Transforming Nanomaterial Synthesis through Advanced Microfluidic Approaches: A Review on Accessing Unrestricted Possibilities
Abstract
:1. Introduction
2. Methods of Preparation of Nanomaterials
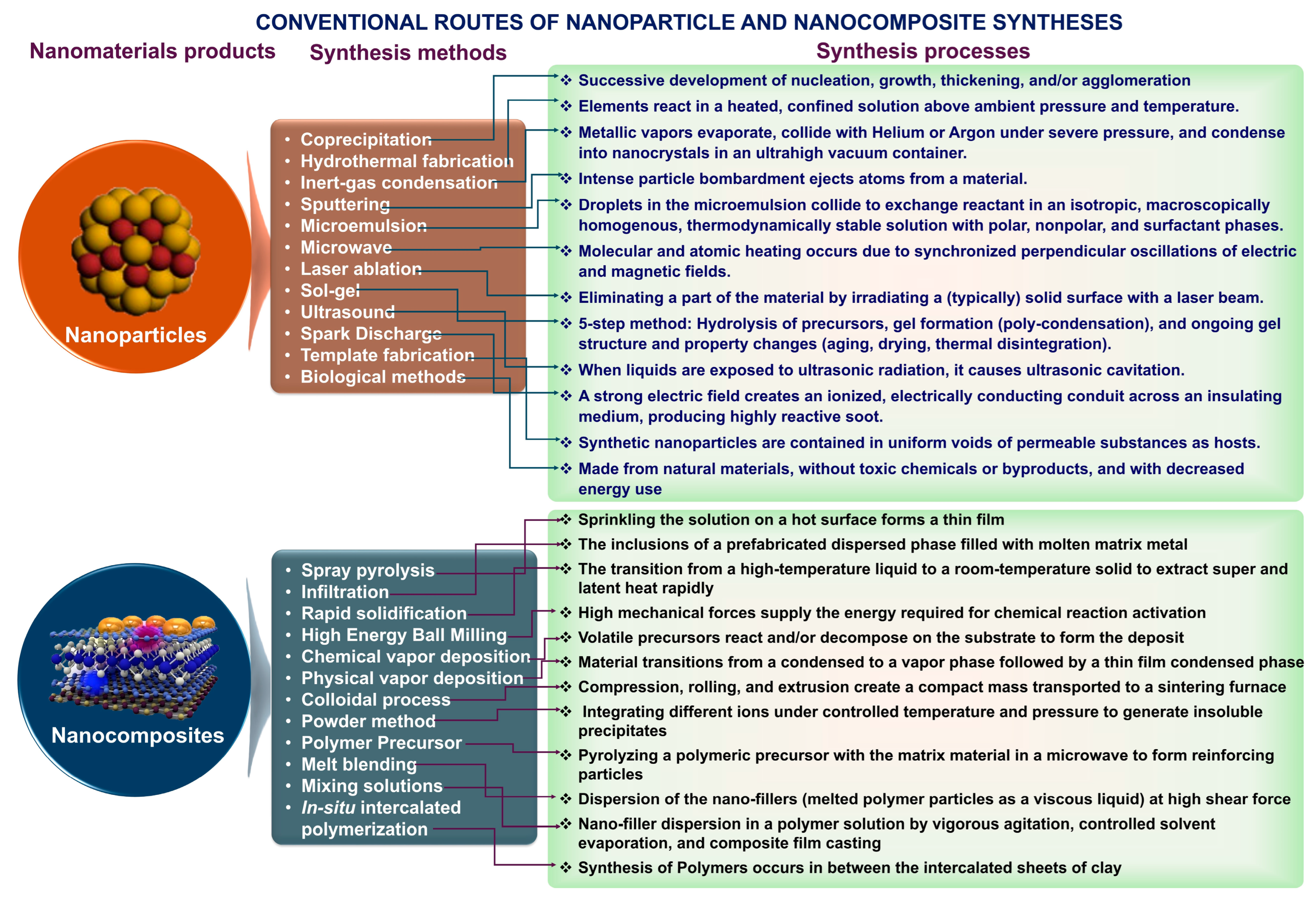
3. Conventional Methods Used in the Synthesis of Nanomaterials
3.1. The Top-Down Method
3.1.1. Mechanical Milling (Ball Milling)
3.1.2. Electrospinning
3.1.3. Lithography
3.1.4. Laser Ablation
3.1.5. Sputtering Method
3.1.6. The Arc Discharge Method
3.2. The Bottom-Up Method
3.2.1. Chemical Vapor Deposition (CVD)
3.2.2. Sol-Gel Method
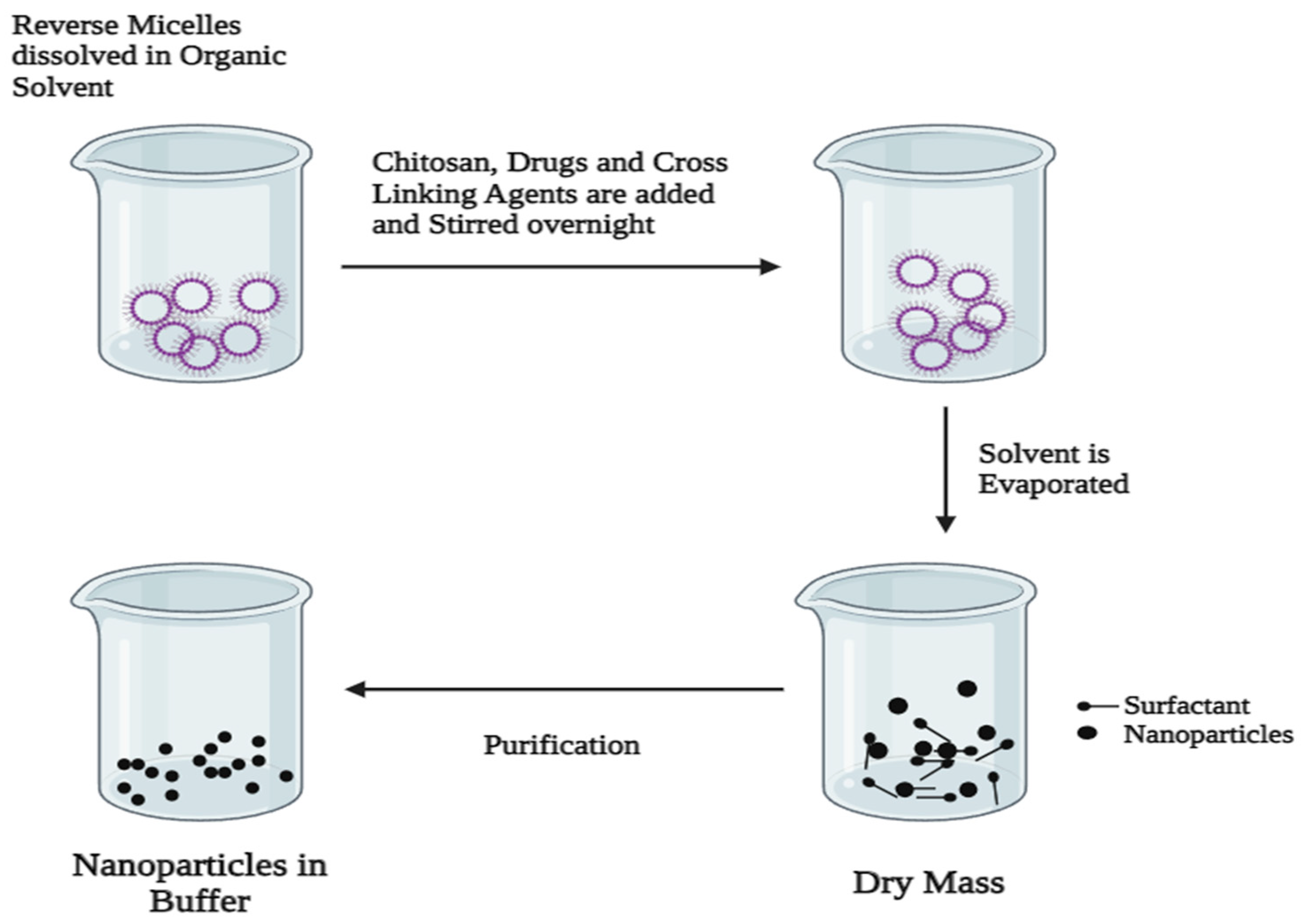
3.2.3. Reverse Micelles Method
3.2.4. Combined Soft-Hard Templating Methods
3.2.5. Microfluidics
Size
Shape and Structure
Rigidity
Surface Modification
4. Microfluidics Methods of Preparation of Nanomaterials
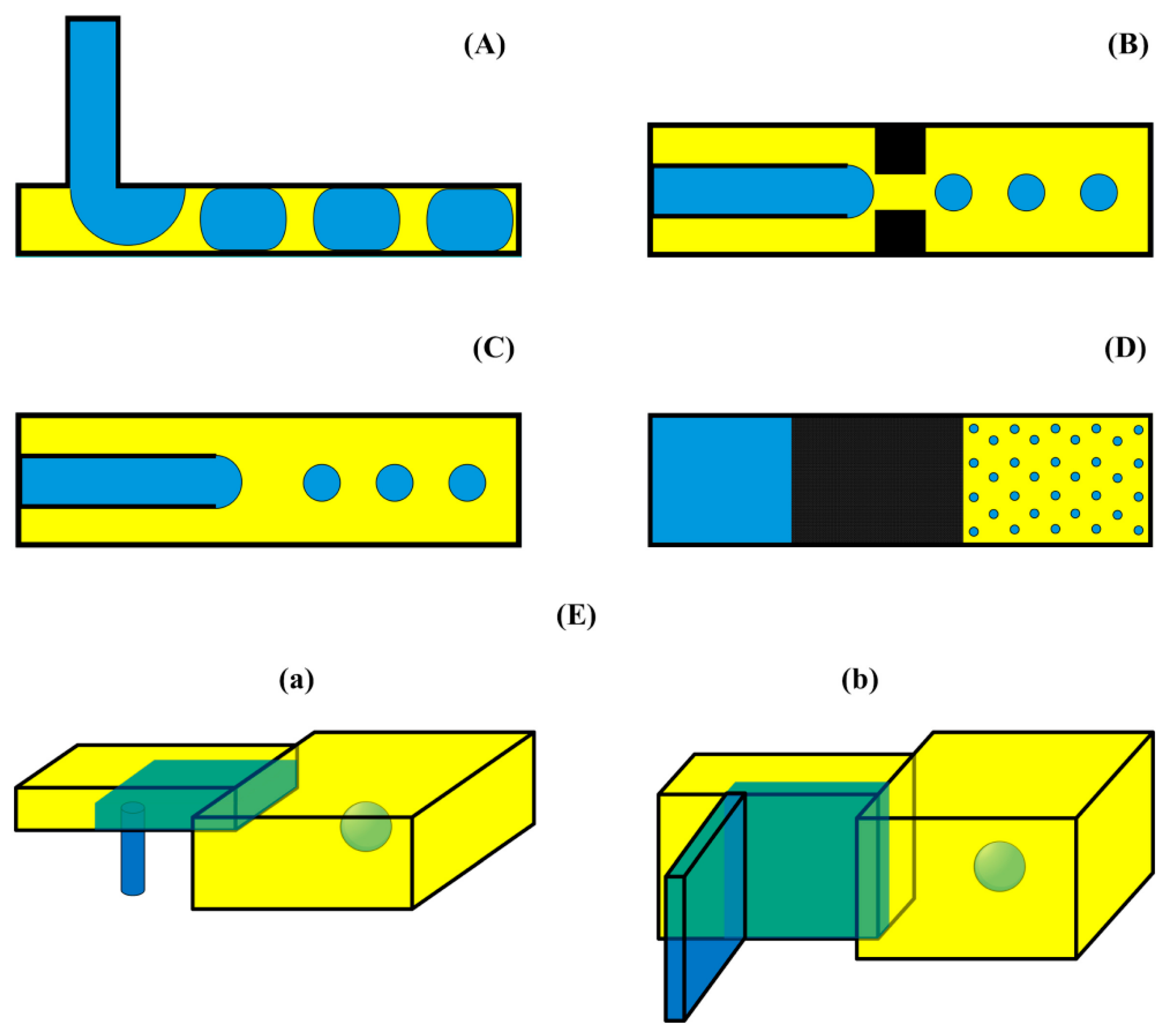
4.1. Single-Phase Flow Systems
4.2. Multi-Phase Flow (Droplet-Based) Systems
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Iqbal, P.; Preece, J.A.; Mendes, P.M. Nanotechnology: The “top-down” and “bottom-up” approaches. In Supramolecular Chemistry: From Molecules to Nanomaterials; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2012. [Google Scholar]
- Yusuf, M.; Kumar, R.; Ali Khan, M.; Ahmed, M.J.; Otero, M.; Muthu Prabhu, S.; Son, M.; Hwang, J.H.; Hyoung Lee, W.; Jeon, B.H. Metal-organic framework-based composites for biogas and natural gas uptake: An overview of adsorption and storage mechanisms of gaseous fuels. Chem. Eng. J. 2023, 478. [Google Scholar] [CrossRef]
- Alhamad, A.A.; Zeghoud, S.; Amor, I.B.; Zaater, A.; Amor, A.B.; Aouadif, A.; Hemmami, A. A short review of nanomaterials: Synthesis methods, properties, and applications. Algerian J. Chem. Eng. 2023, 1, 1–7. [Google Scholar]
- Singh, S.S.; Jena, B.; Roy, S.; Nayak, S.; Behera, S.K.; Chakrabortty, S.; Tripathy, S.K.; Ali Khan, M.; Kumar, R.; Jeon, B.-H.; et al. Sprayable biogenic Ag-collagen nanocomposites with potent antibacterial and antibiofilm activity for Acinetobacter baumannii infected wound healing under hyperglycemic condition. Chem. Eng. J. 2024, 490, 151788. [Google Scholar] [CrossRef]
- Saleh, T.A. Nanomaterials: Classification, properties, and environmental toxicities. Environ. Technol. Innov. 2020, 20, 101067. [Google Scholar] [CrossRef]
- Aljuhani, E.; Al-Ahmed, Z.A. Evaluation of the physical parameters of nano-sized tetrachlorosilane as an inorganic material a mixed solvent using Fuoss-Shedlovsky and Fuoss-Hsia-Fernandez-Prini techniques. Biointerface Res. Appl. Chem. 2020, 10, 5741–5746. [Google Scholar]
- Ejtemaee, P.; Khamehchi, E. Experimental investigation of rheological properties and formation damage of water-based drilling fluids in the presence of Al2O3, Fe3O4, and TiO2 nanoparticles. Biointerface Res. Appl. Chem 2020, 10, 5886–5894. [Google Scholar]
- Zhao, C.-X.; He, L.; Qiao, S.Z.; Middelberg, A.P. Nanoparticle synthesis in microreactors. Chem. Eng. Sci. 2011, 66, 1463–1479. [Google Scholar] [CrossRef]
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef]
- Agha, A.; Waheed, W.; Stiharu, I.; Nerguizian, V.; Destgeer, G.; Abu-Nada, E.; Alazzam, A. A review on microfluidic-assisted nanoparticle synthesis, and their applications using multiscale simulation methods. Discov. Nano 2023, 18, 18. [Google Scholar] [CrossRef]
- Bally, F.; Serra, C.A.; Hessel, V.; Hadziioannou, G. Micromixer-assisted polymerization processes. Chem. Eng. Sci. 2011, 66, 1449–1462. [Google Scholar] [CrossRef]
- Olanrewaju, A.; Beaugrand, M.; Yafia, M.; Juncker, D. Capillary microfluidics in microchannels: From microfluidic networks to capillaric circuits. Lab Chip 2018, 18, 2323–2347. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Cho, Y.H.; Park, M.S.; Kim, B.H. Microchannel fabrication on glass materials for microfluidic devices. Int. J. Precis. Eng. Manuf. 2019, 20, 479–495. [Google Scholar] [CrossRef]
- Preetam, S.; Nahak, B.K.; Patra, S.; Toncu, D.C.; Park, S.; Syväjärvi, M.; Orive, G.; Tiwari, A. Emergence of microfluidics for next generation biomedical devices. Biosens. Bioelectron. X 2022, 10, 100106. [Google Scholar] [CrossRef]
- Lai, X.; Lu, B.; Zhang, P.; Zhang, X.; Pu, Z.; Yu, H.; Li, D. Sticker microfluidics: A method for fabrication of customized monolithic microfluidics. ACS Biomater. Sci. Eng. 2019, 5, 6801–6810. [Google Scholar] [CrossRef]
- Ma, J.; Lee, S.M.-Y.; Yi, C.; Li, C.-W. Controllable synthesis of functional nanoparticles by microfluidic platforms for biomedical applications–a review. Lab Chip 2017, 17, 209–226. [Google Scholar] [CrossRef]
- Shrimal, P.; Jadeja, G.; Patel, S. A review on novel methodologies for drug nanoparticle preparation: Microfluidic approach. Chem. Eng. Res. Des. 2020, 153, 728–756. [Google Scholar] [CrossRef]
- Sebastian Cabeza, V. Chapter High and Efficient Production of Nanomaterials by Microfluidic Reactor Approaches; InTechOpen: London, UK, 2016. [Google Scholar]
- Huang, Y.; Liu, C.; Feng, Q.; Sun, J. Microfluidic synthesis of nanomaterials for biomedical applications. Nanoscale Horiz. 2023, 8, 610–1627. [Google Scholar] [CrossRef]
- Kamat, V.; Dey, P.; Bodas, D.; Kaushik, A.; Boymelgreen, A.; Bhansali, S. Active microfluidic reactor-assisted controlled synthesis of nanoparticles and related potential biomedical applications. J. Mater. Chem. B 2023, 11, 5650–5667. [Google Scholar] [CrossRef]
- Almeida, D.R.; Gil, J.F.; Guillot, A.J.; Li, J.; Pinto, R.J.; Santos, H.A.; Gonçalves, G. Advances in Microfluidic-based Core@ Shell Nanoparticles Fabrication for Cancer Applications. Adv. Healthcare Mater. 2024, 13, 2400946. [Google Scholar] [CrossRef]
- Udepurkar, A.P.; Mampaey, L.; Clasen, C.; Cabeza, V.S.; Kuhn, S. Microfluidic synthesis of PLGA nanoparticles enabled by an ultrasonic microreactor. React. Chem. Eng. 2024, 9, 2208–2217. [Google Scholar] [CrossRef]
- Mehraji, S.; DeVoe, D.L. Microfluidic synthesis of lipid-based nanoparticles for drug delivery: Recent advances and opportunities. Lab Chip 2024, 24, 1154–1174. [Google Scholar] [CrossRef] [PubMed]
- Chircov, C.; Dumitru, I.A.; Vasile, B.S.; Oprea, O.-C.; Holban, A.M.; Popescu, R.C. Microfluidic Synthesis of Magnetite Nanoparticles for the Controlled Release of Antibiotics. Pharmaceutics 2023, 15, 2215. [Google Scholar] [CrossRef] [PubMed]
- Saikia, A.; Newar, R.; Das, S.; Singh, A.; Deuri, D.J.; Baruah, A. Scopes and challenges of microfluidic technology for nanoparticle synthesis, photocatalysis and sensor applications: A comprehensive review. Chem. Eng. Res. Des. 2023, 193, 516–539. [Google Scholar] [CrossRef]
- Zhang, Y.-F.; Zhang, S.; Zhan, L.-W.; Tang, W.-Y.; Hou, J.; Li, B.-D. Application of microfluidic technology on preparation of nano LLM-105. J. Energetic Mater. 2024, 42, 391–405. [Google Scholar] [CrossRef]
- Niculescu, A.-G.; Mihaiescu, D.E.; Grumezescu, A.M. A review of microfluidic experimental designs for nanoparticle synthesis. Int. J. Mol. Sci. 2022, 23, 8293. [Google Scholar] [CrossRef]
- Khizar, S.; Zine, N.; Errachid, A.; Jaffrezic-Renault, N.; Elaissari, A. Microfluidic-based nanoparticle synthesis and their potential applications. Electrophoresis 2022, 43, 819–838. [Google Scholar] [CrossRef]
- Hamdallah, S.I.; Zoqlam, R.; Erfle, P.; Blyth, M.; Alkilany, A.M.; Dietzel, A.; Qi, S. Microfluidics for pharmaceutical nanoparticle fabrication: The truth and the myth. Int. J. Pharm. 2020, 584, 119408. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, X. Why microfluidics? Merits and trends in chemical synthesis. Lab Chip 2017, 17, 3960–3978. [Google Scholar] [CrossRef]
- Augustine, R.; Hasan, A. Multimodal applications of phytonanoparticles. In Phytonanotechnology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 195–219. [Google Scholar]
- Chatterjee, A.; Kwatra, N.; Abraham, J. Nanoparticles fabrication by plant extracts. In Phytonanotechnology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 143–157. [Google Scholar]
- Fajar, M.N.; Endarko, E.; Rubiyanto, A.; Malek, N.A.N.N.; Hadibarata, T.; Syafiuddin, A. A green deposition method of silver nanoparticles on textiles and their antifungal activity. Biointerface Res. Appl. Chem. 2020, 10, 4902–4907. [Google Scholar]
- Fahmy, A.; Zaid, H.; Ibrahim, M. Optimizing the electrospun parameters which affect the preparation of nanofibers. Biointerface Res. Appl. Chem 2019, 9, 4463–4473. [Google Scholar]
- Liu, D.; Cito, S.; Zhang, Y.; Wang, C.F.; Sikanen, T.M.; Santos, H.A. A versatile and robust microfluidic platform toward high throughput synthesis of homogeneous nanoparticles with tunable properties. Adv. Mater. 2015, 27, 2298–2304. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.A.; Wu, H.; Williams, K.R.; Cao, Y.C. Synthesis of CdSe and CdTe nanocrystals without precursor injection. Angew. Chem. Int. Ed. 2005, 44, 6712–6715. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Bernuz, C.R.; Fan, J.; Li, W.; Correia, A.; Hirvonen, J.; Santos, H.A. A nano-in-nano vector: Merging the best of polymeric nanoparticles and drug nanocrystals. Adv. Funct. Mater. 2017, 27, 1604508. [Google Scholar] [CrossRef]
- Ahmed, B.S.; Mostafa, A.A.; Darwesh, O.M.; Abdel-Rahim, E.A. Development of specific nano-antibody for application in selective and rapid environmental diagnoses of Salmonella arizonae. Biointerface Res. Appl. Chem. 2020, 10, 7198–7208. [Google Scholar]
- Monajjemi, M.; Naghsh, F.; Mollaamin, F. Bio-lipid nano capacitors: Resonance with helical myeline proteins. Biointerface Res. Appl. Chem 2020, 10, 6695–6705. [Google Scholar]
- Zhao, X.; Bian, F.; Sun, L.; Cai, L.; Li, L.; Zhao, Y. Microfluidic generation of nanomaterials for biomedical applications. Small 2020, 16, 1901943. [Google Scholar] [CrossRef]
- Niculescu, A.-G.; Chircov, C.; Bîrcă, A.C.; Grumezescu, A.M. Nanomaterials synthesis through microfluidic methods: An updated overview. Nanomaterials 2021, 11, 864. [Google Scholar] [CrossRef]
- Baig, N.; Kammakakam, I.; Falath, W. Nanomaterials: A review of synthesis methods, properties, recent progress, and challenges. Mater. Adv. 2021, 2, 1821–1871. [Google Scholar] [CrossRef]
- Sabdono, P.; Sustiawan, F.; Fadlillah, D.A. The effect of nano-cement content to the compressive strength of mortar. Procedia Eng. 2014, 95, 386–395. [Google Scholar] [CrossRef]
- Shah, M.; Sahoo, K.L.; Das, S.K.; Das, G. Wear mechanism of high chromium white cast iron and its microstructural evolutions during the comminution process. Tribol. Lett. 2020, 68, 77. [Google Scholar] [CrossRef]
- Benjamin, J.S. Mechanical alloying—A perspective. Met. Powder Rep. 1990, 45, 122–127. [Google Scholar] [CrossRef]
- Zhang, D.; Bi, H.; Liu, B.; Qiao, L. Detection of pathogenic microorganisms by microfluidics based analytical methods. Anal. Chem. 2018, 90, 5512–5520. [Google Scholar] [CrossRef] [PubMed]
- Mei, Q.; Lu, K. Melting and superheating of crystalline solids: From bulk to nanocrystals. Prog. Mater. Sci. 2007, 52, 1175–1262. [Google Scholar] [CrossRef]
- Jia, Y.; Yang, C.; Chen, X.; Xue, W.; Hutchins-Crawford, H.J.; Yu, Q.; Topham, P.D.; Wang, L. A review on electrospun magnetic nanomaterials: Methods, properties and applications. J. Mater. Chem. C 2021, 9, 9042–9082. [Google Scholar] [CrossRef]
- Song, X.; Cheng, G.; Cheng, B.; Xing, J. Electrospun polyacrylonitrile/magnetic Fe3O4–polyhedral oligomeric silsesquioxanes nanocomposite fibers with enhanced filter performance for electrets filter media. J. Mater. Res. 2016, 31, 2662–2671. [Google Scholar] [CrossRef]
- Cheng, C.; Dai, J.; Li, Z.; Feng, W. Preparation and magnetic properties of CoFe2O4 oriented fiber arrays by electrospinning. Materials 2020, 13, 3860. [Google Scholar] [CrossRef]
- Kumar, P.S.; Sundaramurthy, J.; Sundarrajan, S.; Babu, V.J.; Singh, G.; Allakhverdiev, S.I.; Ramakrishna, S. Hierarchical electrospun nanofibers for energy harvesting, production and environmental remediation. Energy Environ. Sci. 2014, 7, 3192–3222. [Google Scholar]
- Du, P.; Song, L.; Xiong, J.; Li, N.; Xi, Z.; Wang, L.; Jin, D.; Guo, S.; Yuan, Y. Coaxial electrospun TiO2/ZnO core–sheath nanofibers film: Novel structure for photoanode of dye-sensitized solar cells. Electrochim. Acta 2012, 78, 392–397. [Google Scholar] [CrossRef]
- Paramasivam, G.; Palem, V.V.; Sundaram, T.; Sundaram, V.; Kishore, S.C.; Bellucci, S. Nanomaterials: Synthesis and applications in theranostics. Nanomaterials 2021, 11, 3228. [Google Scholar] [CrossRef]
- Qu, C.; Kinzel, E.C. Infrared metasurfaces created with off-normal incidence microsphere photolithography. Opt. Express 2017, 25, 12632–12639. [Google Scholar] [CrossRef]
- Kuo, C.W.; Shiu, J.Y.; Cho, Y.H.; Chen, P. Fabrication of large-area periodic nanopillar arrays for nanoimprint lithography using polymer colloid masks. Adv. Mater. 2003, 15, 1065–1068. [Google Scholar] [CrossRef]
- Yin, Y.; Gates, B.; Xia, Y. A soft lithography approach to the fabrication of nanostructures of single crystalline silicon with well-defined dimensions and shapes. Adv. Mater. 2000, 12, 1426–1430. [Google Scholar] [CrossRef]
- Crivellaro, S.; Guadagnini, A.; Arboleda, D.M.; Schinca, D.; Amendola, V. A system for the synthesis of nanoparticles by laser ablation in liquid that is remotely controlled with PC or smartphone. Rev. Sci. Instrum. 2019, 90, 033902. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Osone, S.; Kim, T.; Higashi, H.; Seto, T. Synthesis of nanoparticles by laser ablation: A review. KONA Powder Part. J. 2017, 34, 80–90. [Google Scholar] [CrossRef]
- Alheshibri, M. Fabrication of Au–Ag bimetallic nanoparticles using pulsed laser ablation for medical applications: A review. Nanomaterials 2023, 13, 2940. [Google Scholar] [CrossRef]
- Duque, J.S.; Madrigal, B.M.; Riascos, H.; Avila, Y.P. Colloidal metal oxide nanoparticles prepared by laser ablation technique and their antibacterial test. Colloids Interfaces 2019, 3, 25. [Google Scholar] [CrossRef]
- Park, H.; Reddy, D.A.; Kim, Y.; Lee, S.; Ma, R.; Kim, T.K. Synthesis of ultra-small palladium nanoparticles deposited on CdS nanorods by pulsed laser ablation in liquid: Role of metal nanocrystal size in the photocatalytic hydrogen production. Chem.–A Eur. J. 2017, 23, 13112–13119. [Google Scholar] [CrossRef]
- Son, H.H.; Seo, G.H.; Jeong, U.; Kim, S.J. Capillary wicking effect of a Cr-sputtered superhydrophilic surface on enhancement of pool boiling critical heat flux. Int. J. Heat Mass Transf. 2017, 113, 115–128. [Google Scholar] [CrossRef]
- Wender, H.; Migowski, P.; Feil, A.F.; Teixeira, S.R.; Dupont, J. Sputtering deposition of nanoparticles onto liquid substrates: Recent advances and future trends. Coord. Chem. Rev. 2013, 257, 2468–2483. [Google Scholar] [CrossRef]
- Muñoz-García, J.; Vázquez, L.; Cuerno, R.; Sánchez-García, J.; Castro, M.; Gago, R.; Wang, Z. Toward Functional Nanomaterials; Wang, Z.W., Ed.; Springer: Dordrecht, The Netherlands, 2009; p. 323. [Google Scholar]
- Nam, J.H.; Jang, M.J.; Jang, H.Y.; Park, W.; Wang, X.; Choi, S.M.; Cho, B. Room-temperature sputtered electrocatalyst WSe2 nanomaterials for hydrogen evolution reaction. J. Energy Chem. 2020, 47, 107–111. [Google Scholar] [CrossRef]
- Nie, M.; Sun, K.; Meng, D.D. Formation of metal nanoparticles by short-distance sputter deposition in a reactive ion etching chamber. J. Appl. Phys. 2009, 106, 054314. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, K.; Yao, Y.; Liang, F.; Qu, T.; Ma, W.; Yang, B.; Dai, Y.; Lei, Y. Intercalation and exfoliation syntheses of high specific surface area graphene and FeC2O4/graphene composite for anode material of lithium ion battery. Fuller. Nanotub. Carbon Nanostruct. 2019, 27, 746–754. [Google Scholar] [CrossRef]
- Liehr, A.D. Solid State Physics. Advances in Research and Applications. J. Am. Chem. Soc. 1960, 82, 2658–2659. [Google Scholar] [CrossRef]
- Liang, F.; Tanaka, M.; Choi, S.; Watanabe, T. Formation of different arc-anode attachment modes and their effect on temperature fluctuation for carbon nanomaterial production in DC arc discharge. Carbon 2017, 117, 100–111. [Google Scholar] [CrossRef]
- Liang, F.; Shimizu, T.; Tanaka, M.; Choi, S.; Watanabe, T. Selective preparation of polyhedral graphite particles and multi-wall carbon nanotubes by a transferred arc under atmospheric pressure. Diam. Relat. Mater. 2012, 30, 70–76. [Google Scholar] [CrossRef]
- Li, N.; Wang, Z.; Zhao, K.; Shi, Z.; Gu, Z.; Xu, S. Synthesis of single-wall carbon nanohorns by arc-discharge in air and their formation mechanism. Carbon 2010, 48, 1580–1585. [Google Scholar] [CrossRef]
- Wu, Z.-S.; Ren, W.; Gao, L.; Zhao, J.; Chen, Z.; Liu, B.; Tang, D.; Yu, B.; Jiang, C.; Cheng, H.-M. Synthesis of graphene sheets with high electrical conductivity and good thermal stability by hydrogen arc discharge exfoliation. ACS Nano 2009, 3, 411–417. [Google Scholar] [CrossRef]
- Dion, C.D.; Tavares, J.R. Photo-initiated chemical vapor deposition as a scalable particle functionalization technology (a practical review). Powder Technol. 2013, 239, 484–491. [Google Scholar] [CrossRef]
- Jones, A.C.; Hitchman, M.L. Overview of chemical vapour deposition. Chem. Vap. Depos. Precursors Process. Appl. 2009, 1, 1–36. [Google Scholar]
- Mandracci, P. Chemical Vapor Deposition for Nanotechnology; IntechOpen: London, UK, 2019. [Google Scholar]
- Parashar, M.; Shukla, V.K.; Singh, R. Metal oxides nanoparticles via sol–gel method: A review on synthesis, characterization and applications. J. Mater. Sci. Mater. Electron. 2020, 31, 3729–3749. [Google Scholar] [CrossRef]
- Bokov, D.; Turki Jalil, A.; Chupradit, S.; Suksatan, W.; Javed Ansari, M.; Shewael, I.H.; Valiev, G.H.; Kianfar, E. Nanomaterial by sol-gel method: Synthesis and application. Adv. Mater. Sci. Eng. 2021, 2021, 5102014. [Google Scholar] [CrossRef]
- Gupta, S.M.; Tripathi, M. A review on the synthesis of TiO2 nanoparticles by solution route. Cent. Eur. J. Chem. 2012, 10, 279–294. [Google Scholar] [CrossRef]
- Arya, S.; Mahajan, P.; Mahajan, S.; Khosla, A.; Datt, R.; Gupta, V.; Young, S.-J.; Oruganti, S.K. Influence of processing parameters to control morphology and optical properties of Sol-Gel synthesized ZnO nanoparticles. ECS J. Solid State Sci. Technol. 2021, 10, 023002. [Google Scholar] [CrossRef]
- Ristić, M.; Musić, S.; Ivanda, M.; Popović, S. Sol–gel synthesis and characterization of nanocrystalline ZnO powders. J. Alloys Compd. 2005, 397, L1–L4. [Google Scholar] [CrossRef]
- Yue, S.; Yan, Z.; Shi, Y.; Ran, G. Synthesis of zinc oxide nanotubes within ultrathin anodic aluminum oxide membrane by sol–gel method. Mater. Lett. 2013, 98, 246–249. [Google Scholar] [CrossRef]
- Lakshmi, B.B.; Dorhout, P.K.; Martin, C.R. Sol−gel template synthesis of semiconductor nanostructures. Chem. Mater. 1997, 9, 857–862. [Google Scholar] [CrossRef]
- Lone, I.H.; Radwan, N.R.; Aslam, J.; Akhter, A. Concept of reverse micelle method for the synthesis of nano-structured materials. Curr. Nanosci. 2019, 15, 129–136. [Google Scholar] [CrossRef]
- Ghosh, S. Comparative studies on brij reverse micelles prepared in benzene/surfactant/ethylammonium nitrate systems: Effect of head group size and polarity of the hydrocarbon chain. J. Colloid Interface Sci. 2011, 360, 672–680. [Google Scholar] [CrossRef]
- Nasi, R.; Esposito, S.; Freyria, F.S.; Armandi, M.; Gadhi, T.A.; Hernandez, S.; Rivolo, P.; Ditaranto, N.; Bonelli, B. Application of reverse micelle sol–gel synthesis for bulk doping and heteroatoms surface enrichment in mo-doped TiO2 nanoparticles. Materials 2019, 12, 937. [Google Scholar] [CrossRef]
- Chandra, P.; Doke, D.S.; Umbarkar, S.B.; Biradar, A.V. One-pot synthesis of ultrasmall MoO3 nanoparticles supported on SiO2, TiO2, and ZrO2 nanospheres: An efficient epoxidation catalyst. J. Mater. Chem. A 2014, 2, 19060–19066. [Google Scholar] [CrossRef]
- Liu, Y.; Goebl, J.; Yin, Y. Themed issue: Chemistry of functional nanomaterials. Chem. Soc. Rev 2013, 42, 2610–2653. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhao, D. An overview of the synthesis of ordered mesoporous materials. Chem. Commun. 2013, 49, 943–946. [Google Scholar] [CrossRef] [PubMed]
- Poolakkandy, R.R.; Menamparambath, M.M. Soft-template-assisted synthesis: A promising approach for the fabrication of transition metal oxides. Nanoscale Adv. 2020, 2, 5015–5045. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yang, T.; Wang, D.-W.; Lu, G.Q.; Zhao, D.; Qiao, S.Z. A facile soft-template synthesis of mesoporous polymeric and carbonaceous nanospheres. Nat. Commun. 2013, 4, 2798. [Google Scholar] [CrossRef]
- Tang, T.; Zhang, T.; Li, W.; Huang, X.; Wang, X.; Qiu, H.; Hou, Y. Mesoporous N-doped graphene prepared by a soft-template method with high performance in Li–S batteries. Nanoscale 2019, 11, 7440–7446. [Google Scholar] [CrossRef]
- Szczęśniak, B.; Choma, J.; Jaroniec, M. Major advances in the development of ordered mesoporous materials. Chem. Commun. 2020, 56, 7836–7848. [Google Scholar] [CrossRef]
- Hurst, S.J.; Payne, E.K.; Qin, L.; Mirkin, C.A. Multisegmented one-dimensional nanorods prepared by hard-template synthetic methods. Angew. Chem. Int. Ed. 2006, 45, 2672–2692. [Google Scholar] [CrossRef]
- Kumar, M.; Xiong, X.; Wan, Z.; Sun, Y.; Tsang, D.C.; Gupta, J.; Gao, B.; Cao, X.; Tang, J.; Ok, Y.S. Ball milling as a mechanochemical technology for fabrication of novel biochar nanomaterials. Bioresour. Technol. 2020, 312, 123613. [Google Scholar] [CrossRef]
- Schrittwieser, S.; Haslinger, M.J.; Mitteramskogler, T.; Mühlberger, M.; Shoshi, A.; Brückl, H.; Bauch, M.; Dimopoulos, T.; Schmid, B.; Schotter, J. Multifunctional nanostructures and nanopocket particles fabricated by nanoimprint lithography. Nanomaterials 2019, 9, 1790. [Google Scholar] [CrossRef]
- Chakrabartty, I.; Hakeem, K.R.; Mohanta, Y.K.; Varma, R.S. Greener nanomaterials and their diverse applications in the energy sector. Clean Technol. Environ. 2022, 24, 3237–3252. [Google Scholar] [CrossRef]
- Gu, F.; Wang, S.F.; Song, C.F.; Lü, M.K.; Qi, Y.X.; Zhou, G.J.; Xu, D.; Yuan, D.R. Synthesis and luminescence properties of SnO2 nanoparticles. Chem. Phys. Lett. 2003, 372, 451–454. [Google Scholar] [CrossRef]
- Mahato, T.; Prasad, G.; Singh, B.; Acharya, J.; Srivastava, A.; Vijayaraghavan, R. Nanocrystalline zinc oxide for the decontamination of sarin. J. Hazard. Mater. 2009, 165, 928–932. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, J.-G.; Kamiyama, H.; Ishigaki, T. Fe-doped TiO2 nanopowders by oxidative pyrolysis of organometallic precursors in induction thermal plasma: Synthesis and structural characterization. Thin Solid Films 2006, 506, 278–282. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, Q.; Ma, Y.; Sun, J. Microfluidic methods for fabrication and engineering of nanoparticle drug delivery systems. ACS Appl. Bio Mater. 2019, 3, 107–120. [Google Scholar] [CrossRef]
- Ren, K.; Zhou, J.; Wu, H. Materials for microfluidic chip fabrication. Acc. Chem. Res. 2013, 46, 2396–2406. [Google Scholar] [CrossRef]
- Liao, S.; He, Y.; Chu, Y.; Liao, H.; Wang, Y. Solvent-resistant and fully recyclable perfluoropolyether-based elastomer for microfluidic chip fabrication. J. Mater. Chem. A 2019, 7, 16249–16256. [Google Scholar] [CrossRef]
- Nielsen, J.B.; Hanson, R.L.; Almughamsi, H.M.; Pang, C.; Fish, T.R.; Woolley, A.T. Microfluidics: Innovations in materials and their fabrication and functionalization. Anal. Chem. 2019, 92, 150–168. [Google Scholar] [CrossRef]
- Guckenberger, D.J.; De Groot, T.E.; Wan, A.M.; Beebe, D.J.; Young, E.W. Micromilling: A method for ultra-rapid prototyping of plastic microfluidic devices. Lab Chip 2015, 15, 2364–2378. [Google Scholar] [CrossRef]
- Waldbaur, A.; Rapp, H.; Länge, K.; Rapp, B.E. Let there be chip—Towards rapid prototyping of microfluidic devices: One-step manufacturing processes. Anal. Methods 2011, 3, 2681–2716. [Google Scholar] [CrossRef]
- Carrell, C.; Kava, A.; Nguyen, M.; Menger, R.; Munshi, Z.; Call, Z.; Nussbaum, M.; Henry, C. Beyond the lateral flow assay: A review of paper-based microfluidics. Microelectron. Eng. 2019, 206, 45–54. [Google Scholar] [CrossRef]
- Sachdeva, S.; Davis, R.W.; Saha, A.K. Microfluidic point-of-care testing: Commercial landscape and future directions. Front. Bioeng. Biotechnol. 2021, 8, 602659. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Cai, L.; Chang, J.; Li, S.; Liu, C.; Li, T.; Sun, J. Label-free isolation of rare tumor cells from untreated whole blood by interfacial viscoelastic microfluidics. Lab Chip 2018, 18, 3436–3445. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ding, B.; Chen, Q.; Feng, Q.; Lin, L.; Sun, J. Point-of-care-testing of nucleic acids by microfluidics. TrAC Trends Anal. Chem. 2017, 94, 106–116. [Google Scholar] [CrossRef]
- Belliveau, N.M.; Huft, J.; Lin, P.J.; Chen, S.; Leung, A.K.; Leaver, T.J.; Wild, A.W.; Lee, J.B.; Taylor, R.J.; Tam, Y.K. Microfluidic synthesis of highly potent limit-size lipid nanoparticles for in vivo delivery of siRNA. Molecular Therapy-Nucleic Acids 2012, 1, e37. [Google Scholar] [CrossRef]
- Valencia, P.M.; Farokhzad, O.C.; Karnik, R.; Langer, R. Microfluidic technologies for accelerating the clinical translation of nanoparticles. In Nano-Enabled Medical Applications; Jenny Stanford Publishing: Singapore, 2020; pp. 93–112. [Google Scholar]
- Valencia, P.M.; Pridgen, E.M.; Rhee, M.; Langer, R.; Farokhzad, O.C.; Karnik, R. Microfluidic platform for combinatorial synthesis and optimization of targeted nanoparticles for cancer therapy. ACS Nano 2013, 7, 10671–10680. [Google Scholar] [CrossRef]
- Zhang, S.; Gao, H.; Bao, G. Physical principles of nanoparticle cellular endocytosis. ACS Nano 2015, 9, 8655–8671. [Google Scholar] [CrossRef]
- Kimura, N.; Maeki, M.; Sato, Y.; Note, Y.; Ishida, A.; Tani, H.; Harashima, H.; Tokeshi, M. Development of the iLiNP device: Fine tuning the lipid nanoparticle size within 10 nm for drug delivery. ACS Omega 2018, 3, 5044–5051. [Google Scholar] [CrossRef]
- Thiermann, R.; Mueller, W.; Montesinos-Castellanos, A.; Metzke, D.; Löb, P.; Hessel, V.; Maskos, M. Size controlled polymersomes by continuous self-assembly in micromixers. Polymer 2012, 53, 2205–2210. [Google Scholar] [CrossRef]
- Mijajlovic, M.; Wright, D.; Zivkovic, V.; Bi, J.; Biggs, M. Microfluidic hydrodynamic focusing based synthesis of POPC liposomes for model biological systems. Colloids Surf. B Biointerfaces 2013, 104, 276–281. [Google Scholar] [CrossRef]
- Gunduz, O.; Ahmad, Z.; Stride, E.; Edirisinghe, M. Continuous generation of ethyl cellulose drug delivery nanocarriers from microbubbles. Pharm. Res. 2013, 30, 225–237. [Google Scholar] [CrossRef]
- Zhang, L.; Feng, Q.; Wang, J.; Zhang, S.; Ding, B.; Wei, Y.; Dong, M.; Ryu, J.-Y.; Yoon, T.-Y.; Shi, X. Microfluidic synthesis of hybrid nanoparticles with controlled lipid layers: Understanding flexibility-regulated cell–nanoparticle interaction. ACS Nano 2015, 9, 9912–9921. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Park, K. Effects of the microparticle shape on cellular uptake. Mol. Pharm. 2016, 13, 2164–2171. [Google Scholar] [CrossRef] [PubMed]
- Dashtimoghadam, E.; Mirzadeh, H.; Taromi, F.A.; Nyström, B. Microfluidic self-assembly of polymeric nanoparticles with tunable compactness for controlled drug delivery. Polymer 2013, 54, 4972–4979. [Google Scholar] [CrossRef]
- Hui, Y.; Yi, X.; Hou, F.; Wibowo, D.; Zhang, F.; Zhao, D.; Gao, H.; Zhao, C.-X. Role of nanoparticle mechanical properties in cancer drug delivery. ACS Nano 2019, 13, 7410–7424. [Google Scholar] [CrossRef]
- Feng, Q.; Liu, J.; Li, X.; Chen, Q.; Sun, J.; Shi, X.; Ding, B.; Yu, H.; Li, Y.; Jiang, X. One-step microfluidic synthesis of nanocomplex with tunable rigidity and acid-switchable surface charge for overcoming drug resistance. Small 2017, 13, 1603109. [Google Scholar] [CrossRef]
- Di Santo, R.; Digiacomo, L.; Palchetti, S.; Palmieri, V.; Perini, G.; Pozzi, D.; Papi, M.; Caracciolo, G. Microfluidic manufacturing of surface-functionalized graphene oxide nanoflakes for gene delivery. Nanoscale 2019, 11, 2733–2741. [Google Scholar] [CrossRef]
- Damiati, S.; Kompella, U.B.; Damiati, S.A.; Kodzius, R. Microfluidic devices for drug delivery systems and drug screening. Genes 2018, 9, 103. [Google Scholar] [CrossRef]
- Yu, B.; Lee, R.J.; Lee, L.J. Microfluidic methods for production of liposomes. Methods Enzymol. 2009, 465, 129–141. [Google Scholar]
- Fick, A. Ueber Diffusion. Ann. Der Phys. 1855, 170, 59–86. [Google Scholar] [CrossRef]
- Reynolds, O. XXIX. An experimental investigation of the circumstances which determine whether the motion of water shall be direct or sinuous, and of the law of resistance in parallel channels. Philos. Trans. R. Soc. Lond. 1997, 174, 935–982. [Google Scholar] [CrossRef]
- Wang, C.-T.; Hu, Y.-C.; Hu, T.-Y. Biophysical Micromixer. Sensors 2009, 9, 5379–5389. [Google Scholar] [CrossRef] [PubMed]
- Gimondi, S.; Ferreira, H.; Reis, R.L.; Neves, N.M. Microfluidic devices: A tool for nanoparticle synthesis and performance evaluation. ACS Nano 2023, 17, 14205–14228. [Google Scholar] [CrossRef] [PubMed]
- Rasouli, M.R.; Tabrizian, M. An ultra-rapid acoustic micromixer for synthesis of organic nanoparticles. Lab Chip 2019, 19, 3316–3325. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.O.; Park, K.-S.; Kim, D.-Y.; Lee, S.S.; Lee, C.-S.; Kim, J.M. Gear-shaped micromixer for synthesis of silica particles utilizing inertio-elastic flow instability. Lab Chip 2021, 21, 513–520. [Google Scholar] [CrossRef]
- Singh, A.D.; Limaye, M.V.; Singh, S.B.; Lalla, N.P.; Malek, C.K.; Kulkarni, S.K. A facile and fast approach for the synthesis of doped nanoparticles using a microfluidic device. Nanotechnology 2008, 19, 245613. [Google Scholar] [CrossRef]
- Chen, X.; Arruebo, M.; Yeung, K.L. Flow-synthesis of mesoporous silicas and their use in the preparation of magnetic catalysts for Knoevenagel condensation reactions. Catal. Today 2013, 204, 140–147. [Google Scholar] [CrossRef]
- Han, S.-Y.; Paul, B.K.; Chang, C.-h. Nanostructured ZnO as biomimetic anti-reflective coatings on textured silicon using a continuous solution process. J. Mater. Chem. 2012, 22, 22906–22912. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Z.; Cai, Y.; Song, Q.; Wang, B. Synthesis of Cu2O Nanoparticles by Ellipse Curve Micromixer. ACS Omega 2023, 8, 29758–29769. [Google Scholar] [CrossRef]
- Chang, M.; Gabayno, J.L.F.; Ye, R.; Huang, K.-W.; Chang, Y.-J. Mixing efficiency enhancing in micromixer by controlled magnetic stirring of Fe3O4 nanomaterial. Microsyst. Technol. 2017, 23, 457–463. [Google Scholar] [CrossRef]
- Patil, G.A.; Bari, M.L.; Bhanvase, B.A.; Ganvir, V.; Mishra, S.; Sonawane, S.H. Continuous synthesis of functional silver nanoparticles using microreactor: Effect of surfactant and process parameters. Chem. Eng. Process. Process Intensif. 2012, 62, 69–77. [Google Scholar] [CrossRef]
- Song, Y.; Li, R.; Sun, Q.; Jin, P. Controlled growth of Cu nanoparticles by a tubular microfluidic reactor. Chem. Eng. J. 2011, 168, 477–484. [Google Scholar] [CrossRef]
- Liu, G.; Li, Z.; Li, X.; Luo, Y.; Wang, X.; Zhu, D.; Yang, Z.; Wang, J. Controllable Synthesis of Silver Nanoparticles Using a Double-Layer Y-Shaped SAR Micromixer. Nano 2020, 15, 2050068. [Google Scholar] [CrossRef]
- Magdalene, D.J.; Muthuselvam, D.; Pravinraj, T. Microfluidics-based green synthesis of silver nanoparticle from the aqueous leaf extract of Ipomea quamoclit L. Appl. Nanosci. 2021, 11, 2073–2084. [Google Scholar] [CrossRef]
- Maged, A.; Abdelbaset, R.; Mahmoud, A.A.; Elkasabgy, N.A. Merits and advances of microfluidics in the pharmaceutical field: Design technologies and future prospects. Drug Deliv. 2022, 29, 1549–1570. [Google Scholar] [CrossRef] [PubMed]
- Cai, G.; Xue, L.; Zhang, H.; Lin, J. A Review on Micromixers. Micromachines 2017, 8, 274. [Google Scholar] [CrossRef]
- Tata Rao, L.; Goel, S.; Kumar Dubey, S.; Javed, A. Performance Investigation of T-Shaped Micromixer with Different Obstacles. J. Phys. Conf. Ser. 2019, 1276, 012003. [Google Scholar] [CrossRef]
- Niculescu, A.-G.; Munteanu, O.M.; Bîrcă, A.C.; Moroșan, A.; Purcăreanu, B.; Vasile, B.Ș.; Istrati, D.; Mihaiescu, D.E.; Hadibarata, T.; Grumezescu, A.M. New 3D Vortex Microfluidic System Tested for Magnetic Core-Shell Fe3O4-SA Nanoparticle Synthesis. Nanomaterials 2024, 14, 902. [Google Scholar] [CrossRef]
- Jahangir, R.; Munir, I.; Yesiloz, G. One-Step Synthesis of Ultrasmall Nanoparticles in Glycerol as a Promising Green Solvent at Room Temperature Using Omega-Shaped Microfluidic Micromixers. Anal Chem 2023, 95, 17177–17186. [Google Scholar] [CrossRef]
- Guo, K.; Chen, Y.; Zhou, Z.; Zhu, S.; Ni, Z.; Xiang, N. A novel 3D Tesla valve micromixer for efficient mixing and chitosan nanoparticle production. Electrophoresis 2022, 43, 2184–2194. [Google Scholar] [CrossRef]
- Zeng, C.; Wang, C.; Wang, F.; Zhang, Y.; Zhang, L. A novel vapor–liquid segmented flow based on solvent partial vaporization in microstructured reactor for continuous synthesis of nickel nanoparticles. Chem. Eng. J. 2012, 204–206, 48–53. [Google Scholar] [CrossRef]
- Chung, C.K.; Shih, T.R.; Chang, C.K.; Lai, C.W.; Wu, B.H. Design and experiments of a short-mixing-length baffled microreactor and its application to microfluidic synthesis of nanoparticles. Chem. Eng. J. 2011, 168, 790–798. [Google Scholar] [CrossRef]
- Sattari, A.; Hanafizadeh, P.; Hoorfar, M. Multiphase flow in microfluidics: From droplets and bubbles to the encapsulated structures. Adv. Colloid Interface Sci. 2020, 282, 102208. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, I.; Ricouvier, J.; Yazhgur, P.; Tabeling, P.; Leshansky, A. Droplet generation at Hele-Shaw microfluidic T-junction. Phys. Fluids 2019, 31, 022010. [Google Scholar] [CrossRef]
- Anna, S.L.; Bontoux, N.; Stone, H.A. Formation of dispersions using “flow focusing” in microchannels. Appl. Phys. Lett. 2003, 82, 364–366. [Google Scholar] [CrossRef]
- Hamlington, B.D.; Steinhaus, B.; Feng, J.J.; Link, D.; Shelley, M.J.; Shen, A.Q. Liquid crystal droplet production in a microfluidic device. Liq. Cryst. 2007, 34, 861–870. [Google Scholar] [CrossRef]
- Tanudjaja, H.J.; Hejase, C.A.; Tarabara, V.V.; Fane, A.G.; Chew, J.W. Membrane-based separation for oily wastewater: A practical perspective. Water Res. 2019, 156, 347–365. [Google Scholar] [CrossRef]
- Nisisako, T.; Torii, T. Microfluidic large-scale integration on a chip for mass production of monodisperse droplets and particles. Lab Chip 2008, 8, 287–293. [Google Scholar] [CrossRef]
- Kobayashi, I.; Takano, T.; Maeda, R.; Wada, Y.; Uemura, K.; Nakajima, M. Straight-through microchannel devices for generating monodisperse emulsion droplets several microns in size. Microfluid. Nanofluid. 2008, 4, 167–177. [Google Scholar] [CrossRef]
- Wong, V.-L.; Ng, C.-A.I.; Teo, L.-R.I.; Lee, C.-W. Microfluidic Synthesis of Functional Materials as Potential Sorbents for Water Remediation and Resource Recovery. In Advances in Microfluidic Technologies for Energy and Environmental Applications; IntechOpen: London, UK, 2020; p. 39. [Google Scholar]
- Ding, Y.; Howes, P.D.; deMello, A.J. Recent advances in droplet microfluidics. Anal. Chem. 2019, 92, 132–149. [Google Scholar] [CrossRef]
- Singh, A.; Shirolkar, M.; Lalla, N.P.; Malek, C.K.; Kulkarni, S. Room temperature, water-based, microreactor synthesis of gold and silver nanoparticles. Int. J. Nanotechnol. 2009, 6, 541–551. [Google Scholar] [CrossRef]
- Ye, Z.; Wang, K.; Lou, M.; Jia, X.; Xu, F.; Ye, G. Consecutive synthesis of gold nanobipyramids with controllable morphologies using a microfluidic platform. Microfluid. Nanofluid. 2020, 24, 1–8. [Google Scholar] [CrossRef]
- Iyer, K.S.; Raston, C.L.; Saunders, M. Continuous flow nano-technology: Manipulating the size, shape, agglomeration, defects and phases of silver nano-particles. Lab Chip 2007, 7, 1800–1805. [Google Scholar] [CrossRef] [PubMed]
- Kašpar, O.; Koyuncu, A.; Pittermannová, A.; Ulbrich, P.; Tokárová, V. Governing factors for preparation of silver nanoparticles using droplet-based microfluidic device. Biomed. Microdevices 2019, 21, 88. [Google Scholar] [CrossRef] [PubMed]
- Hong, T.; Lu, A.; Liu, W.; Chen, C. Microdroplet synthesis of silver nanoparticles with controlled sizes. Micromachines 2019, 10, 274. [Google Scholar] [CrossRef]
- Sue, K.; Kimura, K.; Arai, K. Hydrothermal synthesis of ZnO nanocrystals using microreactor. Mater. Lett. 2004, 58, 3229–3231. [Google Scholar] [CrossRef]
- Luo, H.; Leprince-Wang, Y.; Jing, G. Tunable growth of ZnO nanostructures on the inner wall of capillary tubes. J. Phys. Chem. C 2019, 123, 7408–7415. [Google Scholar] [CrossRef]
- Wang, H.; Nakamura, H.; Uehara, M.; Miyazaki, M.; Maeda, H. Preparation of titania particles utilizing the insoluble phase interface in a microchannel reactor. Chem. Commun. 2002, 14, 1462–1463. [Google Scholar] [CrossRef]
- Hao, N.; Nie, Y.; Zhang, J.X. Microfluidic flow synthesis of functional mesoporous silica nanofibers with tunable aspect ratios. Acs Sustain. Chem. Eng. 2018, 6, 1522–1526. [Google Scholar] [CrossRef]
- Hao, N.; Nie, Y.; Xu, Z.; Closson, A.B.; Usherwood, T.; Zhang, J.X. Microfluidic continuous flow synthesis of functional hollow spherical silica with hierarchical sponge-like large porous shell. Chem. Eng. J. 2019, 366, 433–438. [Google Scholar] [CrossRef]
- Song, Y.; Modrow, H.; Henry, L.L.; Saw, C.K.; Doomes, E.; Palshin, V.; Hormes, J.; Kumar, C.S. Microfluidic synthesis of cobalt nanoparticles. Chem. Mater. 2006, 18, 2817–2827. [Google Scholar] [CrossRef]
- Suryawanshi, P.L.; Sonawane, S.H.; Bhanvase, B.A.; Ashokkumar, M.; Pimplapure, M.S.; Gogate, P.R. Synthesis of iron oxide nanoparticles in a continuous flow spiral microreactor and Corning® advanced flow™ reactor. Green Process. Synth. 2018, 7, 1–11. [Google Scholar] [CrossRef]
- Liu, J.; Gu, Y.; Wu, Q.; Wang, X.; Zhao, L.; DeMello, A.; Wen, W.; Tong, R.; Gong, X. Synthesis and study of CdSe QDs by a microfluidic method and via a bulk reaction. Crystals 2019, 9, 368. [Google Scholar] [CrossRef]
- Xu, P.-F.; Liu, Z.-H.; Duan, Y.-H.; Sun, Q.; Wang, D.; Zeng, X.-F.; Wang, J.-X. Microfluidic controllable synthesis of monodispersed sulfur nanoparticles with enhanced antibacterial activities. Chem. Eng. J. 2020, 398, 125293. [Google Scholar] [CrossRef]
- Hood, R.R.; DeVoe, D.L. High-throughput continuous flow production of nanoscale liposomes by microfluidic vertical flow focusing. Small 2015, 11, 5790–5799. [Google Scholar] [CrossRef]
- Kawamura, J.; Kitamura, H.; Otake, Y.; Fuse, S.; Nakamura, H. Size-controllable and scalable production of liposomes using a v-shaped mixer micro-flow reactor. Org. Process Res. Dev. 2020, 24, 2122–2127. [Google Scholar] [CrossRef]
- Huang, X.; Caddell, R.; Yu, B.; Xu, S.; Theobald, B.; Lee, L.J.; Lee, R.J. Ultrasound-enhanced microfluidic synthesis of liposomes. Anticancer Res. 2010, 30, 463–466. [Google Scholar] [CrossRef]
- Shokoohinia, P.; Hajialyani, M.; Sadrjavadi, K.; Akbari, M.; Rahimi, M.; Khaledian, S.; Fattahi, A. Microfluidic-assisted preparation of PLGA nanoparticles for drug delivery purposes: Experimental study and computational fluid dynamic simulation. Res. Pharm. Sci. 2019, 14, 459–470. [Google Scholar]
- Min, K.-I.; Lee, H.-J.; Kim, D.-P. Three-dimensional flash flow microreactor for scale-up production of monodisperse PEG–PLGA nanoparticles. Lab Chip 2014, 14, 3987–3992. [Google Scholar] [CrossRef]
- Heshmatnezhad, F.; Solaimany Nazar, A.R. On-chip controlled synthesis of polycaprolactone nanoparticles using continuous-flow microfluidic devices. J. Flow Chem. 2020, 10, 533–543. [Google Scholar] [CrossRef]
- Bicudo, R.C.S.; Santana, M.H.A. Production of hyaluronic acid (HA) nanoparticles by a continuous process inside microchannels: Effects of non-solvents, organic phase flow rate, and HA concentration. Chem. Eng. Sci. 2012, 84, 134–141. [Google Scholar] [CrossRef]




| S. No | Method | Process | Materials | Technical Aspects | Results | References |
|---|---|---|---|---|---|---|
| 1 | Top-Down | Ball Milling |
| The kinetic energy produced by the movement of moving balls causes the chemical bonds between the molecules to break, resulting in a reduction in particle size. |
| [1,42,94] |
| Electro-Spinning | A wide range of materials. |
|
| [1,42,48] | ||
| Lithography | A stamp containing both hydrophobic and hydrophilic areas. |
|
| [1,42,53,95,96] | ||
| Laser Ablation | A high intensity Laser. |
|
| [1,38,42,57] | ||
| 2 | Bottom-Up | Chemical Vapor Deposition | Volatile matter to be turned into solid products. |
|
| [1,40,42,73,74,75] |
| Sol-Gel Method |
Alkoxides
IV Isopropoxide.
Acetate dehydrates oxalic acid and ethanol.
chloride pentahydrate and Ammonia solution. |
| Synthesis of TiO2, ZnO, SnO2, CaO Nanoparticles. | [42,76,78,79,97,98] | ||
| Reverse Micelles Method | There are at least three components, two of which are immiscible and the other a surfactant with amphi-phallic qualities. |
|
| [42,83,84,85,86,87,88,89,90,91,92,93,94,95,96,99] | ||
| Microfluidic Approach | Typical substances include ceramics, glass, silicon, metals, and polymers. |
| Development, manufacture, and use of disposable, portable, and economical equipment. | [9,41,42,100] |
| Fabrication of Nanomaterials | Type of Micro-Size Reactor | Utilization of Chemicals | Fabrication Conditions | Morphology | References |
|---|---|---|---|---|---|
| Inorganic nanoparticles | |||||
| AuNPs | Polydimethylsiloxane microreactor | NaBH4 for reduction, Na3C6H5O7 for capping and HAuCl4 | Operating time of 5 min at ambient temperature | ~2 nm of particle size | [158] |
| AuNPs | Polydimethylsiloxane microchannel | Utilization of HAuCl4 and NaBH4 for gold seeds, AgNO3, ascorbic acid | Proper stirring followed by a controlled flow rate | Desired morphology of Au-nano-bipyramids | [159] |
| AgNPs | Flash fabrication in the spinning disc process | AgNO3, ascorbic acid, starch, and polymers (polyethylene glycol (PEG) and poly(4-vinyl pyridine)) | Vigorous stirring forms a thin film of fluid at ambient temperature on a rotating disc | Particle size is controlled by disc speed | [160] |
| AgNPs | Polydimethylsiloxane device with small volume (micro to femto liter) of reaction mixture | AGNO3, Na3C6H5O7, tannic acid, mineral oil, PDMS | Vigorous mixing, fast reaction, colloid formation at ambient temperature | Controlled particle size uniformity and polydispersity in droplet PDMS chip | [161] |
| AgNPs | Polydimethylsiloxane microchip with droplet seed formation | AgNO3 and NaBH4 used to fabricate Ag-seeds, Na3C6H5O7, molten paraffin | Microdroplets in microreactor at 60 °C for seed formation | Particle size is controlled by adjusting temperature, time, and Ag+ ion concentration | [162] |
| ZnO NPs | Used flow-through equipment and microreactor under hydrothermal fabrication | ZnSO4 (0.001 mol/kg) and KOH (0.004 mol/kg) solutions | Hydrothermal fabrication at 400 °C in a furnace followed by crystal separation and drying at 60 °C | Achieved particle diameter of 9 nm | [163] |
| ZnO nanostructures | Microfluidic chip with multifunctional properties | Zn(CH3COO)2·2H2O, HN(CH2CH2OH)2, ZnNO3·6H2O, C6H12N4, 28% v/v, NH3 solution | Dip-coating and annealing to fabricate ZnO seed followed by mixing with other reagents and vigorous mixing at 60 °C for 20 min | Growth of microstructure ZnO of different morphology at channel wall of capillary tubes | [164] |
| TiO2 NPs | Microchannel reactor made up of ceramic material and glass cover | Two solution, titanium(IV) isopropoxide (TTIP) in 1-Hexanol and formamide (CH3NO) in H2O | Reacted at the interface of two insoluble currents (TTIP and CH3NO) in microchannel | Controlled particles of 10 nm of anatase morphology formed | [165] |
| SiO2 nanofibers | Microfluidic process in polydimethylsiloxane reactor (spiral-shaped) | Cetyltrimethylammonium Br (CTAB), NH3 solution, tetraethyl orthosilicate (TEOS), ethyl alcohol | CTAB in NH3 solution and TEOS solution mixed from two in-lets and react to form SiO2 nanofiber at ambient temperature | Fabricated mesoporous SiO2 nanofiber; Morphology control by controlling the flow rates of two solutions or reagent concentration | [166] |
| Spongy, porous, and spherical SiO2 NPs | Spiral PDMS microreactor laminar flow | CTAB, NH3 solution, TEOS, 1,3,5-trimethylbenzene (TMC) | Vigorous mixing of two solutions (CTAB in NH3 solution and TMC in TEOS) to react and form NPs | Fabricated spherical and porous SiO2 NPs with an average diameter of 1200 nm | [167] |
| Cobalt NPs | Microfluidic reactor | CoCl2, tetrahydrofuran (THF), LiBH(C2H5)3, stabilizer 3-dimethylammonio -1-propanesulfonate | Fabricated in the microfluidic reactor with fine tuning of quenching time and flow rates of reactants (0.08 to 0.9 mL/min) | Obtained crystals of CoNPs | [168] |
| Fe2O3 NPs | Spiral-shaped Cu wire microreactor with continuous flow | Fe(NO3)3·9H2O, NaOH, CTAB | The flow of NaOH and Fe(NO3)3·9H2O through a syringe in a spiral microreactor to co-precipitate and reduce at ambient temperature | Obtained 6 nm of particle size at 1 mL/min flow rate of reactants | [169] |
| CdSe quantum dots (QDs) | Polytetrafluoroethylene microreactor | CdO, Se, diphenyl ether, trioctylphosphine-oleic acid (TOP-OA), CHCl3 | Solution mixture of CdO, OA, and diphenyl ether heated at 180 °C and Se TOP prepared by ultrasonication; both solutions reacted to form CdSe QDs | Obtained photoluminescence CdSe QDs | [170] |
| Sulfur NPs | Y- and T-type microfluidic reactors | Dry sulfur (sublimed), CTAB, solvent and anti-solvent as CS2, and ethanol | Solvent (sulfur and CS2) and anti-solvent (CTAB in ethanol) mixed to precipitate and obtain suspension; Spray dry to synthesize sulfur NPs | Obtained 15–50 nm particle size | [171] |
| Organic nanoparticles | |||||
| Liposomes | Microreactor made up of thermoplastic with microfluidic vertical flow and hydrodynamic focusing (VFF and MHF system) | Lipid of 80 mmol/L, solvents | Size controlled by flow rate ration, i.e., aqueous/ lipid-solvent flow | Obtained 80–200 nm of size of monodispersed vesicles | [172] |
| Liposomes | Reactor with V-shaped off-the-shelf Teflon tubing | Ethanol and different types of lipids (1,2-distearoyl-synglycero- 3-phosphocholine, N-carbonylmethoxypolyethyleneglycol 2000)-1,2-distearoylsn- glycerol-3-phosphoethanolamine, Cholesterol) | The reaction of aqueous and ethanol solution of lipids with controlling flow rates at 25 °C | Obtained 50–70 nm of diameter particle size | [173] |
| Liposomes | Used ultrasonication with microfluidic devices | Phosphatidylcholine from egg and cholesterol | Microfluidic devices placed in a sonicator with controlled flow rates for microdispersion | Obtained 66.27 to 189.9 nm of particle size depending upon the flow rates | [174] |
| Polylactic-co-glycolic acid (PLGA) NPs | Computational fluid dynamic-based micromixing in the microfluidic system made up of Teflon material (plus-shaped chip) | Dimethylsulfoxide (DMSO) to dissolve PLGA and aqueous polyvinyl alcohol (PVA) solution | The reaction of PLGA dissolved in DMSO with aqueous PVA for nanoprecipitation in the microdevice | Obtained uniform and harmonious spherical particle size (238 nm) | [175] |
| Polyethylene glycol (PEG)-PLGA NPs | Microreactor of polyimide film with three-dimensional flow | CH3CN dissolved polymers (PEG and PLGA) and H2O | The flash flow of 11 milli second in a unit microchannel of reaction mixture | Obtained 50–85 nm of particle size | [176] |
| Polycaprolactone (PCL) NPs | Microfluidic chip made up of glass; different channel dimensions and confluence angles (CA) | PCL dissolved in THF (organic phase); H2O, PVA, and surfactants (tween-80) (aqueous phase) Aqueous phase: PVA, Tween 80, Milli-Q Water, Organic phase: PCL, THF | Flow rate, ratio, channel width, and its length, and CA of inlet channel affected particle size, distribution, and polydispersity index; Non-solvent precipitation | Obtained 40–370 nm of particle size NPs | [177] |
| Hyaluronic acid (HA) NPs | T-shaped microchannel device made up of glass | Ethanol, isopropyl alcohol, acetone (organic phase); Na-HA, H2O, EDCI, ADH (aqueous phase) | Synthesized HA NPs at a pH of 6.0 at the interface of organic and aqueous phases inside the microchannel | Obtained homogeneous 140–460 nm of particle-sized | [178] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roy, S.; Kumar, R.; Acooli, A.; Roy, S.; Chatterjee, A.; Chattaraj, S.; Nayak, J.; Jeon, B.-H.; Basu, A.; Banerjee, S.; et al. Transforming Nanomaterial Synthesis through Advanced Microfluidic Approaches: A Review on Accessing Unrestricted Possibilities. J. Compos. Sci. 2024, 8, 386. https://doi.org/10.3390/jcs8100386
Roy S, Kumar R, Acooli A, Roy S, Chatterjee A, Chattaraj S, Nayak J, Jeon B-H, Basu A, Banerjee S, et al. Transforming Nanomaterial Synthesis through Advanced Microfluidic Approaches: A Review on Accessing Unrestricted Possibilities. Journal of Composites Science. 2024; 8(10):386. https://doi.org/10.3390/jcs8100386
Chicago/Turabian StyleRoy, Sanjib, Ramesh Kumar, Argha Acooli, Snehagni Roy, Abhrajit Chatterjee, Sujoy Chattaraj, Jayato Nayak, Byong-Hun Jeon, Aradhana Basu, Shirsendu Banerjee, and et al. 2024. "Transforming Nanomaterial Synthesis through Advanced Microfluidic Approaches: A Review on Accessing Unrestricted Possibilities" Journal of Composites Science 8, no. 10: 386. https://doi.org/10.3390/jcs8100386









