-J.; Jea, Y.-S.; Lee, T.-T. Effects of spent mushroom compost meal on growth performance and meat characteristics of grower geese. Rev. Bras. de Zootec. 2016, 45, 281–287. [Google Scholar] [CrossRef] [Green Version]
- Mohan, K.; Padmanaban, A.; Uthayakumar, V.; Chandirasekar, R.; Muralisankar, T.; Santhanam, P. Effect of dietary Ganoderma lucidum polysaccharides on biological and physiological responses of the giant freshwater prawn Macrobrachium rosenbergii. Aquaculture 2016, 464, 42–49. [Google Scholar] [CrossRef]
- Van Doan, H.; Hoseinifar, S.H.; Tapingkae, W.; Chitmanat, C.; Mekchay, S. Effects of Cordyceps militaris spent mushroom substrate on mucosal and serum immune parameters, disease resistance and growth performance of Nile tilapia, (Oreochromis niloticus). Fish Shellfish. Immunol. 2017, 67, 78–85. [Google Scholar] [CrossRef]
- Boontiam, W.; Wachirapakorn, C.; Phaengphairee, P.; Wattanachai, S. Effect of spent mushroom (Cordyceps militaris) on growth performance, immunity, and intestinal microflora in weaning pigs. Animals 2020, 10, 2360. [Google Scholar] [CrossRef] [PubMed]
- Qi, Q.; Peng, Q.; Tang, M.; Chen, D.; Zhang, H. Microbiome Analysis Investigating the Impacts of Fermented Spent Mushroom Substrates on the Composition of Microbiota in Weaned Piglets Hindgut. Front. Veter-Sci. 2020, 7, 584243. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.D.; Borges, V.D.; Rosa, C.E.; Colares, E.P.; Robaldo, R.B.; Martinez, P.E.; Bianchini, A. Effects of increasing temperature on antioxidant defense system and oxidative stress parameters in the Antarctic fish Notothenia coriiceps and Notothenia rossii. J. Therm. Biol. 2017, 68, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Investig. 2017, 114, 1752–1761. [Google Scholar] [CrossRef]
- Bosoi, C.R.; Rose, C.F. Oxidative stress: A systemic factor implicated in the pathogenesis of hepatic encephalopathy. Metab. Brain Dis. 2013, 28, 175–178. [Google Scholar] [CrossRef] [Green Version]
- Cichoż-Lach, H.; Michalak, A. Oxidative stress as a crucial factor in liver diseases. World J. Gastroenterol. WJG 2014, 20, 8082. [Google Scholar] [CrossRef]
- Liu, H.; Yang, Q.; Gao, Z.; Zhu, Y.; Zhang, J.; Jia, L. The Antioxidative, Anti-inflammatory, and Liver-Protective Effects of Mycelia Selenium Polysaccharides from the Deep Root Mushroom, Oudemansiella radicata (Agaricomycetes). Int. J. Med. Mushrooms 2019, 21, 1019–1031. [Google Scholar] [CrossRef]
- Miller, A.-F. Superoxide dismutases: Active sites that save, but a protein that kills. Curr. Opin. Chem. Biol. 2004, 8, 162–168. [Google Scholar] [CrossRef]
- Ezraty, B.; Gennaris, A.; Barras, F.; Collet, J.F. Oxidative stress, protein damage and repair in bacteria. Nat. Rev. Microbiol. 2017, 15, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Kirkman, H.N.; Gaetani, G.F. Mammalian catalase: A venerable enzyme with new mysteries. Trends Biochem. Sci. 2007, 32, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Mahfuz, S.; He, T.; Ma, J.; Liu, H.; Long, S.; Shang, Q.; Zhang, L.; Yin, J.; Piao, X. Mushroom (Flammulina velutipes) stem residue on growth performance, meat quality, antioxidant status and lipid metabolism of broilers. Ital. J. Anim. Sci. 2020, 19, 803–812. [Google Scholar] [CrossRef]
- Drori, A.; Shabat, Y.; Ben Ya’Acov, A.; Danay, O.; Levanon, D.; Zolotarov, L.; Ilan, Y. Extracts from Lentinula edodes (Shiitake) Edible Mushrooms Enriched with Vitamin D Exert an Anti-Inflammatory Hepatoprotective Effect. J. Med. Food 2016, 19, 383–389. [Google Scholar] [CrossRef]
- Kuang, Z.; Jin, T.; Wu, C.; Zong, Y.; Yin, P.; Dong, W.; Lin, X.; You, W.; Zhang, C.; Wang, L.; et al. Lentinan Attenuates Damage of the Small Intestinal Mucosa, Liver, and Lung in Mice with Gut-Origin Sepsis. J. Immunol. Res. 2021, 2021, 2052757. [Google Scholar] [CrossRef]
- Sun, W.; Feng, Y.; Zhang, M.; Song, X.; Jia, L. Protective effects of sulfated polysaccharides from Lentinula edodes on the lung and liver of MODS mice. Food Funct. 2021, 12, 6389–6402. [Google Scholar] [CrossRef] [PubMed]
- Hamza, R.G.; El Shahat, A.; Al-Seeni, M. Studying the antioxidant role of mushroom against hazards of gamma radiation exposure in male rats. Indian J. Anim. Res. 2018, 52, 226–231. [Google Scholar] [CrossRef]
- Dogan, A.; Dalar, A.; Sadullahoglu, C.; Battal, A.; Uzun, Y.; Celik, I.; Demirel, K. Investigation of the protective effects of horse mushroom (Agaricus arvensis Schaeff.) against carbon tetrachloride-induced oxidative stress in rats. Mol. Biol. Rep. 2018, 45, 787–797. [Google Scholar] [CrossRef] [PubMed]
- Chuang, W.Y.; Lin, L.J.; Der Shih, H.; Shy, Y.M.; Chang, S.C.; Lee, T.T. Intestinal Microbiota, Anti-Inflammatory, and Anti-Oxidative Status of Broiler Chickens Fed Diets Containing Mushroom Waste Compost By-Products. Animals 2021, 11, 2550. [Google Scholar] [CrossRef]
- Zhao, H.; Lan, Y.; Liu, H.; Zhu, Y.; Liu, W.; Zhang, J.; Jia, L. Antioxidant and Hepatoprotective Activities of Polysaccharides from Spent Mushroom Substrates (Laetiporus sulphureus) in Acute Alcohol-Induced Mice. Oxidative Med. Cell. Longev. 2017, 2017, 5863523. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Zheng, M.; Zhou, M.; Zhou, L.; Ge, X.; Pang, N.; Li, H.; Li, X.; Li, M.; Zhang, J.; et al. Lentinan Supplementation Protects the Gut–Liver Axis and Prevents Steatohepatitis: The Role of Gut Microbiota Involved. Front. Nutr. 2022, 8, 803691. [Google Scholar] [CrossRef]
- Du, T.; Fang, Q.; Zhang, Z.; Zhu, C.; Xu, R.; Chen, G.; Wang, Y. Lentinan Protects against Nonalcoholic Fatty Liver Disease by Reducing Oxidative Stress and Apoptosis via the PPARα Pathway. Metabolites 2022, 12, 55. [Google Scholar] [CrossRef] [PubMed]
- Uluköy, G.; Baba, E.; Birincioğlu, S.S. Effects of dietary lentinula edodes extract on liver and gut histology of rainbow trout (Oncorhynchus mykiss, Walbaum 1792). Fish Shellfish. Immunol. 2016, 53, 106. [Google Scholar] [CrossRef]
- Song, X.; Cai, W.; Ren, Z.; Jia, L.; Zhang, J. Antioxidant and Hepatoprotective Effects of Acidic-Hydrolysis Residue Polysaccharides from Shiitake Culinary-Medicinal Mushroom Lentinus edodes (Agaricomycetes) in Mice. Int. J. Med. Mushrooms 2021, 23, 85–96. [Google Scholar] [CrossRef]
- Hertli, S.; Zimmermann, P. Molecular interactions between the intestinal microbiota and the host. Mol. Microbiol. 2022, 117, 1297–1307. [Google Scholar] [CrossRef] [PubMed]
- Giannenas, I.; Tontis, D.; Tsalie, E.; Chronis, E.; Doukas, D.; Kyriazakis, I. Influence of dietary mushroom Agaricus bisporus on intestinal morphology and microflora composition in broiler chickens. Res. Veter-Sci. 2010, 89, 78–84. [Google Scholar] [CrossRef]
- Giannenas, I.; Tsalie, E.; Chronis, E.; Mavridis, S.; Tontis, D.; Kyriazakis, I. Consumption of Agaricus bisporus mushroom affects the performance, intestinal microbiota composition and morphology, and antioxidant status of turkey poults. Anim. Feed. Sci. Technol. 2011, 165, 218–229. [Google Scholar] [CrossRef]
- Guo, P.; Zhang, K.; Ma, X.; He, P. Clostridium species as probiotics: Potentials and challenges. J. Anim. Sci. Biotechnol. 2020, 11, 1–10. [Google Scholar] [CrossRef]
- Siddiqui, M.T.; Cresci, G.A.M. The Immunomodulatory Functions of Butyrate. J. Inflamm. Res. 2021, 14, 6025–6041. [Google Scholar] [CrossRef]
- Jesus, G.F.A.; Owatari, M.S.; Pereira, S.A.; Silva, B.C.; Syracuse, N.M.; Lopes, G.R.; Addam, K.; Cardoso, L.; Mouriño, J.L.P.; Martins, M.L. Effects of sodium butyrate and Lippia origanoides essential oil blend on growth, intestinal microbiota, histology, and haemato-immunological response of Nile tilapia. Fish Shellfish. Immunol. 2021, 117, 62–69. [Google Scholar] [CrossRef]
- Zeng, X.; Yang, Y.; Wang, J.; Wang, Z.; Li, J.; Yin, Y.; Yang, H. Dietary butyrate, lauric acid and stearic acid improve gut morphology and epithelial cell turnover in weaned piglets. Anim. Nutr. 2022, 11, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Roberfroid, M.B. Dietary Modulation of the Human Colonic Microbiota: Introducing the Concept of Prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [CrossRef]
- Moreau, M.C.; Coste, M. Immune responses to dietary protein antigens. World Rev. Nutr. Diet. 1993, 74, 22–57. [Google Scholar] [PubMed]
- Sharma, R.; Schumacher, U.; Ronaasen, V.; Coates, M. Rat intestinal mucosal responses to a microbial flora and different diets. Gut 1995, 36, 209–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamann, L.; El-Samalouti, V.; Ulmer, A.J.; Flad, H.-D.; Rietschel, E.T. Components of gut bacteria as immunomodulators. Int. J. Food Microbiol. 1998, 41, 141–154. [Google Scholar] [CrossRef]



| Material | LEF0 | LEF1 | LEF2 | LEF3 | LEF4 | LEF5 |
|---|---|---|---|---|---|---|
| fish meal | 280.0 | 280.0 | 280.0 | 280.0 | 280.0 | 280.0 |
| Soybean protein concentrate | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| Corn gluten meal | 140.0 | 140.0 | 140.0 | 140.0 | 140.0 | 140.0 |
| Soybean meal | 78.0 | 78.0 | 78.0 | 78.0 | 78.0 | 78.0 |
| Peanut bran | 60.0 | 60.0 | 60.0 | 60.0 | 60.0 | 60.0 |
| flour | 205.9 | 205.9 | 205.9 | 205.9 | 205.9 | 205.9 |
| Beer yeast powder | 50.0 | 50.0 | 50.0 | 50.0 | 50.0 | 50.0 |
| fish oil | 51.0 | 51.0 | 51.0 | 51.0 | 51.0 | 51.0 |
| lecithin | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 |
| Vitamin and mineral premix 1 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 |
| Choline chloride (50%) | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 |
| antioxidant | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Calcium dihydrogen phosphate | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 |
| Mushroom leftover fermentation product 2 | 0 | 10 | 20 | 30 | 40 | 50 |
| Proximate composition (% dry matter) | ||||||
| crude protein | 46.05 | 46.15 | 46.26 | 46.36 | 46.46 | 46.57 |
| crude lipid | 10.08 | 10.09 | 10.10 | 10.11 | 10.13 | 10.14 |
| Ash | 7.07 | 7.12 | 7.17 | 7.22 | 7.27 | 7.32 |
| Gross energy (MJ kg−1) 3 | 18.40 | 18.43 | 18.46 | 18.48 | 18.51 | 18.54 |
| Indexes | LEF0 | LEF1 | LEF2 | LEF3 | LEF4 | LEF5 |
|---|---|---|---|---|---|---|
| IBW (g) | 28.8 ± 0.04 | 28.8 ± 0.04 | 28.8 ± 0.04 | 28.8 ± 0.04 | 28.8 ± 0.04 | 28.8 ± 0.04 |
| FBW (g) | 64.58 ± 3.09 ab | 62.99 ± 2.21 ab | 68.24 ± 2.44 a | 64.51 ± 1.45 ab | 59.82 ± 3.35 b | 64.04 ± 1.82 ab |
| WGR (%) | 123.99 ± 10.70 ab | 118.49 ± 7.65 ab | 136.74 ± 8.43 a | 123.77 ± 5.01 ab | 107.50 ± 11.63 b | 122.13 ± 6.32 ab |
| SGR (%day−1) | 1.34 ± 0.08 ab | 1.30 ± 0.06 ab | 1.43 ± 0.06 a | 1.34 ± 0.04 ab | 1.21 ± 0.09 b | 1.33 ± 0.05 ab |
| FCR | 1.45 ± 0.14 | 1.37 ± 0.06 | 1.42 ± 0.11 | 1.29 ± 0.02 | 1.40 ± 0.05 | 1.40 ± 0.06 |
| CF (%) | 1.85 ± 0.03 ab | 1.84 ± 0.02 ab | 1.88 ± 0.03 ab | 1.93 ± 0.03 a | 1.84 ± 0.05 ab | 1.82 ± 0.03 b |
| VSI (%) | 5.57 ± 0.09 | 5.18 ± 0.17 | 5.37 ± 0.13 | 5.36 ± 0.17 | 5.31 ± 0.14 | 5.53 ± 0.11 |
| HSI (%) | 1.48 ± 0.07 | 1.38 ± 0.07 | 1.52 ± 0.07 | 1.52 ± 0.09 | 1.52 ± 0.09 | 1.54 ± 0.06 |
| SR (%) | 89.38 ± 2.77 | 90.63 ± 0.63 | 90.00 ± 5.20 | 91.88 ± 1.57 | 91.25 ± 2.39 | 88.75 ± 2.39 |
| Indexes | LEF0 | LEF1 | LEF2 | LEF3 | LEF4 | LEF5 |
|---|---|---|---|---|---|---|
| Villus number | 27.75 ± 0.85 | 28.25 ± 1.97 | 29.00 ± 2.27 | 28.25 ± 1.71 | 29.75 ± 1.49 | 30.25 ± 0.62 |
| Muscularis (um) | 106.08 ± 4.12 b | 103.51 ± 8.93 b | 114.79 ± 7.27 ab | 104.18 ± 5.91 b | 116.48 ± 7.68 ab | 128.64 ± 5.15 a |
| Goblet cell number | 11.78 ± 1.42 b | 15.11 ± 1.23 ab | 13.89 ± 2.87 b | 14.72 ± 1.68 ab | 15.28 ± 2.252 ab | 20.25 ± 1.42 a |
| Villus length (um) | 613.75 ± 17.67 ab | 634.52 ± 30.79 a | 597.31 ± 24.78 ab | 583.57 ± 15.44 ab | 536.88 ± 32.68 b | 636.95 ± 23.53 a |
| Diets | Sob | Chao1 | ACE | Shannon | Simpson | Goods-Coverage |
|---|---|---|---|---|---|---|
| LEF0 | 255.00 abc | 341.48 | 321.34 | 2.30 | 0.59 | 99.94 |
| LEF1 | 321.00 ab | 376.04 | 389.52 | 2.96 | 0.74 | 99.94 |
| LEF2 | 217.67 bc | 285.29 | 279.94 | 1.91 | 0.55 | 99.94 |
| LEF3 | 226.00 abc | 297.42 | 315.24 | 2.13 | 0.52 | 99.94 |
| LEF4 | 334.67 a | 396.91 | 381.50 | 2.14 | 0.53 | 99.94 |
| LEF5 | 207.00 c | 283.75 | 281.52 | 1.81 | 0.59 | 99.94 |
| Phylum | LEF0 | LEF1 | LEF2 | LEF3 | LEF4 | LEF5 |
|---|---|---|---|---|---|---|
| Fusobacteria | 46.13 | 36.43 | 55.43 | 4.41 | 53.31 | 39.14 |
| Tenericutes | 6.21 | 21.34 | 16.53 | 64.25 | 22.95 | 11.27 |
| Firmicutes | 23.52 | 23.10 | 19.94 | 3.33 | 6.65 | 25.02 |
| Proteobacteria | 13.87 | 15.70 | 2.57 | 22.46 | 12.64 | 23.76 |
| Bacteroidetes | 7.69 | 2.28 | 4.65 | 3.58 | 3.11 | 0.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Yu, Z.; Liu, G.; Li, S.; Zhou, G.; Wang, H.; Dong, Y.; You, C.; Bai, W.; Zhou, M.; et al. Effects of Dietary Lentinus edodes Fermentation Supplementation on Digestive Enzyme Activity, Antioxidant Capacity and Morphology of the Liver and Intestine in Largemouth Bass (Micropterus salmoides) Fed High Plant Protein Diets. Fishes 2023, 8, 329. https://doi.org/10.3390/fishes8060329
Xu J, Yu Z, Liu G, Li S, Zhou G, Wang H, Dong Y, You C, Bai W, Zhou M, et al. Effects of Dietary Lentinus edodes Fermentation Supplementation on Digestive Enzyme Activity, Antioxidant Capacity and Morphology of the Liver and Intestine in Largemouth Bass (Micropterus salmoides) Fed High Plant Protein Diets. Fishes. 2023; 8(6):329. https://doi.org/10.3390/fishes8060329
Chicago/Turabian StyleXu, Jiaming, Zhoulin Yu, Guangye Liu, Sijie Li, Guoyong Zhou, Hanhua Wang, Yewei Dong, Cuihong You, Weidong Bai, Meng Zhou, and et al. 2023. "Effects of Dietary Lentinus edodes Fermentation Supplementation on Digestive Enzyme Activity, Antioxidant Capacity and Morphology of the Liver and Intestine in Largemouth Bass (Micropterus salmoides) Fed High Plant Protein Diets" Fishes 8, no. 6: 329. https://doi.org/10.3390/fishes8060329
APA StyleXu, J., Yu, Z., Liu, G., Li, S., Zhou, G., Wang, H., Dong, Y., You, C., Bai, W., Zhou, M., Huang, Y., & Tan, X. (2023). Effects of Dietary Lentinus edodes Fermentation Supplementation on Digestive Enzyme Activity, Antioxidant Capacity and Morphology of the Liver and Intestine in Largemouth Bass (Micropterus salmoides) Fed High Plant Protein Diets. Fishes, 8(6), 329. https://doi.org/10.3390/fishes8060329







