A Self-Pumping Composite Dressing Improved Hypertrophic Scar Healing with Dual Therapy and Active-Fluid Transport
Abstract
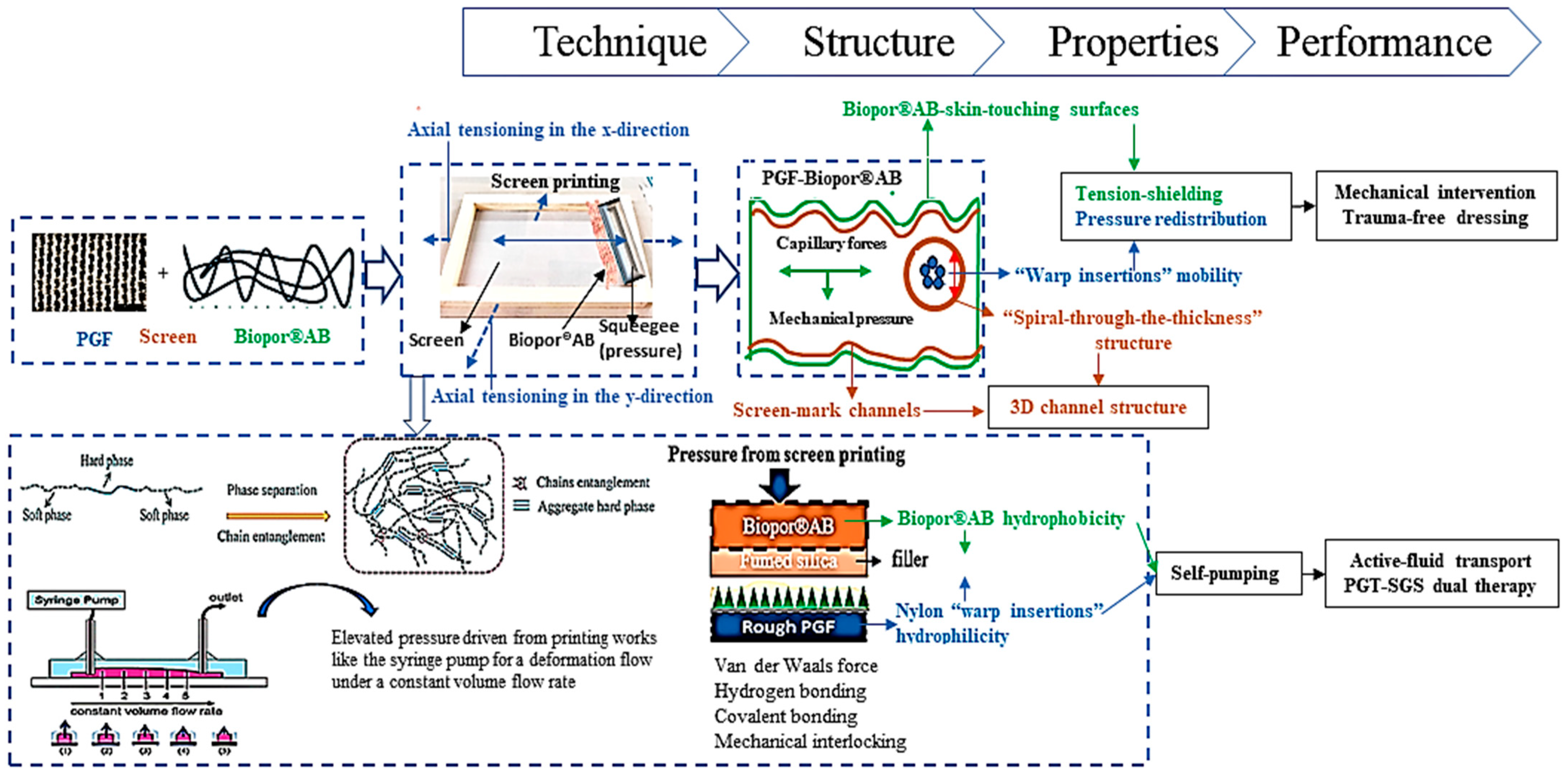
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PGF-Biopor®AB
2.3. Investigations of Physical and Structural Properties
2.4. Trial Patient Study with ATDDS
3. Results and Discussion
3.1. Dressing Structure
3.1.1. Warp Insertion Mobility
3.1.2. Structure of Biopor®AB-Touching-Skin Surfaces
3.1.3. 3D Channel Structure
3.1.4. Active-Fluid Transport
3.2. Physical Properties
3.2.1. Water Uptake Capability
3.2.2. Water Vapor Permeability
3.3. Trial Patient Study
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gauglitz, G.G.; Korting, H.C.; Pavicic, T.; Ruzicka, T.; Jeschke, M.G. Hypertrophic scarring and keloids: Pathomechanisms and current and emerging treatment strategies. Mol. Med. 2011, 17, 113. [Google Scholar] [CrossRef] [PubMed]
- Reportlinker.com Announces the Release of the Report “Medical Aesthetics Market Product, End User—Global Forecast to 2025”; Globe Newswire: New York, NY, USA, 2020.
- Anthonissen, M.; Daly, D.; Janssens, T.; Van den Kerckhove, E. The effects of conservative treatments on burn scars: A systematic review. Burns 2016, 42, 508–518. [Google Scholar] [CrossRef]
- Brown, M.B.; Martin, G.P.; Jones, S.A.; Akomeah, F.K. Dermal and transdermal drug delivery systems: Current and future prospects. Drug Deliv. 2006, 13, 175–187. [Google Scholar] [CrossRef]
- Muir, I.F.K. On the nature of keloid and hypertrophic scars. Br. J. Plast. Surg. 1990, 43, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Mustoe, T.A. Evolution of silicone therapy and mechanism of action in scar management. Aesthetic Plast. Surg. 2008, 32, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhu, C.; Fan, D.; Fu, R.; Ma, P.; Duan, Z.; Li, X.; Lei, H.; Chi, L. A bi-layer PVA/CMC/PEG hydrogel with gradually changing pore sizes for wound dressing. Macromol. Biosci. 2019, 19, 1800424. [Google Scholar] [CrossRef]
- Monstrey, S.; Middelkoop, E.; Vranckx, J.J.; Bassetto, F.; Ziegler, U.E.; Meaume, S.; Téot, L. Updated scar management practical guidelines: Non-invasive and invasive measures. J. Plast. Reconstr. Aesthetic Surg. 2014, 67, 1017–1025. [Google Scholar] [CrossRef]
- Ogawa, R. The most current algorithms for the treatment and prevention of hypertrophic scars and keloids: A 2020 update of the algorithms published 10 years ago. Plast. Reconstr. Surg. 2022, 149, 79. [Google Scholar] [CrossRef]
- Wright, J.A.; Richards, T.; Srai, S.K.S. The role of iron in the skin and cutaneous wound healing. Front Pharmacol. Front. Media S.A. 2014, 5, 156. [Google Scholar] [CrossRef]
- Kolarsick, P.A.J.; Kolarsick, M.A.; Goodwin, C. Anatomy and physiology of the skin. J. Derm. Nurses Assoc. 2011, 3, 203–213. [Google Scholar] [CrossRef]
- Moore, A.; Marshall, C.; Longaker, M. Minimizing skin scarring through biomaterial design. J. Funct. Biomater. 2017, 8, 3. [Google Scholar] [CrossRef]
- Tejiram, S.; Zhang, J.; Travis, T.E.; Carney, B.C.; Alkhalil, A.; Moffatt, L.T.; Shupp, J.W. Compression therapy affects collagen type balance in hypertrophic scar. J. Surg. Res. 2016, 201, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Mokos, Z.B.; Jović, A.; Grgurević, L.; Dumić-Čule, I.; Kostović, K.; Čeović, R.; Marinović, B. Current therapeutic approach to hypertrophic scars. Front. Med. 2017, 4, 83. [Google Scholar] [CrossRef] [PubMed]
- Chow, L.; Yick, K.-L.; Kwan, M.-Y.; Yuen, C.-F.; Ng, S.-P.; Yu, A.; Yip, J. Customized fabrication approach for hypertrophic scar treatment: 3D printed fabric silicone composite. Int. J. Bioprint. 2020, 6, 262. [Google Scholar] [CrossRef] [PubMed]
- Quinn, K.; Courtney, J.; Evans, J.; Gaylor, J.; Reid, W. Principles of burn dressings. Biomaterials 1985, 6, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Lalani, R.; Liu, L. Electrospun zwitterionic poly (sulfobetaine methacrylate) for nonadherent, superabsorbent, and antimicrobial wound dressing applications. Biomacromolecules 2012, 13, 1853–1863. [Google Scholar] [CrossRef]
- Ye, S.; Jiang, L.; Wu, J.; Su, C.; Huang, C.; Liu, X.; Shao, W. Flexible amoxicillin-grafted bacterial cellulose sponges for wound dressing: In vitro and in vivo evaluation. ACS Appl. Mater. Interfaces 2018, 10, 5862–5870. [Google Scholar] [CrossRef]
- Chiang, R.S.; Borovikova, A.A.; King, K.; Banyard, D.A.; Lalezari, S.; Toranto, J.D.; Widgerow, A.D. Current concepts related to hypertrophic scarring in burn injuries. Wound Repair Regen. 2016, 24, 466–477. [Google Scholar] [CrossRef]
- Shen, J.; Xu, F.; Cheng, L.; Pan, W.; Ge, Y.; Li, J.; Zhang, J. Simulation of internal flow characteristics of an axial flow pump with variable tip clearance. Water 2022, 14, 1652. [Google Scholar] [CrossRef]
- Ji, H.; Wei, M.; Liu, R.; Chang, C. Drive Control Simulation and Experimental Studies on the Flow Characteristics of a Pump Injector. IEEE Access 2020, 8, 35672–35681. [Google Scholar] [CrossRef]
- Young, D.F.; Tsai, F.Y. Flow characteristics in models of arterial stenoses—I. Steady flow. J. Biomech. 1973, 6, 395–410. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Hong, S.; Yu, D.; Peng, G.; Du, J.; Yuan, S. Flow Characteristic Analysis of the Self-Priming Pump Based on the Population Balance Model. Front. Energy Res. 2022, 10, 404. [Google Scholar] [CrossRef]
- Akbarzadeh, P. Numerical study of thermohydrodynamic characteristics of oil tilting-pad journal bearings with a self-pumping fluid flow circulation. Tribol. Trans. 2015, 58, 18–30. [Google Scholar] [CrossRef]
- Sidgwick, G.P.; McGeorge, D.; Bayat, A. A comprehensive evidence-based review on the role of topicals and dressings in the management of skin scarring. Arch. Dermatol. Res. 2015, 307, 461–477. [Google Scholar] [CrossRef]
- Klare, M.; Frank, G.; Markus, K.; Veit, T. Elastomer Materials. Germany Patent DE 10 2011 115 061, 11 April 2013. [Google Scholar]
- Verdier-Sévrain, S.; Bonté, F. Skin hydration: A review on its molecular mechanisms. J. Cosmet. Dermatol. 2007, 6, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Li, A.; Wang, R.; Zhang, J.; Ding, Y.; Pan, D.; Shen, Z. Elastic Janus film for wound dressings: Unidirectional biofluid transport and effectively promoting wound healing. Adv. Funct. Mater. 2021, 31, 2105265. [Google Scholar] [CrossRef]
- Zhang, Y.; Tian, M.; Wang, L.; Zhao, H.; Qu, L. Flexible Janus textile-based electroosmotic pump for large-area unidirectional positive water transport. Adv. Mater. Interfaces 2020, 7, 1902133. [Google Scholar] [CrossRef]
- Zhou, L.; Xu, P.; Dong, P.; Ou, X.; Du, X.; Chen, Y.; Zhang, X.; Gao, W.; Gao, G. A self-pumping dressing with in situ modification of non-woven fabric for promoting diabetic wound healing. Chem. Eng. J. 2023, 457, 141108. [Google Scholar] [CrossRef]
- Shao, Z.; Chen, J.; Ke, L.-J.; Wang, Q.; Wang, X.; Li, W.; Zheng, G. Directional transportation in a self-pumping dressing based on a melt electrospinning hydrophobic mesh. ACS Biomater. Sci. Eng. 2021, 7, 5918–5926. [Google Scholar] [CrossRef]
- Qi, L.; Ou, K.; Hou, Y.; Yuan, P.; Yu, W.; Li, X.; Wang, B.; He, J.; Cui, S.; Chen, X. Unidirectional water-transport antibacterial trilayered nanofiber-based wound dressings induced by hydrophilic-hydrophobic gradient and self-pumping effects. Mater. Des. 2021, 201, 109461. [Google Scholar] [CrossRef]
- Si, Y.; Chen, L.; Yang, F.; Guo, F.; Guo, Z. Stable Janus superhydrophilic/hydrophobic nickel foam for directional water transport. J. Colloid Interface Sci. 2018, 509, 346–352. [Google Scholar] [CrossRef]
- Zhu, H.; Zhi, C.; Meng, J.; Wang, Y.; Liu, Y.; Wei, L.; Fu, S.; Miao, M.; Yu, L. A self-pumping dressing with multiple liquid transport channels for wound microclimate management. Macromol. Biosci. 2022, 23, 2200356. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Zhang, Z.; Fan, W.; Gao, W.; Su, H.; Li, Z. Fabrication and evaluation of polydimethylsiloxane modified gelatin/silicone rubber asymmetric bilayer membrane with porous structure. Mater. Des. 2018, 158, 28–38. [Google Scholar] [CrossRef]
- Gencer, Z.A.; Odabas, S.; Sasmazel, H.T.; Piskin, E. Microporous silicone biomaterials with modified surface chemistry: Production and characterization. J. Bioact. Compat. Polym. 2012, 27, 419–428. [Google Scholar] [CrossRef]
- Magniez, K.; Zaidi, B.M.; Zhang, J.; Miao, M. Prestrained twistless flax yarn as reinforcement for polymer-matrix composites. Polym. Compos. 2020, 41, 930–938. [Google Scholar] [CrossRef]
- Jaffe, M.; Hammond, W.; Tolias, P.; Arinzeh, T. (Eds.) Characterization of Biomaterials; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Gu, R.; Ngan, A.H.W. Effects of pre-straining and coating on plastic deformation of aluminium micropillars. Acta Mater. 2012, 60, 6102–6111. [Google Scholar] [CrossRef]
- Ly, N.G.; Denby, E.F. A CSIRO inter-laboratory trial of the KES-F for measuring fabric properties. J. Text. Inst. 1988, 79, 198–219. [Google Scholar] [CrossRef]
- McNeely, M.R.; Sputea, M.K.; Tusneem, N.A.; Oliphant, A.R. Sample processing with hydrophobic microfluidics. J. Assoc. Lab. Autom. 1999, 4, 30–33. [Google Scholar] [CrossRef]
- Hong, S.; Minary-Jolandan, M.; Naraghi, M. Controlling the wettability and adhesion of carbon fibers with polymer interfaces via grafted nanofibers. Compos. Sci. Technol. 2015, 117, 130–138. [Google Scholar] [CrossRef]
- Andersson, C.-H.; Dartman, T.; Gredinger, P.; Asplund, J.; Strandqvist, H. Flexible composites, strength, deformation, and fracture processes. 1. reinforcement structures and tensile strength. Mech. Compos. Mater. 1998, 34, 525–536. [Google Scholar] [CrossRef]
- Schüth, F.; Sing, K.S.W.; Weitkamp, J. Handbook of Porous Solids; Wiley: Hoboken, NJ, USA, 2002. [Google Scholar]
- Wang, L.; Felder, M.; Cai, Y. Study of properties of medical compression garments fabrics. J. Fiber Bioeng. Inform. 2011, 4, 15–22. [Google Scholar] [CrossRef]
- Choi, F.K. Modeling of Singles and Two-Ply Staple Fiber Yarns. Ph.D. Thesis, Institute of Textiles and Clothing of the Hong Kong Polytechnic University, Department of Applied Mathematics, Hong Kong Polytechnic University, Hong Kong, China, 1997. [Google Scholar]
- Choi, F.K. Mechanical properties of wrapped yarn. Text. Asia 1991, 4, 33–37. [Google Scholar]
- Crookston, J.J.; Long, A.C.; Jones, I.A. A summary review of mechanical properties prediction methods for textile reinforced polymer composites. Proceedings of the Institution of Mechanical Engineers, Part L. J. Mater. Des. Appl. 2005, 219, 91–109. [Google Scholar]
- Wu, W.L.; Hamada, H.; Kotaki, M.; Maekawa, Z. Design of knitted fabric reinforced composites. J. Reinf. Plast. Compos. 1995, 14, 786–798. [Google Scholar] [CrossRef]
- Nasser, J.; Lin, J.; Steinke, K.; Sodano, H.A. Enhanced interfacial strength of aramid fiber reinforced composites through adsorbed aramid nanofiber coatings. Compos. Sci. Technol. 2019, 174, 125–133. [Google Scholar] [CrossRef]
- McCoy, R.J.; Jungreuthmayer, C.; O’Brien, F.J. Influence of flow rate and scaffold pore size on cell behaviour during mechanical stimulation in a flow perfusion bioreactor. Biotechnol. Bioeng. 2012, 109, 1583–1594. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Lei, M.; Lei, J.; Li, Z. A scalable hybrid fiber and its textile with pore and wrinkle structures for passive personal cooling. Adv. Mater. Technol. 2020, 5, 2000287. [Google Scholar] [CrossRef]
- Dai, N.-T.; Williamson, M.; Khammo, N.; Adams, E.; Coombes, A. Composite cell support membranes based on collagen and polycaprolactone for tissue engineering of skin. Biomaterials 2004, 25, 4263–4271. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Luo, G.; Xia, H.; He, W.; Zhao, J.; Liu, B.; Tan, J.; Zhou, J.; Liu, D.; Wang, Y.; et al. Novel bilayer wound dressing composed of silicone rubber with particular micropores enhanced wound re-epithelialization and contraction. Biomaterials 2015, 40, 1–11. [Google Scholar] [CrossRef]
- Zeng, W.; Shu, L.; Li, Q.; Chen, S.; Wang, F.; Tao, X.M. Fiber-based wearable electronics: A review of materials, fabrication, devices, and applications. Adv. Mater. 2014, 26, 5310–5336. [Google Scholar] [CrossRef]
- Jang, B.Z. Control of interfacial adhesion in continuous carbon and Kevlar fiber reinforced polymer composites. Compos. Sci. Technol. 1992, 44, 333–349. [Google Scholar] [CrossRef]
- Comyn, J. What are adhesives and sealants and how do they work? In Adhesive Bonding; Woodhead Publishing: Cambridge, UK, 2021; pp. 41–78. [Google Scholar]
- Wang, H.; Ding, J.; Dai, L.; Wang, X.; Lin, T. Directional water-transfer through fabrics induced by asymmetric wettability. J. Mater. Chem. 2010, 20, 7938–7940. [Google Scholar] [CrossRef]
- Wang, H.; Wang, X.; Lin, T. Unidirectional water transfer effect from fabrics having a superhydrophobic-to-hydrophilic gradient. J. Nanosci. Nanotechnol. 2013, 13, 839–842. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Chen, Z.; Zhu, L.; Du, Z.; Wang, X.; Naebe, M. Directional trans-planar and different in-plane water transfer properties of composite structured bifacial fabrics modified by a facile three-step plasma treatment. Coatings 2017, 7, 132. [Google Scholar] [CrossRef]
- Jin, M.; Chen, W.; Li, Z.; Zhang, Y.; Zhang, M.; Chen, S. Patterned bacterial cellulose wound dressing for hypertrophic scar inhibition behavior. Cellulose 2018, 25, 6705–6717. [Google Scholar] [CrossRef]
- Hu, G.; Kang, J.; Ng, L.W.T.; Zhu, X.; Howe, R.C.T.; Jones, C.G.; Hersam, M.C.; Hasan, T. Functional inks and printing of two-dimensional materials. Chem. Soc. Rev. 2018, 47, 3265–3300. [Google Scholar] [CrossRef]
- Zheng, S.; Jiang, Y.; Wu, X.; Xu, Z.; Liu, Z.; Yang, W.; Yang, M. Highly sensitive pressure sensor with broad linearity via constructing a hollow structure in polyaniline/polydimethylsiloxane composite. Compos. Sci. Technol. 2021, 201, 108546. [Google Scholar] [CrossRef]
- Ji, J.; Ge, X.; Pang, X.; Liu, R.; Wen, S.; Sun, J.; Liang, W.; Ge, J.; Chen, X. Synthesis and characterization of room temperature vulcanized silicone rubber using methoxyl-capped MQ silicone resin as self-reinforced cross-linker. Polymers 2019, 11, 1142. [Google Scholar] [CrossRef]
- Zou, C.; Lao, L.; Chen, Q.; Fan, J.; Shou, D. Nature-inspired moisture management fabric for unidirectional liquid transport and surface repellence and resistance. Energy Build. 2021, 248, 111203. [Google Scholar] [CrossRef]
- O’connor, S.M.; Hanson, I.B.; Bente IV, P.F.; Clemente, M.J. Fluid Restriction Mechanisms for Drug Delivery Pumps. U.S. Patent No. 10,617,820, 14 April 2020. [Google Scholar]
- Comanns, P. Passive water collection with the integument: Mechanisms and their biomimetic potential. J. Exp. Biol. 2018, 221, jeb153130. [Google Scholar] [CrossRef]
- Hollies, N.R.; Kaessinger, M.M.; Watson, B.S.; Bogaty, H. Water transport mechanisms in textile materials: Part II: Capillary-type penetration in yarns and fabrics. Text. Res. J. 1957, 27, 8–13. [Google Scholar] [CrossRef]
- Gupta, M.; Singh, R. PLA-coated sisal fibre-reinforced polyester composite: Water absorption, static and dynamic mechanical properties. J. Compos. Mater. 2019, 53, 65–72. [Google Scholar] [CrossRef]
- Hollies, N.R.S.; Kaessinger, M.M.; Bogaty, H. Water transport mechanisms in textile materials1 Part I: The role of yarn roughness in capillary-type penetration. Text. Res. J. 1956, 26, 829–835. [Google Scholar] [CrossRef]
- Jhanji, Y.; Gupta, D.; Kothari, V.K. Liquid transfer properties and drying behavior of plated knitted fabrics with varying fibre types. Indian J. Fibre Text. Res. 2015, 40, 162–169. [Google Scholar]
- Abdul-Bari, M.M.; McQueen, R.H.; Nguyen, H.; Wismer, W.V.; de la Mata, A.P.; Harynuk, J.J. Synthetic clothing and the problem with odor: Comparison of nylon and polyester fabrics. Cloth. Text. Res. J. 2018, 36, 251–266. [Google Scholar] [CrossRef]
- Sweeney, I.R.; Miraftab, M.; Collyer, G. Absorbent alginate fibres modified with hydrolysed chitosan for wound care dressings–II. pilot scale development. Carbohydr. Polym. 2014, 102, 920–927. [Google Scholar] [CrossRef]
- Lou, C.W.; Lin, C.W.; Chen, Y.S.; Yao, C.H.; Lin, Z.S.; Chao, C.Y.; Lin, J.H. Properties evaluation of Tencel/cotton nonwoven fabric coated with chitosan for wound dressing. Text. Res. J. 2008, 78, 248–253. [Google Scholar] [CrossRef]
- Lamke, L.-O.; Nilsson, G.; Reithner, H. The evaporative water loss from burns and the water-vapour permeability of grafts and artificial membranes used in the treatment of burns. Burns 1977, 3, 159–165. [Google Scholar] [CrossRef]
- Liu, X.; Liu, Y.; Du, J.; Li, X.; Yu, J.; Ding, B. Breathable, stretchable, and adhesive nanofibrous hydrogels as wound dressing materials. Eng. Regen. 2021, 2, 63–69. [Google Scholar] [CrossRef]
- Berman, B.; Perez, O.A.; Konda, S.; Kohut, B.E.; Viera, M.H.; Delgado, S.; Li, Q. A review of the biological effects, clinical efficacy, and safety of silicone elastomer sheeting for hypertrophic and keloid scar treatment and management. Dermatol. Surg. 2007, 33, 1291–1303. [Google Scholar]
- Kannon, G.A.; Garrett, A.B. Moist wound healing with occlusive dressings: A clinical review. Dermatol. Surg. 1995, 21, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Tran, B.; Wu, J.J.; Ratner, D.; Han, G. Topical scar treatment products for wounds: A systematic review. Dermatol. Surg. 2020, 46, 1564–1571. [Google Scholar] [CrossRef] [PubMed]
- Meyers, M.A.; Chen, P.Y. Biological Materials Science: Biological Materials, Bioinspired Materials, and Biomaterials; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Meyers, M.A.; Chen, P.Y.; Lin, A.Y.M.; Seki, Y. Biological materials: Structure and mechanical properties. Prog. Mater. Sci. 2008, 53, 1–206. [Google Scholar] [CrossRef]










| Sampling Groups | Apparent Porosity/% | Diameter of Average Pore Size/μm | S.D. for Pore Size Uniformity | # of Pores for Pore Distribution |
|---|---|---|---|---|
| HSP–2–1000 | 0.35 | 47 | 0.019 | 83 |
| HSP–4–1000 | 0.085 | 31 | 0.011 | 48 |
| HSP–6–1000 | 5.44 | 41 | 0.013 | 130 |
| HSP–8–1000 | 0.47 | 47 | 0.017 | 115 |
| HSP–25–1000 | 0.22 | 41 | 0.015 | 73 |
| HSP–50–1000 | 0.33 | 35 | 0.010 | 156 |
| HSP–75–1000 | 0.52 | 67 | 0.020 | 66 |
| HSP–100–1000 | 0.14 | 43 | 0.013 | 44 |
| HSP–2–2000 | 0.35 | 47 | 0.019 | 86 |
| HSP–4–2000 | 0.45 | 55 | 0.026 | 74 |
| HSP–6–2000 | 0.37 | 51 | 0.017 | 78 |
| HSP–8–2000 | 0.20 | 40 | 0.011 | 70 |
| HSP–25–2000 | 1.03 | 58 | 0.019 | 170 |
| HSP–50–2000 | 0.64 | 56 | 0.028 | 101 |
| HSP–75–2000 | 1.63 | 59 | 0.021 | 260 |
| HSP–100–2000 | 1.19 | 67 | 0.029 | 139 |
| Range analysis | 0.085–5.44 | 31–67 | 0.010–0.029 | 44–260 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lui, K.-C.; Noor, N.; Kan, C.-W.; Wang, X. A Self-Pumping Composite Dressing Improved Hypertrophic Scar Healing with Dual Therapy and Active-Fluid Transport. J. Compos. Sci. 2023, 7, 192. https://doi.org/10.3390/jcs7050192
Lui K-C, Noor N, Kan C-W, Wang X. A Self-Pumping Composite Dressing Improved Hypertrophic Scar Healing with Dual Therapy and Active-Fluid Transport. Journal of Composites Science. 2023; 7(5):192. https://doi.org/10.3390/jcs7050192
Chicago/Turabian StyleLui, Kam-Che, Nuruzzaman Noor, Chi-Wai Kan, and Xungai Wang. 2023. "A Self-Pumping Composite Dressing Improved Hypertrophic Scar Healing with Dual Therapy and Active-Fluid Transport" Journal of Composites Science 7, no. 5: 192. https://doi.org/10.3390/jcs7050192
APA StyleLui, K. -C., Noor, N., Kan, C. -W., & Wang, X. (2023). A Self-Pumping Composite Dressing Improved Hypertrophic Scar Healing with Dual Therapy and Active-Fluid Transport. Journal of Composites Science, 7(5), 192. https://doi.org/10.3390/jcs7050192









