Nanostructured C@CuS Core–Shell Framework with High Lithium-Ion Storage Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of CuS Nanoparticles and Nanostructured C@CuS Core–Shell Framework
2.3. Materials Characterization
2.4. Electrochemical Measurements
3. Results
3.1. XRD and Raman Spectroscopy Characterization
3.2. Morphology and Structure Analysis
3.3. Lithium Storage Mechanism
3.4. Cycling Performance
3.5. Rate Performance, Galvanostatic Charge–Discharge, and Ex Situ Analyses after Cycling
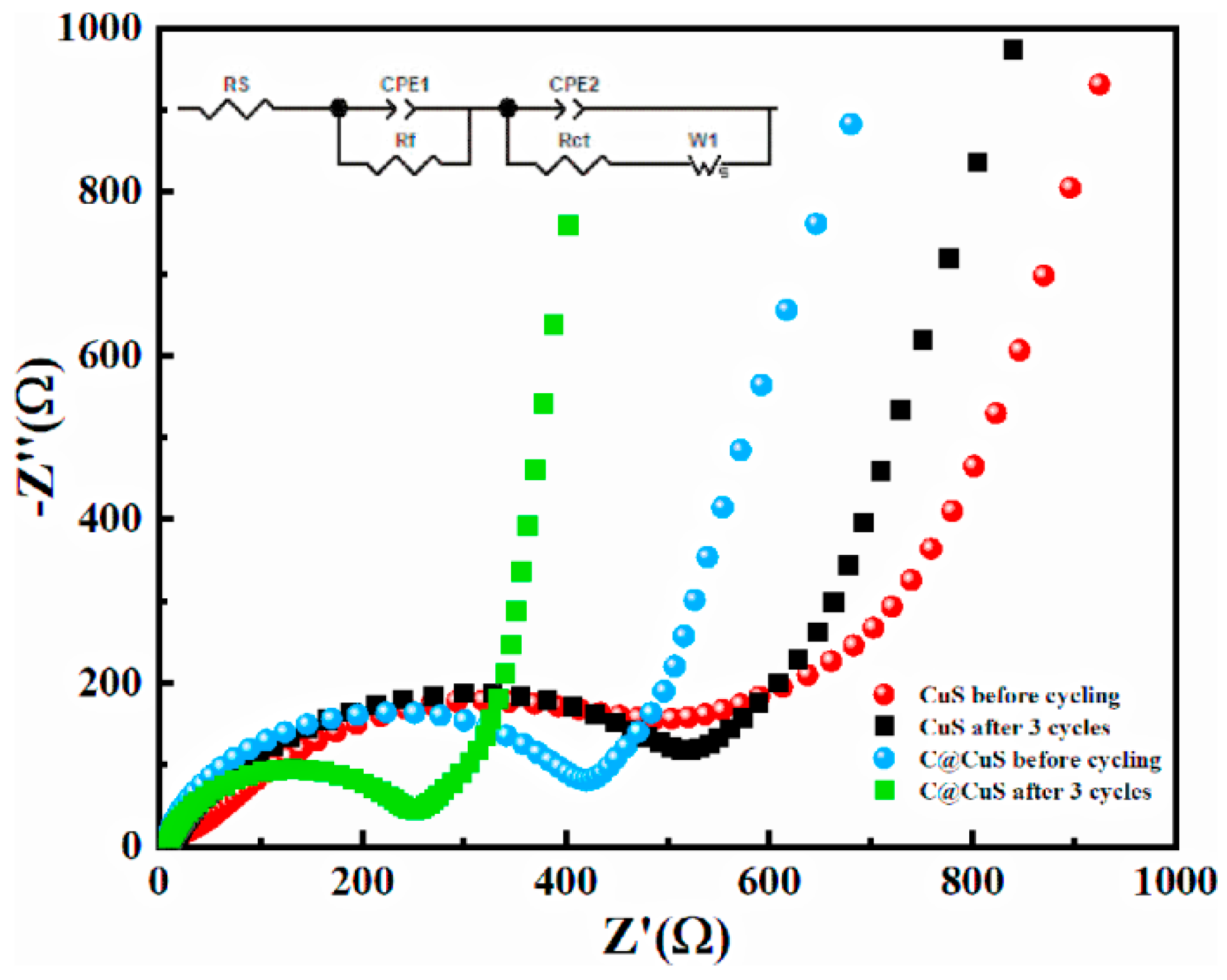
3.6. EIS Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lin, D.; Liu, Y.; Cui, Y. Reviving the lithium metal anode for high-energy batteries. Nat. Nanotechnol. 2017, 12, 194–206. [Google Scholar] [CrossRef] [PubMed]
- Zou, F.; Manthiram, A. A Review of the design of advanced binders for high performance batteries. Adv. Energy Mater. 2020, 10, 2002508. [Google Scholar] [CrossRef]
- Liu, W.; Song, M.S.; Kong, B.; Cui, Y. Flexible and stretchable energy storage: Recent advances and future perspectives. Adv. Mater. 2017, 29, 1603436. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Wang, P.; Fang, W.; Guo, X.; Bao, W.; Yang, Y.; Shen, S.; Qiu, F. Interface engineering of Silicon/Carbon thin-film anodes for high-rate lithium-ion batteries. ACS Appl. Mater. Interfaces 2020, 12, 29242–29252. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Zheng, S.; He, S.; Ye, Y.; Liang, J.; Shi, A.; Wang, Z.; Zheng, Z. Preparation of micron Si@C anodes for lithium ion battery by recycling the lamellar submicron silicon in the kerf slurry waste from photovoltaic industry. Diam. Relat. Mater. 2020, 107, 107898. [Google Scholar] [CrossRef]
- Nguyen, T.X.; Patra, J.; Chang, J.-K.; Ting, J.-M. High entropy spinel oxide nanoparticles for superior lithiation–delithiation performance. J. Mater. Chem. A 2020, 8, 18963–18973. [Google Scholar] [CrossRef]
- Agostini, M.; Hwang, J.Y.; Kim, H.M.; Bruni, P.; Brutti, S.; Croce, F.; Matic, A.; Sun, Y.K. Minimizing the Electrolyte Volume in Li–S Batteries: A Step Forward to High Gravimetric Energy Density. Adv. Energy Mater. 2018, 8, 1801560. [Google Scholar] [CrossRef]
- Maroni, F.; Bruni, P.; Giuli, G.; Brutti, S.; Croce, F. Electrospun Carbon/CuxO Nanocomposite material as Sustainable and High Performance Anode for Lithium-Ion Batteries. ChemistryOpen 2019, 8, 781–787. [Google Scholar] [CrossRef]
- Geng, P.; Zheng, S.; Tang, H.; Zhu, R.; Zhang, L.; Cao, S.; Xue, H.; Pang, H. Transition metal sulfides based on graphene for electrochemical energy storage. Adv. Energy Mater. 2018, 8, 1703259. [Google Scholar] [CrossRef]
- Lim, Y.V.; Li, X.L.; Yang, H.Y. Recent tactics and advances in the application of metal sulfides as high-performance anode materials for rechargeable sodium-ion batteries. Adv. Funct. Mater. 2020, 31, 2006761. [Google Scholar] [CrossRef]
- Theerthagiri, J.; Senthil, R.A.; Nithyadharseni, P.; Lee, S.J.; Durai, G.; Kuppusami, P.; Madhavan, J.; Choi, M.Y. Recent progress and emerging challenges of transition metal sulfides based composite electrodes for electrochemical supercapacitive energy storage. Ceram. Int. 2020, 10, 14317–14345. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, Y.; Wang, Y.; Li, H.; Peng, Y. The application of nanostructured transition metal sulfides as anodes for lithium ion batteries. J. Energy Chem. 2018, 27, 1536–1554. [Google Scholar] [CrossRef]
- Jiang, K.; Chen, Z.; Meng, X. CuS and Cu2S as cathode materials for lithium batteries: A Review. ChemElectroChem 2019, 6, 2825–2840. [Google Scholar] [CrossRef]
- Lai, C.-H.; Huang, K.-W.; Cheng, J.-H.; Lee, C.-Y.; Hwang, B.-J.; Chen, L.-J. Direct growth of high-rate capability and high capacity copper sulfide nanowire array cathodes for lithium-ion batteries. J. Mater. Chem. A 2010, 20, 6638–6645. [Google Scholar] [CrossRef]
- Jing, P.; Wang, Q.; Wang, B.; Gao, X.; Zhang, Y.; Wu, H. Encapsulating yolk-shell FeS2@carbon microboxes into interconnected graphene framework for ultrafast lithium/sodium storage. Carbon 2020, 159, 366–377. [Google Scholar] [CrossRef]
- Cheng, J.; Pan, Y.; Zhu, J.; Li, Z.; Pan, J.; Ma, Z. Hybrid network CuS monolith cathode materials synthesized via facile in situ melt-diffusion for Li-ion batteries. J. Power Sources 2014, 257, 192–197. [Google Scholar] [CrossRef]
- Chen, Y.; Li, J.; Lei, Z.; Huo, Y.; Yang, L.; Zeng, S.; Ding, H.; Qin, Y.; Jie, Y.; Huang, F.; et al. Hollow CuS nanoboxes as Li-Free cathode for high-rate and long-life lithium metal batteries. Adv. Energy Mater. 2020, 10, 1903401. [Google Scholar] [CrossRef]
- Iqbal, S.; Bahadur, A.; Saeed, A.; Zhou, K.; Shoaib, M.; Waqas, M. Electrochemical performance of 2D polyaniline anchored CuS/Graphene nano-active composite as anode material for lithium-ion battery. J. Colloid Interface Sci. 2017, 502, 16–23. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; Huang, J.; Zhang, Y.; Zhao, J. Microwave-assisted synthesis of CuS/Graphene composite for enhanced lithium storage properties. Electrochim. Acta 2017, 225, 443–451. [Google Scholar] [CrossRef]
- Wang, W.; Yang, Y.; NuLi, Y.; Zhou, J.; Yang, J.; Wang, J. Metal organic framework (MOF)-derived carbon-encapsulated cuprous sulfide cathode based on displacement reaction for hybrid Mg2+/Li+ batteries. J. Power Sources 2020, 445, 227325. [Google Scholar] [CrossRef]
- Li, L.; Liu, Y.; Han, Y.; Qi, X.; Li, X.; Fan, H.; Meng, L. Metal-organic framework-derived carbon coated copper sulfide nanocomposites as a battery-type electrode for electrochemical capacitors. Mater. Lett. 2019, 236, 131–134. [Google Scholar] [CrossRef]
- Wang, P.; Shen, M.; Zhou, H.; Meng, C.; Yuan, A. MOF-derived CuS@Cu-BTC composites as high-performance anodes for lithium-ion batteries. Small 2019, 15, 1903522. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Li, H.; Peng, Y.; Li, J.; Wang, J.; Hwang, B.-J.; Zhao, J. Realizing high reversible capacity: 3D intertwined CNTs inherently conductive network for CuS as an anode for lithium ion batteries. Chem. Eng. J. 2018, 332, 49–56. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, Y.; Zhang, Y.; Li, J.; Babaa, M.R.; Liu, N.; Bakenov, Z. Synthesis of microflower-like vacancy defective copper sulfide/reduced graphene oxide composites for highly efficient lithium-ion batteries. Nanotechnology 2019, 31, 095405. [Google Scholar] [CrossRef]
- Hwa, Y.; Zhao, J.; Cairns, E.J. Lithium sulfide (Li2S)/graphene oxide nanospheres with conformal carbon coating as a high-rate, long-life cathode for Li/S cells. Nano Lett. 2015, 15, 3479–3486. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, H.; Zhang, W.; Feng, Y.; Ma, J. S-doped carbon-coated FeS2/C@C nanorods for potassium storage. Acta Metall. Sin-Engl. 2020, 34, 321–328. [Google Scholar] [CrossRef]
- Li, X.; He, X.; Shi, C.; Liu, B.; Zhang, Y.; Wu, S.; Zhu, Z.; Zhao, J. Synthesis of one-dimensional copper sulfide nanorods as high-performance anode in lithium ion batteries. ChemSusChem 2014, 7, 3328–3333. [Google Scholar] [CrossRef]
- Xu, X.; Li, L.; Chen, H.; Guo, X.; Zhang, Z.; Liu, J.; Mao, C.; Li, G. Constructing heterostructured FeS2/CuS nanospheres as high rate performance lithium ion battery anodes. Inorg. Chem. Front. 2020, 7, 1900–1908. [Google Scholar] [CrossRef]
- Ding, C.; Su, D.; Ma, W.; Zhao, Y.; Yan, D.; Li, J.; Jin, H. Design of hierarchical CuS/graphene architectures with enhanced lithium storage capability. Appl. Surf. Sci. 2017, 403, 1–8. [Google Scholar] [CrossRef]
- Zhou, M.; Peng, N.; Liu, Z.; Xi, Y.; He, H.; Xia, Y.; Liu, Z.; Okada, S. Synthesis of sub-10nm copper sulphide rods as high-performance anode for long-cycle life Li-ion batteries. J. Power Sources 2016, 306, 408–412. [Google Scholar] [CrossRef]
- Li, S.; Ge, P.; Jiang, F.; Foster, C.W.; Banks, C.E.; Xu, W.; Zhang, Y.; Hong, W.; Zhang, C.; Sun, W.; et al. Molecular-level CuS@S hybrid nanosheets constructed by mineral chemistry for energy storage systems. ACS Appl. Mater. Interfaces 2018, 10, 43669–43681. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Zhang, L.; Yang, M.; Song, X.; Zhao, H.; Jia, Z.; Sun, K.; Liu, G. One-pot synthesis of copper sulfide nanowires/reduced graphene oxide nanocomposites with excellent lithium-storage properties as anode materials for lithium-ion batteries. ACS Appl. Mater. Interfaces 2015, 7, 15726–15734. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Ying, H.; Huang, P.; Wang, J.; Zhang, Z.; Yang, T.; Han, W.Q. Rational design of pillared SnS/Ti3C2Tx MXene for superior lithium-ion storage. ACS Nano 2020, 14, 17665–17674. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ning, P.; Huang, S.; Wang, W.; Fei, S.; He, Q.; Zai, J.; Jiang, Y.; Hu, Z.; Qian, X.; et al. Multi-functional NiS2/FeS2/N-doped carbon nanorods derived from metal-organic frameworks with fast reaction kinetics for high performance overall water splitting and lithium-ion batteries. J. Power Sources 2019, 436, 226857. [Google Scholar] [CrossRef]
- Chen, Q.; Chen, W.; Ye, J.; Wang, Z.; Lee, J.Y. l-Cysteine-assisted hydrothermal synthesis of nickel disulfide/graphene composite with enhanced electrochemical performance for reversible lithium storage. J. Power Sources 2015, 294, 51–58. [Google Scholar] [CrossRef]
- Hu, Q.; Meng, Y.; Zhang, H.; Zhao, G.; Hu, J.; Zhu, F.; Zhang, Y. Encapsulated Ni3S2 nanoparticles with N, S dual-doped carbon nanotubes: A robust structure for lithium storage. J. Electroanal. Chem. 2020, 873, 114383. [Google Scholar] [CrossRef]
- Pi, W.; Mei, T.; Li, J.; Wang, J.; Li, J.; Wang, X. Durian-like NiS2@rGO nanocomposites and their enhanced rate performance. Chem. Eng. J. 2018, 335, 275–281. [Google Scholar] [CrossRef]
- Xu, X.; Ji, S.; Gu, M.; Liu, J. In situ synthesis of MnS hollow microspheres on reduced graphene oxide sheets as high-capacity and long-life anodes for Li- and Na-ion batteries. ACS Appl. Mater. Interfaces 2015, 7, 20957–20964. [Google Scholar] [CrossRef]
- Wang, R.; Li, B.; Lai, L.; Hou, M.; Gao, J.; Wu, R. 3D urchin-like architectures assembled by MnS nanorods encapsulated in N-doped carbon tubes for superior lithium storage capability. Chem. Eng. J. 2019, 355, 752–759. [Google Scholar] [CrossRef]
- Liu, H.; Wei, C.; Ai, Z.; Li, M.; Xu, M.; Ma, C.; Shi, J. The positive effect of 3D interpenetrating network porous structure by carbon membranes on alleviating the volume expansion of SnS2 nanosheets for enhancing lithium and sodium storage. Colloids Surf. B 2021, 610, 125937. [Google Scholar] [CrossRef]
- Xu, Q.-T.; Xue, H.-G.; Guo, S.-P. FeS2 walnut-like microspheres wrapped with rGO as anode material for high-capacity and long-cycle lithium-ion batteries. Electrochim. Acta 2018, 292, 1–9. [Google Scholar] [CrossRef]
- Lazanas, A.C.; Prodromidis, M.I. Electrochemical Impedance Spectroscopy—A Tutorial. ACS Meas. Sci. Au 2023, 3, 162–193. [Google Scholar] [CrossRef] [PubMed]






| Electrode Materials | Initial Discharge Capacity (mAh g−1) | Discharge Capacity (mAh g−1) | Current Density (mA g−1) | Refs. |
|---|---|---|---|---|
| CuS nanorods | 735 | 390 (250 cycles) | 112 | [30] |
| CuS nanorods | 550 | 472 (100 cycles) | 100 | [27] |
| CuS monolith | 310 | 468 (100 cycles) | 112 | [16] |
| CuS/2.5%CNT | 430 | 558 (100 cycles) | 400 | [23] |
| Vs-CuS/rGO | 882 | 500 (100 cycles) | 112 | [24] |
| 513 (450 cycles) | 500 | |||
| CuS@S | 790 | 513.2 (200 cycles) | 100 | [31] |
| 402 (200 cycles) | 1120 | |||
| CuS-GO | 625 | 497 (100 cycles) | 200 | [19] |
| 348 (1000 cycles) | 2000 | |||
| CuS NWs/rGO | 908 | 620 (100 cycles) | 280 | [32] |
| 320 (430 cycles) | 2240 | |||
| C@CuS | 1108 | 811 (100 cycles) | 100 | this work |
| 463 (800 cycles) | 1000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, C.; Peng, Z.; Wei, Y.; Nan, R.; Yang, Z.; Jian, Z.; Ding, Q. Nanostructured C@CuS Core–Shell Framework with High Lithium-Ion Storage Performance. J. Compos. Sci. 2024, 8, 375. https://doi.org/10.3390/jcs8090375
Jin C, Peng Z, Wei Y, Nan R, Yang Z, Jian Z, Ding Q. Nanostructured C@CuS Core–Shell Framework with High Lithium-Ion Storage Performance. Journal of Composites Science. 2024; 8(9):375. https://doi.org/10.3390/jcs8090375
Chicago/Turabian StyleJin, Changqing, Zaidong Peng, Yongxing Wei, Ruihua Nan, Zhong Yang, Zengyun Jian, and Qingping Ding. 2024. "Nanostructured C@CuS Core–Shell Framework with High Lithium-Ion Storage Performance" Journal of Composites Science 8, no. 9: 375. https://doi.org/10.3390/jcs8090375









