Digestion and Absorption of Dietary Phosphorus in Fish
Abstract
:1. Digestion of Dietary Phosphorus
1.1. Digestibility and Related Factors
1.2. Gastric Acid and Bone P
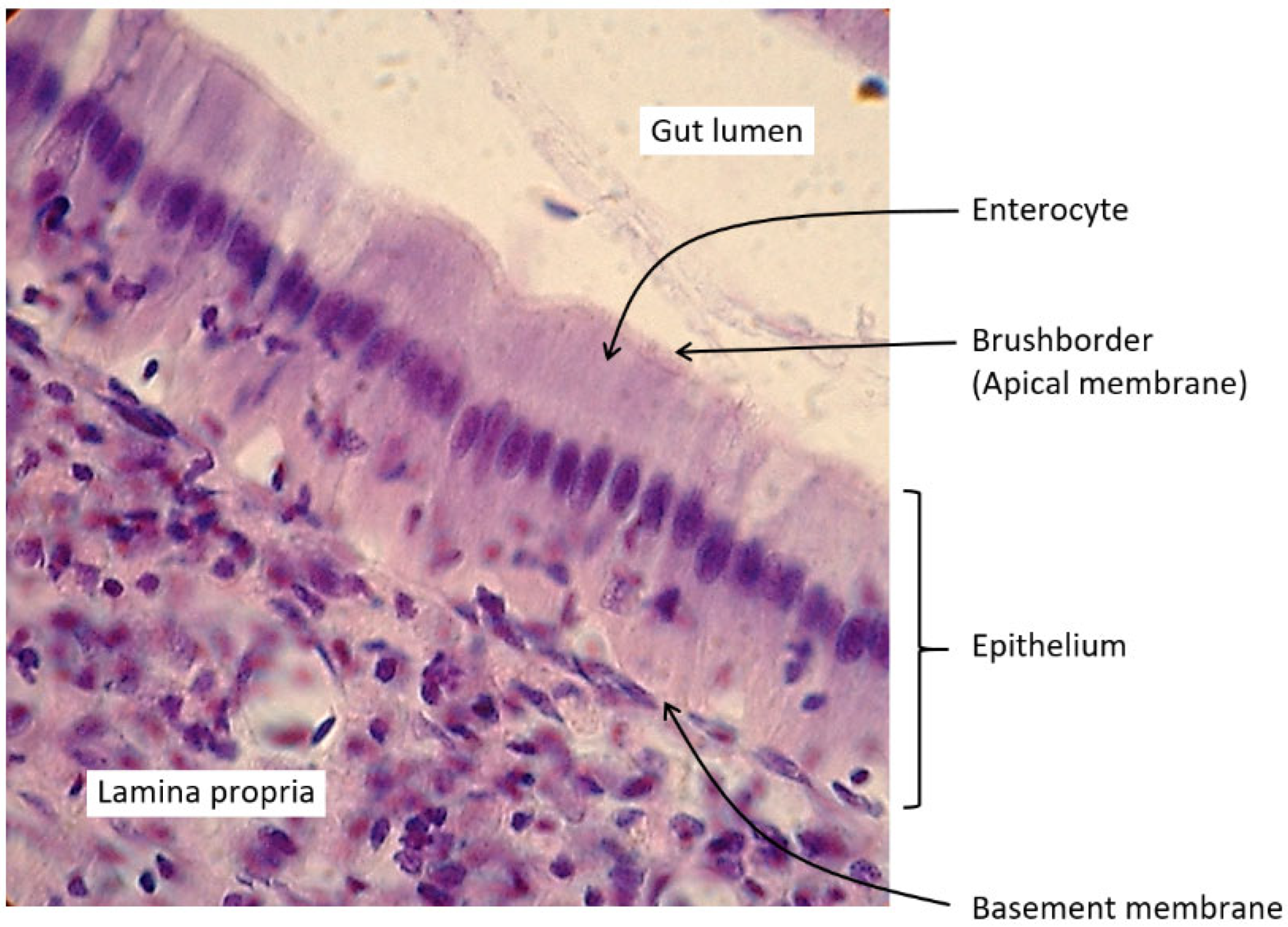
1.3. Intestinal Digestion
1.4. Phytates
1.5. P-Binders
2. Absorption of Dietary Phosphorus
2.1. Physiology of Pi Transport in the Intestine
2.2. Active Pi Transport
2.3. NaPi Transporters
2.4. Tissue Distribution of NaPi
2.5. Dietary Regulation of NaPi
2.6. Paracellular Diffusion and Related Factors
2.7. Sites of Pi Absorption
2.8. Hormones Regulating P Homeostasis
2.9. Renal Handling of Pi
2.10. Pi Absorption from Ambient Water
3. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cho, C.Y.; Bureau, D.P. A review of diet formulation strategies and feeding systems to reduce excretory and feed wastes in aquaculture. Aquacult. Res. 2001, 32, 349–360. [Google Scholar] [CrossRef]
- Sugiura, S.H.; Hardy, R.W. Environmentally friendly feeds. In Encyclopedia of Aquaculture; Stickney, R.R., Ed.; Wiley & Sons, Inc.: Hoboken, NJ, USA, 2000; pp. 299–310. [Google Scholar]
- Hua, K.; Bureau, D.P. Modelling digestible phosphorus content of salmonid fish feeds. Aquaculture 2006, 254, 455–465. [Google Scholar] [CrossRef]
- Sugiura, S.H. Phosphorus in Fish Nutrition; Bookway Academic Publishing: Hyogo, Japan, 2018; 420p. [Google Scholar]
- Sugiura, S.H.; Roy, P.K.; Ferraris, R.P. Dietary acidification enhances phosphorus digestibility but decreases H+/K+-ATPase expression in rainbow trout. J. Exp. Biol. 2006, 209, 3719–3728. [Google Scholar] [CrossRef] [PubMed]
- Luckstadt, C. The use of acidifiers in fish nutrition. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 2008, 3, 1–8. [Google Scholar] [CrossRef]
- Kang, J.; Badger, T.M.; Ronis, M.J.; Wu, X. Non-isoflavone phytochemicals in soy their health effects. J. Agric. Food Chem. 2010, 58, 8119–8133. [Google Scholar] [CrossRef] [PubMed]
- Schlemmer, U.; Frølich, W.; Prieto, R.M.; Grases, F. Phytate in foods and significance for humans: Food sources, intake, processing, bioavailability, protective role and analysis. Mol. Nutr. Food Res. 2009, 53, S330–S375. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, A.; Chanez-Paredes, S.D.; Haest, X.; Turner, J.R. Paracellular permeability and tight junction regulation in gut health and disease. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 417–432. [Google Scholar] [CrossRef] [PubMed]
- Karasov, W.H. Integrative physiology of transcellular paracellular intestinal absorption. J. Exp. Biol. 2017, 220, 2495–2501. [Google Scholar] [CrossRef] [PubMed]
- Wood, C.M.; Grosell, M. Independence of net water flux from paracellular permeability in the intestine of Fundulus heteroclitus, a euryhaline teleost. J. Exp. Biol. 2012, 215, 508–517. [Google Scholar] [CrossRef]
- Raboy, V. Approaches and challenges to engineering seed phytate and total phosphorus. Plant Sci. 2009, 177, 281–296. [Google Scholar] [CrossRef]
- Skrede, G.; Storebakken, T.; Skrede, A.; Sahlstrøm, S.; Sørensen, M.; Shearer, K.D.; Slinde, E. Lactic acid fermentation of wheat and barley whole meal flours improves digestibility of nutrients and energy in Atlantic salmon (Salmo salar L.) diets. Aquaculture 2002, 210, 305–321. [Google Scholar] [CrossRef]
- Tanemura, N.; Akiyoshi, Y.; Okano, K.; Sugiura, S. Effects of culturing rapeseed meal, soybean meal, macrophyte meal, and algal meal with three species of white-rot fungi on their in vitro and in vivo digestibilities evaluated using rainbow trout. Aquaculture 2016, 453, 130–134. [Google Scholar] [CrossRef]
- Kawamoto, K.; Sakuma, M.; Tanaka, S.; Masuda, M.; Nakao-Muraoka, M.; Niida, Y.; Nakamatsu, Y.; Ito, M.; Taketani, Y.; Arai, H. High-fat diets provoke phosphorus absorption from the small intestine in rats. Nutrition 2020, 72, 110694. [Google Scholar] [CrossRef] [PubMed]
- Levi, M.; Gratton, E.; Forster, I.C.; Hernando, N.; Wagner, C.A.; Biber, J.; Sorribas, V.; Murer, H. Mechanisms of phosphate transport. Nat. Rev. Nephrol. 2019, 15, 482–500. [Google Scholar] [CrossRef]
- Manghat, P.; Sodi, R.; Swaminathan, R. Phosphate homeostasis and disorders. Ann. Clin. Biochem. 2014, 51, 631–656. [Google Scholar] [CrossRef]
- Sabbagh, Y.; Giral, H.; Caldas, Y.; Levi, M.; Schiavi, S.C. Intestinal phosphate transport. Adv. Chronic Kidney Dis. 2011, 18, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Knöpfel, T.; Himmerkus, N.; Günzel, D.; Bleich, M.; Hernando, N.; Wagner, C.A. Paracellular transport of phosphate along the intestine. Am. J. Physiol. 2019, 317, G233–G241. [Google Scholar] [CrossRef]
- Saurette, M.; Alexander, R.T. Intestinal phosphate absorption: The paracellular pathway predominates? Exp. Biol. Med. 2019, 244, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Danisi, G.; Murer, H. Inorganic phosphate absorption in small intestine. In Handbook of Physiology, The Gastrointestinal System; American Physiological Society: Bethesda, MD, USA, 1991; Volume 4, pp. 323–336. [Google Scholar] [CrossRef]
- Marks, J.; Debnam, E.S.; Unwin, R.J. Phosphate homeostasis and the renal-gastrointestinal axis. Am. J. Physiol. 2010, 299, F285–F296. [Google Scholar] [CrossRef]
- Sugiura, S.H.; Ferraris, R.P. Contributions of different NaPi cotransporter isoforms to dietary regulation of P transport in the pyloric caeca and intestine of rainbow trout. J. Exp. Biol. 2004, 207, 2055–2064. [Google Scholar] [CrossRef]
- Murer, H.; Hernando, N.; Forster, L.; Biber, J. Molecular mechanisms in proximal tubular and small intestinal phosphate reabsorption (plenary lecture). Mol. Membr. Biol. 2001, 18, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Takeda, E.; Taketani, Y.; Morita, K.; Miyamoto, K. Sodium-dependent phosphate co-transporters. Int. J. Biochem. Cell Biol. 1999, 31, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y. In vitro absorption of inorganic phosphate and other electrolytes in the carp intestine. Comp. Biochem. Phys. A 1985, 80, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y. Sodium-dependent absorption of inorganic phosphate by the carp intestine. Comp. Biochem. Phys. A 1985, 80, 437–439. [Google Scholar] [CrossRef] [PubMed]
- Avila, E.M.; Tu, H.; Basantes, S.; Ferraris, R.P. Dietary phosphorus regulates intestinal transport and plasma concentrations of phosphate in rainbow trout. J. Comp. Physiol. B 2000, 170, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Forster, I.C.; Wagner, C.A.; Busch, A.E.; Lang, F.; Biber, J.; Hernando, N.; Murer, H.; Werner, A. Electrophysiological characterization of the flounder type II Na+/Pi cotransporter (NaPi-5) expressed in Xenopus laevis oocytes. J. Membr. Biol. 1997, 160, 9–25. [Google Scholar] [CrossRef]
- Graham, C.; Nalbant, P.; Scholermann, B.; Hentschel, H.; Kinne, R.K.; Werner, A. Characterization of a type IIb sodium-phosphate cotransporter from zebrafish (Danio rerio) kidney. Am. J. Physiol. 2003, 284, F727–F736. [Google Scholar] [CrossRef] [PubMed]
- Kohl, B.; Herter, P.; Hulseweh, B.; Elger, M.; Hentschel, H.; Kinne, R.K.; Werner, A. Na-Pi cotransport in flounder: Same transport system in kidney and intestine. Am. J. Physiol. 1996, 270, F937–F944. [Google Scholar] [CrossRef]
- Nalbant, P.; Boehmer, C.; Dehmelt, L.; Wehner, F.; Werner, A. Functional characterization of a Na+-phosphate cotransporter (NaPi-II) from zebrafish and identification of related transcripts. J. Physiol. 1999, 520, 79–89. [Google Scholar] [CrossRef]
- Sugiura, S. Identification of intestinal phosphate transporters in fishes and shellfishes. Fish. Sci. 2009, 75, 99–108. [Google Scholar] [CrossRef]
- Verri, T.; Werner, A. Type II Na+-phosphate cotransporters and phosphate balance in teleost fish. Pflügers Arch. Eur. J. Physiol. 2019, 471, 193–212. [Google Scholar] [CrossRef] [PubMed]
- Werner, A.; Kinne, R.K. Evolution of the Na-Pi cotransport systems. Am. J. Physiol. 2001, 280, R301–R312. [Google Scholar] [CrossRef]
- Chen, P.; Tang, Q.; Wang, C. Characterizing and evaluating the expression of the type IIb sodium-dependent phosphate cotransporter (slc34a2) gene and its potential influence on phosphorus utilization efficiency in yellow catfish (Pelteobagrus fulvidraco). Fish Physiol. Biochem. 2016, 42, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Huang, Y.; Bayir, A.; Wang, C. Characterization of the isoforms of type IIb sodium-dependent phosphate cotransporter (Slc34a2) in yellow catfish, Pelteobagrus fulvidraco, and their vitamin D3-regulated expression under low-phosphate conditions. Fish Physiol. Biochem. 2017, 43, 229–244. [Google Scholar] [CrossRef]
- Carlile, M.; Nalbant, P.; Preston-Fayers, K.; McHaffie, G.S.; Werner, A. Processing of naturally occurring sense/antisense transcripts of the vertebrate Slc34a gene into short RNAs. Physiol. Genom. 2008, 34, 95–100. [Google Scholar] [CrossRef]
- Forster, I.; Hernando, N.; Sorribas, V.; Werner, A. Phosphate transporters in renal, gastrointestinal, and other tissues. Adv. Chronic Kidney Dis. 2011, 18, 63–76. [Google Scholar] [CrossRef]
- Piatek, M.J.; Henderson, V.; Zynad, H.S.; Werner, A. Natural antisense transcription from a comparative perspective. Genomics 2016, 108, 56–63. [Google Scholar] [CrossRef]
- Werner, A. Natural antisense transcripts. RNA Biol. 2005, 2, 53–62. [Google Scholar] [CrossRef]
- Werner, A.; Carlile, M.; Swan, D. What do natural antisense transcripts regulate? RNA Biol. 2009, 6, 43–48. [Google Scholar] [CrossRef]
- Ichida, Y.; Hosokawa, N.; Takemoto, R.; Koike, T.; Nakatogawa, T.; Hiranuma, M.; Arakawa, H.; Miura, Y.; Azabu, H.; Ohtomo, S.; et al. Significant species differences in intestinal phosphate absorption between dogs, rats, and monkeys. J. Nutr. Sci. Vitaminol. 2020, 66, 60–67. [Google Scholar] [CrossRef]
- Marks, J.; Srai, S.K.; Biber, J.; Murer, H.; Unwin, R.J.; Debnam, E.S. Intestinal phosphate absorption and the effect of vitamin D: A comparison of rats with mice. Exp. Physiol. 2006, 91, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Radanovic, T.; Wagner, C.A.; Murer, H.; Biber, J. Regulation of intestinal phosphate transport I. Segmental expression and adaptation to low-Pi diet of the type IIb Na+-Pi cotransporter in mouse small intestine. Am. J. Physiol. 2005, 288, G496–G500. [Google Scholar] [CrossRef]
- Huber, K.; Walter, C.; Schroder, B.; Breves, G. Phosphate transport in the duodenum and jejunum of goats and its adaptation by dietary phosphate and calcium. Am. J. Physiol. 2002, 283, R296–R302. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Angel, R.; Ashwell, C.M. Characterization of the chicken small intestine type IIb sodium phosphate cotransporter. Poult. Sci. 2007, 86, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, M.; Naito, S. Tissue-specific mRNA expression profiles of human solute carrier transporter superfamilies. Drug Metab. Pharmacok. 2008, 23, 22–44. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, S.H.; McDaniel, N.K.; Ferraris, R.P. In vivo fractional Pi absorption and NaPi-II mRNA expression in rainbow trout are upregulated by dietary P restriction. Am. J. Physiol. 2003, 285, R770–R781. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, S.H.; Kelsey, K.; Ferraris, R.P. Molecular conventional responses of large rainbow trout to dietary phosphorus restriction. J. Comp. Physiol. B 2007, 177, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Laverty, G.; Clark, N.B. The kidney. In Vertebrate Endocrinology: Fundamentals and Biomedical Implications; Academic Press: New York, NY, USA, 1989; Volume 3, pp. 277–317. [Google Scholar]
- Møbjerg, N.; Werner, A.; Hansen, S.M.; Novak, I. Physiological and molecular mechanisms of inorganic phosphate handling in the toad Bufo bufo. Pflügers Arch. Eur. J. Physiol. 2007, 454, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Hattenhauer, O.; Traebert, M.; Murer, H.; Biber, J. Regulation of small intestinal Na-Pi type IIb cotransporter by dietary phosphate intake. Am. J. Physiol. 1999, 277, G756–G762. [Google Scholar] [CrossRef]
- Huber, K.; Walter, C.; Schroder, B.; Biber, J.; Murer, H.; Breves, G. Epithelial phosphate transporters in small ruminants. Ann. N. Y. Acad. Sci. 2000, 915, 95–97. [Google Scholar] [CrossRef]
- Katai, K.; Miyamoto, K.; Kishida, S.; Segawa, H.; Nii, T.; Tanaka, H.; Tani, Y.; Arai, H.; Tatsumi, S.; Morita, K.; et al. Regulation of intestinal Na+-dependent phosphate co-transporters by a low-phosphate diet and 1,25-dihydroxyvitamin D3. Biochem. J. 1999, 343, 705–712. [Google Scholar] [CrossRef]
- Fleet, J.C.; Peacock, M. Physiology of vitamin D, calcium, and phosphate absorption. In The Physiological Basis of Metabolic Bone Disease; CRC Press: Boca Raton, FL, USA, 2014; pp. 13–40. [Google Scholar] [CrossRef]
- Candeal, E.; Caldas, Y.A.; Guillén, N.; Levi, M.; Sorribas, V. Intestinal phosphate absorption is mediated by multiple transport systems in rats. Am. J. Physiol. 2017, 312, G355–G366. [Google Scholar] [CrossRef]
- Coloso, R.M.; Basantes, S.P.; King, K.; Hendrix, M.A.; Fletcher, J.W.; Weis, P.; Ferraris, R.P. Effect of dietary phosphorus and vitamin D3 on phosphorus levels in effluent from the experimental culture of rainbow trout (Oncorhynchus mykiss). Aquaculture 2001, 202, 145–161. [Google Scholar] [CrossRef]
- Coloso, R.M.; King, K.; Fletcher, J.W.; Weis, P.; Werner, A.; Ferraris, R.P. Dietary P regulates phosphate transporter expression, phosphatase activity, and effluent P partitioning in trout culture. J. Comp. Physiol. B 2003, 173, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Lake, J.; Gravel, C.; Koko, G.K.D.; Robert, C.; Vandenberg, G.W. Combining suppressive subtractive hybridization and cDNA microarrays to identify dietary phosphorus-responsive genes of the rainbow trout (Oncorhynchus mykiss) kidney. Comp. Biochem. Phys. D 2010, 5, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Jiang, W.D.; Wu, P.; Liu, Y.; Kuang, S.Y.; Tang, L.; Tang, W.N.; Zhang, Y.A.; Zhou, X.Q.; Feng, L. Effect of dietary phosphorus deficiency on the growth, immune function and structural integrity of head kidney, spleen and skin in young grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol. 2017, 63, 103–126. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.S.; Pei, W.L.; Wang, Y.Y.; Wang, Z.; Zhuo, M.Q. Topology, tissue distribution, and transcriptional level of SLC34s in response to Pi and pH in grass carp Ctenopharyngodon idella. Fish Physiol. Biochem. 2021, 47, 1383–1393. [Google Scholar] [CrossRef]
- Zhuo, M.Q.; Lv, W.H.; Xu, Y.H.; Luo, Z. Isolation and characterization of three sodium-phosphate cotransporter genes and their transcriptional regulation in the grass carp Ctenopharyngodon idella. Int. J. Mol. Sci. 2020, 21, 8228. [Google Scholar] [CrossRef]
- Wang, Y.; Geng, Y.; Shi, X.; Wang, S.; Yang, Z.; Zhang, P.; Liu, H. Effects of dietary phosphorus levels on growth performance, phosphorus utilization and intestinal calcium and phosphorus transport-related genes expression of juvenile Chinese soft-shelled turtle (Pelodiscus sinensis). Animals 2022, 12, 3101. [Google Scholar] [CrossRef]
- IOM: Institute of Medicine. Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride; National Academies Press: Washington, DC, USA, 1997. [Google Scholar]
- Sabbagh, Y.; O’Brien, S.P.; Song, W.; Boulanger, J.H.; Stockmann, A.; Arbeeny, C.; Schiavi, S.C. Intestinal npt2b plays a major role in phosphate absorption and homeostasis. J. Am. Soc. Nephrol. 2009, 20, 2348–2358. [Google Scholar] [CrossRef]
- Nakamura, Y.; Hirano, T. Effect of hypophysectomy on absorption of inorganic phosphate by the eel intestine. Comp. Biochem. Phys. A 1986, 84, 595–599. [Google Scholar] [CrossRef]
- Christakos, S.; Dhawan, P.; Porta, A.; Mady, L.J.; Seth, T. Vitamin D and intestinal calcium absorption. Mol. Cell. Endocrinol. 2011, 347, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Christakos, S.; Dhawan, P.; Verstuyf, A.; Verlinden, L.; Carmeliet, G. Vitamin D: Metabolism, molecular mechanism of action, and pleiotropic effects. Physiol. Rev. 2016, 96, 365–408. [Google Scholar] [CrossRef] [PubMed]
- Fujita, H.; Sugimoto, K.; Inatomi, S.; Maeda, T.; Osanai, M.; Uchiyama, Y.; Yamamoto, Y.; Wada, T.; Kojima, T.; Yokozaki, H.; et al. Tight junction proteins claudin-2 and-12 are critical for vitamin D-dependent Ca2+ absorption between enterocytes. Mol. Biol. Cell 2008, 19, 1912–1921. [Google Scholar] [CrossRef]
- Zhang, Y.G.; Wu, S.; Lu, R.; Zhou, D.; Zhou, J.; Carmeliet, G.; Petrof, E.; Claud, E.C.; Sun, J. Tight junction CLDN2 gene is a direct target of the vitamin D receptor. Sci. Rep. 2015, 5, 10642. [Google Scholar] [CrossRef]
- Chen, K.; Zhou, X.Q.; Jiang, W.D.; Wu, P.; Liu, Y.; Jiang, J.; Kuang, S.Y.; Tang, L.; Tang, W.N.; Zhang, Y.A.; et al. Impaired intestinal immune barrier and physical barrier function by phosphorus deficiency: Regulation of TOR, NF-κB, MLCK, JNK and Nrf2 signalling in grass carp (Ctenopharyngodon idella) after infection with Aeromonas hydrophila. Fish Shellfish Immunol. 2018, 74, 175–189. [Google Scholar] [CrossRef]
- Chen, K.; Zhou, X.Q.; Jiang, W.D.; Wu, P.; Liu, Y.; Jiang, J.; Kuang, S.Y.; Tang, L.; Tang, W.N.; Feng, L. Dietary phosphorus deficiency caused alteration of gill immune and physical barrier function in the grass carp (Ctenopharyngodon idella) after infection with Flavobacterium columnare. Aquaculture 2019, 506, 1–13. [Google Scholar] [CrossRef]
- Merrifield, D.L.; Ringø, E.; Myklebust, R.; Olsen, R.E. Dietary effect of soybean (Glycine max) products on gut histology and microbiota of fish. In Soybean and Nutrition; El-Shemy, H., Ed.; INTECH Open Access Publisher: London, UK, 2011; pp. 231–250. [Google Scholar]
- Kotler, B.M.; Kerstetter, J.E.; Insogna, K.L. Claudins, dietary milk proteins, and intestinal barrier regulation. Nutr. Rev. 2013, 71, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Ulluwishewa, D.; Anderson, R.C.; McNabb, W.C.; Moughan, P.J.; Wells, J.M.; Roy, N. Regulation of tight junction permeability by intestinal bacteria and dietary components. J. Nutr. 2011, 141, 769–776. [Google Scholar] [CrossRef]
- Robertson, D.R. Regulation of calcium and phosphate by intestine. In Vertebrate Endocrinology: Fundamentals and Biomedical Implications; Academic Press: San Diego, CA, USA, 1989; pp. 243–275. [Google Scholar]
- Razzaque, M.S. Phosphate toxicity: New insights into an old problem. Clin. Sci. 2011, 120, 91–97. [Google Scholar] [CrossRef]
- Partridge, I.G. Studies on digestion and absorption in the intestines of growing pigs. 3. Net movements of mineral nutrients in the digestive tract. Br. J. Nutr. 1978, 39, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Dabrowski, K.R.; Schwarz, F.J. Minerals content in the digestive tract of stomachless fish. Zool. Jb. Physiol. 1986, 90, 193–200. [Google Scholar]
- Snellgrove, D.L. Dietary Availability and Retention of Selected Minerals Associated with the Intensive Production of Rainbow Trout (Oncorhynchus mykiss). Ph.D. Thesis, University of Plymouth, Plymouth, UK, 2003; 294p. [Google Scholar]
- Nakajima, M.; Sugiura, S. Effects of dietary NaCl on the in vivo apparent absorption of dietary nutrients determined in rainbow trout (Oncorhynchus mykiss). Aquaculture 2016, 460, 1–7. [Google Scholar] [CrossRef]
- Bakke-McKellep, A.M.; Nordrum, S.; Krogdahl, Å.; Buddington, R.K. Absorption of glucose, amino acids, and dipeptides by the intestines of Atlantic salmon (Salmo salar L.). Fish Physiol. Biochem. 2000, 22, 33–44. [Google Scholar] [CrossRef]
- Tani, Y.; Sato, T.; Yamanaka-Okumura, H.; Yamamoto, H.; Arai, H.; Sawada, N.; Genjida, K.; Taketani, Y.; Takeda, E. Effects of prolonged high phosphorus diet on phosphorus and calcium balance in rats. J. Clin. Biochem. Nutr. 2007, 40, 221–228. [Google Scholar] [CrossRef]
- Werner, A.; Murer, H.; Kinne, R.K. Cloning and expression of a renal Na-Pi cotransport system from flounder. Am. J. Physiol. 1994, 267, F311–F317. [Google Scholar] [CrossRef] [PubMed]
- Elger, M.; Werner, A.; Herter, P.; Kohl, B.; Kinne, R.K.H.; Hentschel, H. Na-Pi cotransport sites in proximal tubule and collecting tubule of winter flounder (Pleuronectes americanus). Am. J. Physiol. 1998, 274, F374–F383. [Google Scholar] [CrossRef]
- Kohl, B.; Wagner, C.A.; Huelseweh, B.; Busch, A.E.; Werner, A. The Na+-phosphate cotransport system (NaPi-II) with a cleaved protein backbone: Implications on function and membrane insertion. J. Physiol. 1998, 508, 341–350. [Google Scholar] [CrossRef]
- Renfro, J.L. Solute transport by flounder renal cells in primary culture. Fish Physiol. 1995, 14, 147–171. [Google Scholar] [CrossRef]
- Renfro, J.L. Recent developments in teleost renal transport. J. Exp. Zool. A 1999, 283, 653–661. [Google Scholar] [CrossRef]
- Suyama, T.; Okada, S.; Ishijima, T.; Iida, K.; Abe, K.; Nakai, Y. High phosphorus diet-induced changes in NaPi-IIb phosphate transporter expression in the rat kidney: DNA microarray analysis. PLoS ONE 2012, 7, e29483. [Google Scholar] [CrossRef] [PubMed]
- Schultz, A.G.; Guffey, S.C.; Clifford, A.M.; Goss, G.G. Phosphate absorption across multiple epithelia in the Pacific hagfish (Eptatretus stoutii). Am. J. Physiol. 2014, 307, R643–R652. [Google Scholar] [CrossRef] [PubMed]


| Ingredients and Total P Concentrations (%) * | Agastric Fish ** | Monogastric Fish ** | Other Species ** |
|---|---|---|---|
| Barley (whole grain): 0.33–0.42 | t37–79, a81, | p28–51, k35–72, p28–43 | |
| Blood meal: 0.08–0.30 | t100, m60, w58–72, | k100, p92 | |
| Canola meal: 1.01–1.11 | t12, v55, | p21 | |
| Casein: 0.75–0.82 | h97, | a92, t90, f90, v70, c53, | k100 |
| Corn (whole grain): 0.26–0.34 | g71, | n63, t36–37, f25–34, | p12–48, k0–80, p9–29 |
| Corn gluten meal: 0.34–0.54 | h0, | m57, a26, t2–17, v28–79, n97, e48, c16–19, r22, z83, j33, w70, q56, | p20, k50–70, p15–59 |
| Cottonseed meal: 0.98–1.17 | m41, n65, t53–56, f73, v53, d40, i28, q22, | k10–80, p0–42 | |
| Egg albumin: 0.11 | f71, b81, | ||
| Feather meal: 0.29–1.83 | m56, a77, e23, t28–84, c75, w61, | k100, p31 | |
| Fish meal (anchovy, sardine): 2.43–2.90 | g33, | t49–59, f40, v28, c47, z72, j58, | k100 |
| Fish meal (herring, capelin): 1.67–2.41 | a52–53, t44–58, e80–82, c57, | k100 | |
| Fish meal (menhaden): 2.88–3.61 | a87, t35–47, f39–46, c40, d23–48 | k100, p94 | |
| Fish meal (Peruvian): 2.92 | m65, e54, t44, v74–97, w71–79, q66, | ||
| Fish meal (brown fish meal) | h13,25,33, h1, | e75–78, t54–81, w82, i59, q53, | k100 |
| Fish meal (whitefish, cod): 3.03–3.50 | h10,18,26, h0, h4, | a79, t8–72, v65, c55–71, b29, w80, r60, i61, o64 | k78–100 |
| Fish meal (whitefish, high-ash): 7.41 | t12–17 | ||
| Fish meal (general, various) | n55, e49–63, t0, v73, w68 | p85, k83–102, p100 | |
| Lupin meal, extruded: 0.51 | a26, t62–100, u100, b91–100 | ||
| Meat and bone meal: 2.68–5.59 | m40, n53, e43, t22–46, f53, v58, d66, w62–64, q60, | k67–102, p64–102 | |
| Meat and bone meal (low-ash): 2.49 | t35, | k100 | |
| Meat meal: 2.76–3.88 | t3, e80–83, w64, | k100 | |
| Meat meal (low ash): 2.28 | t45, | ||
| Peas, extruded: 0.39 | t43, u100, | p45 | |
| Peanut meal: 0.59–0.65 | g70, | m45, v53, w58, o55, | k0–33, p12 |
| Poultry byproduct meal: 2.17–2.50 | t35–64, m57, n87, a81, e30, c68, d27, z94, | k100–101 | |
| Poultry byproduct meal (low-ash): 1.65–2.09 | t47–56, e60, v63, w60–62, | ||
| Rapeseed meal (solv. ext.): 1.01–1.11 | h35, | t26, m39, u49, i26, w56, q26, o52, | p23–32, k0–29, p16–21 |
| Rapeseed meal (heated) | t42, u65, | ||
| Rice bran: 1.61–2.06 | h25, | t19, n90, f51, | p12, k4–16, p25 |
| Sorghum: 0.27–0.29 | f36, | k0–33, p28 | |
| Soybean meal (solv. ext.): 0.64–0.76 | h11, g67, | m47, n44–88, a8–36, e36–59, t14–57, f27–54, v30–77, c28–43, d47, b82, r13, z88, i39, j28, w60, q42, o56, | p24–42, k3–43, p18–38 |
| Soybean meal (full fat): 0.59 | t20, q41, | k42 | |
| Soybean meal (de-phytinized) | t93, | ||
| Wheat: 0.32–0.39 | t17–69, f56, c50, d79, j31, | p40–74, k38–62, p49–69 | |
| Wheat middling: 0.53–0.93 | g54, | n71, a32, e–10, t55–61, f28–78, c41, | p28–41, k26, p34–41 |
| Wheat bran: 0.99–1.20 | p41, k23–49, p29–55 | ||
| Wheat germ: 0.99 | h57, | e19, t58, | |
| Wheat gluten meal: 0.26 | t62–75, c57, b73, | ||
| Yeast (brewer’s, dried): 1.44–1.72 | h93, | a79, e39, t69–91 | |
| Phosphoric acid H3PO4: 31.6 | k128, p100 | ||
| KH2PO4: 22.8, NaH2PO4∙nH2O: | h90–98, g90–91 | a94–95, t96–98, f90, b72–97, c93, y96, | s68, x67, p87–96, k96, p100 |
| Ca(H2PO4)2∙H2O: 24.6 | h93–94, g90, | a90, e47–65, t94, f94, b92, y92, i91, | s46, x73, p91, k99–100, p100–121 |
| Monocalcium phosphate (feed-grade) | p82–84 | ||
| Na2HPO4∙nH2O: K2HPO4: 17.8 | h97, | t64–94, b95, | |
| CaHPO4∙2H2O: 18.0 | h46–62, g60, | n46, a72, e66–71, t71, f65, b65, y59, | s19, x28, p32–87, k86–99, p62–107 |
| Dicalcium phosphate (feed-grade) | p74–82, k74–100 | ||
| Ca3(PO4)2: 20.0 | h6–13, g3, | a56, e42–60, t64, b44, y49, | s10, k93–100 |
| Tricalcium phosphate (feed-grade) | p48, k84 | ||
| Soft rock phosphate | p28–57, k38 | ||
| Defluorinated phosphate | p41, k69–96, p87–100 | ||
| Superphosphate, Triple superphosphate (agri-grade) | k93–96 | ||
| Monoammonium phosphate (feed-grade, agri-grade) | k100 | ||
| Ammonium polyphosphate | k95–118 | ||
| Bone meal (fish bone): 7.69 | t13–66, | ||
| Bone meal (unspecified): 12.9 | p68–85, k90–100, p82 | ||
| Phytates, phytin, phytic acid (IP6) | h5–38 | a0–15, e2, t0–19, f1, v50, b32, | k0–60, p25–40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sugiura, S.H. Digestion and Absorption of Dietary Phosphorus in Fish. Fishes 2024, 9, 324. https://doi.org/10.3390/fishes9080324
Sugiura SH. Digestion and Absorption of Dietary Phosphorus in Fish. Fishes. 2024; 9(8):324. https://doi.org/10.3390/fishes9080324
Chicago/Turabian StyleSugiura, Shozo H. 2024. "Digestion and Absorption of Dietary Phosphorus in Fish" Fishes 9, no. 8: 324. https://doi.org/10.3390/fishes9080324






