Three-Component Condensation of β-Ketonitriles, 4-Fluorobenzaldehyde, and Secondary Cyclic Amines
Abstract
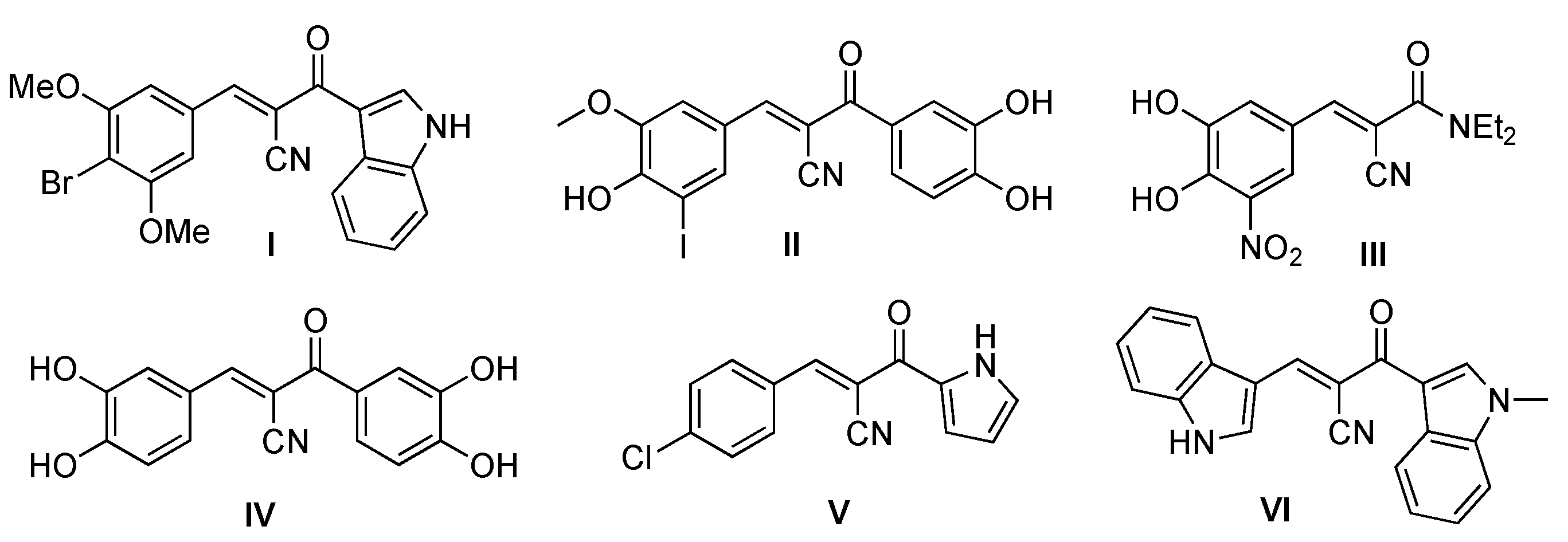
:1. Introduction
2. Materials and Methods
2.1. Materials and Instrumentation
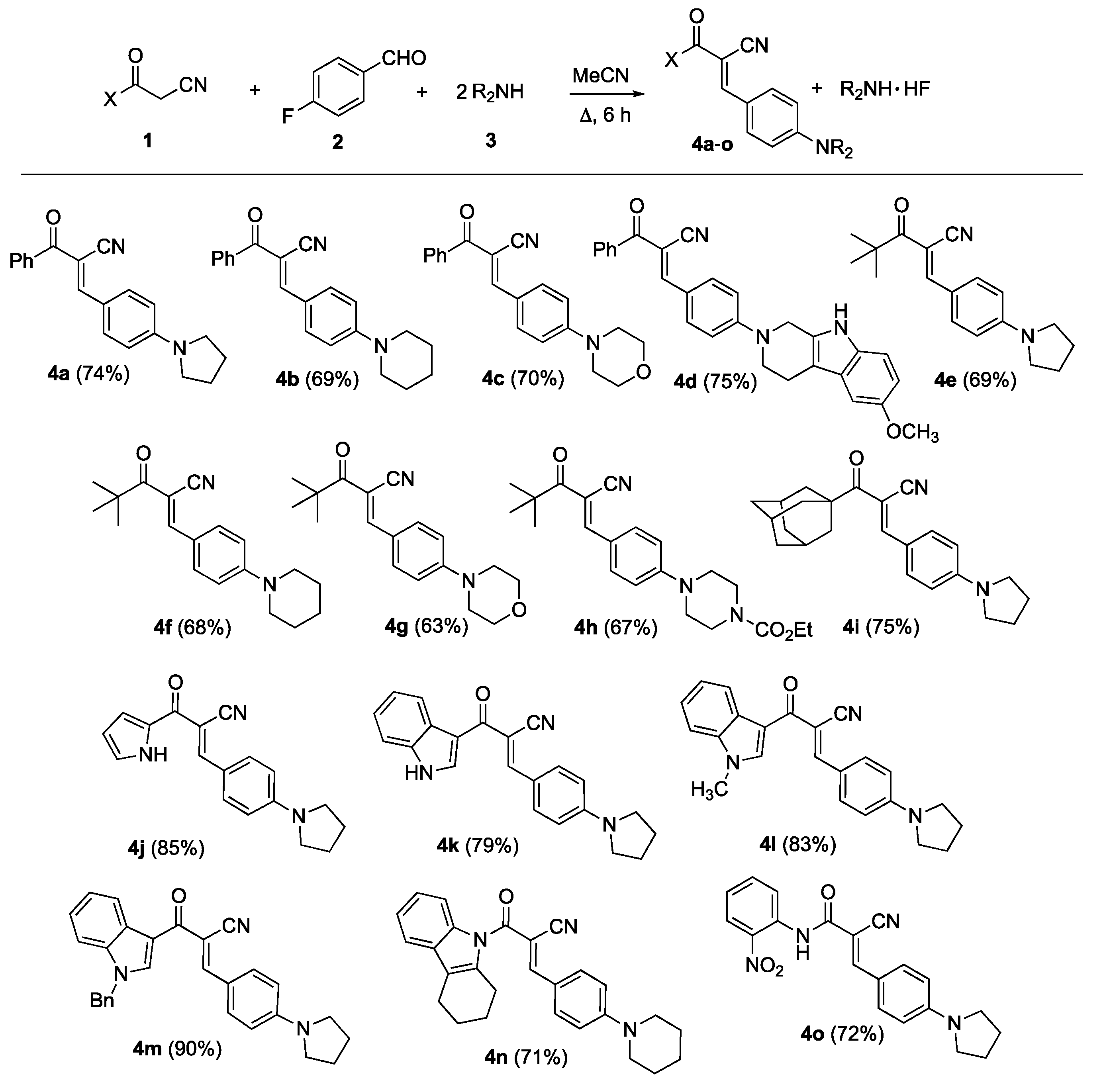
2.2. General Procedure for Preparation of α-Arylidenenitriles 4a–c,e–o and Products 7a,b, 9
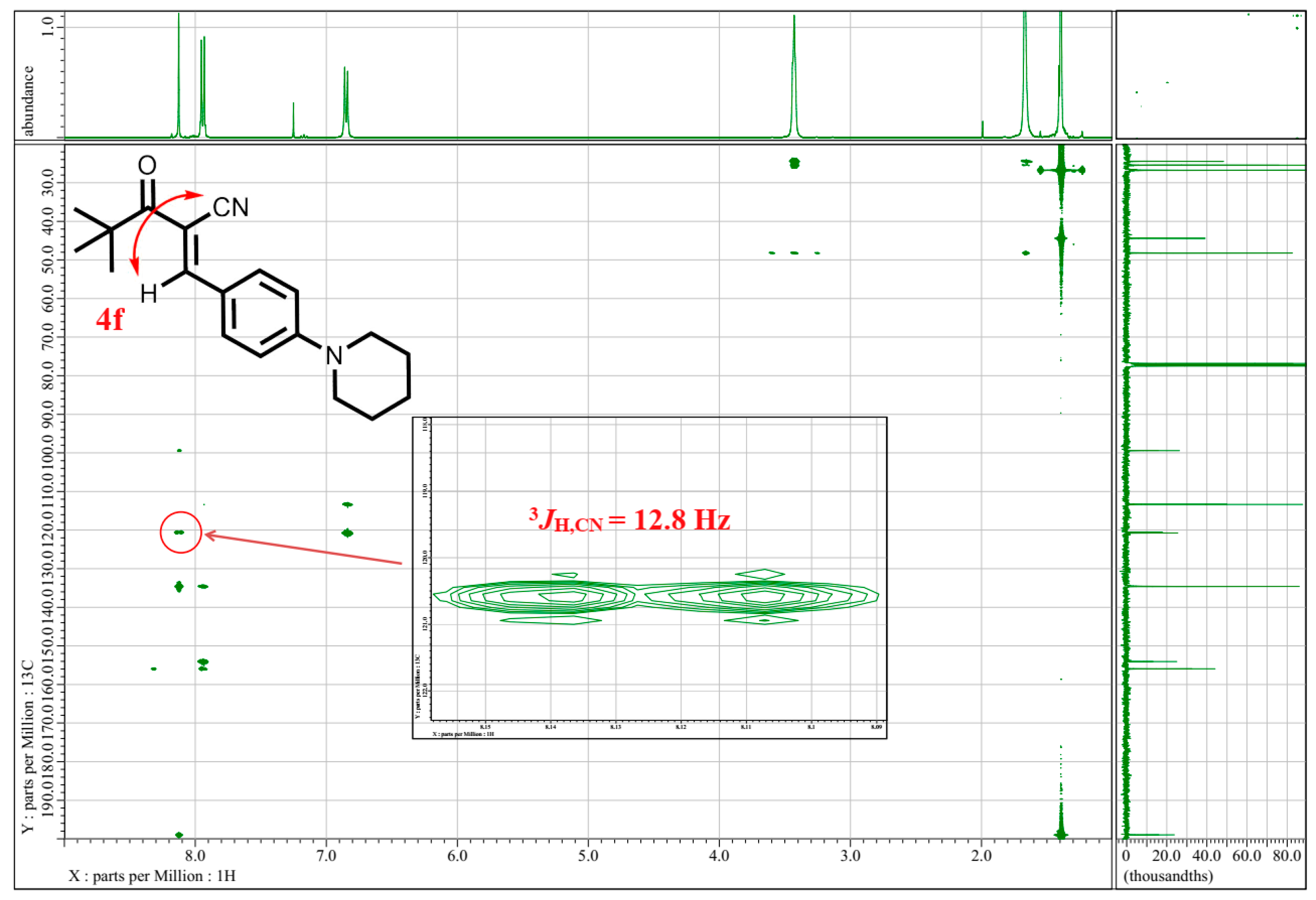
2.3. Spectroscopic Characterization
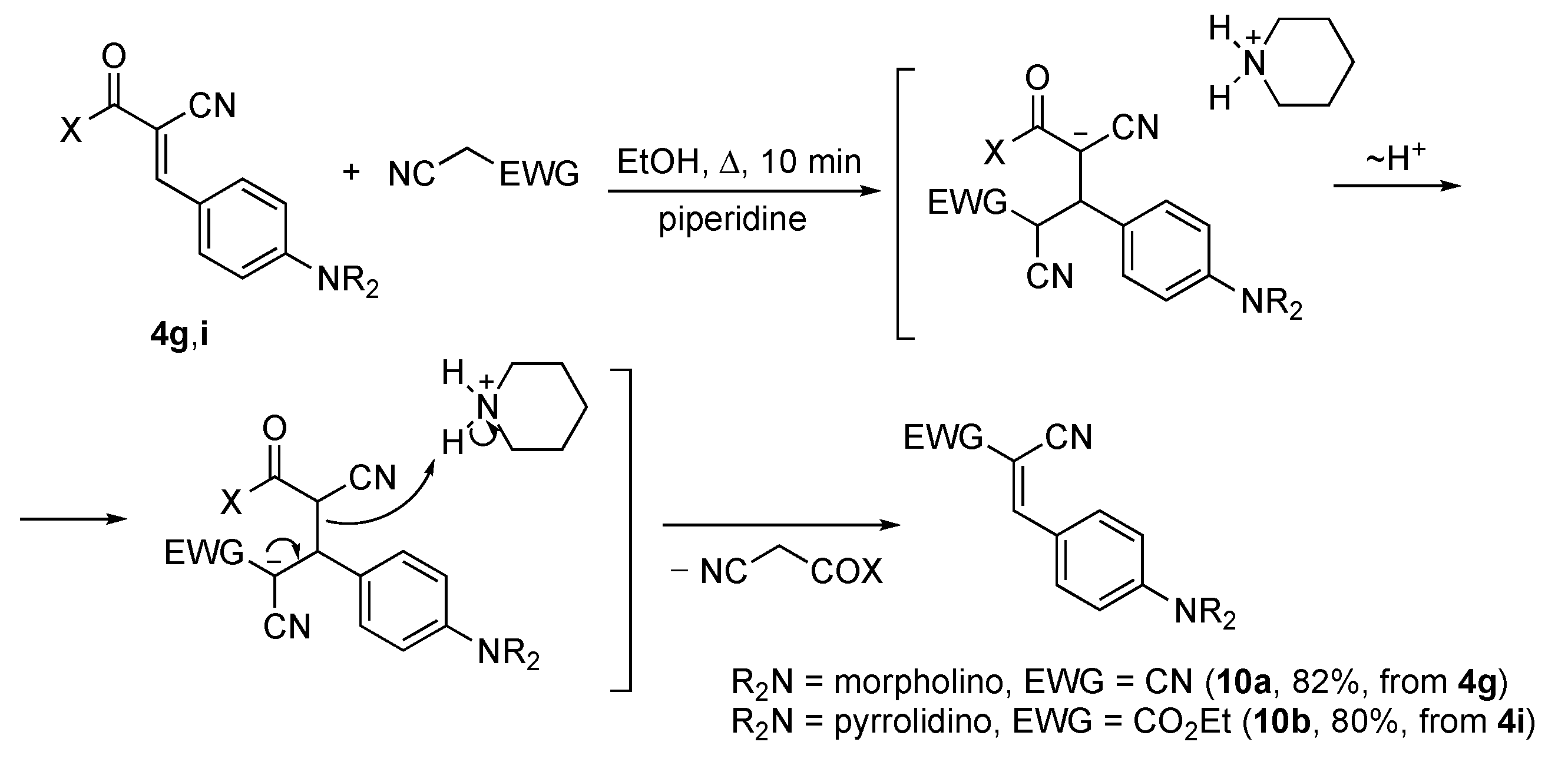
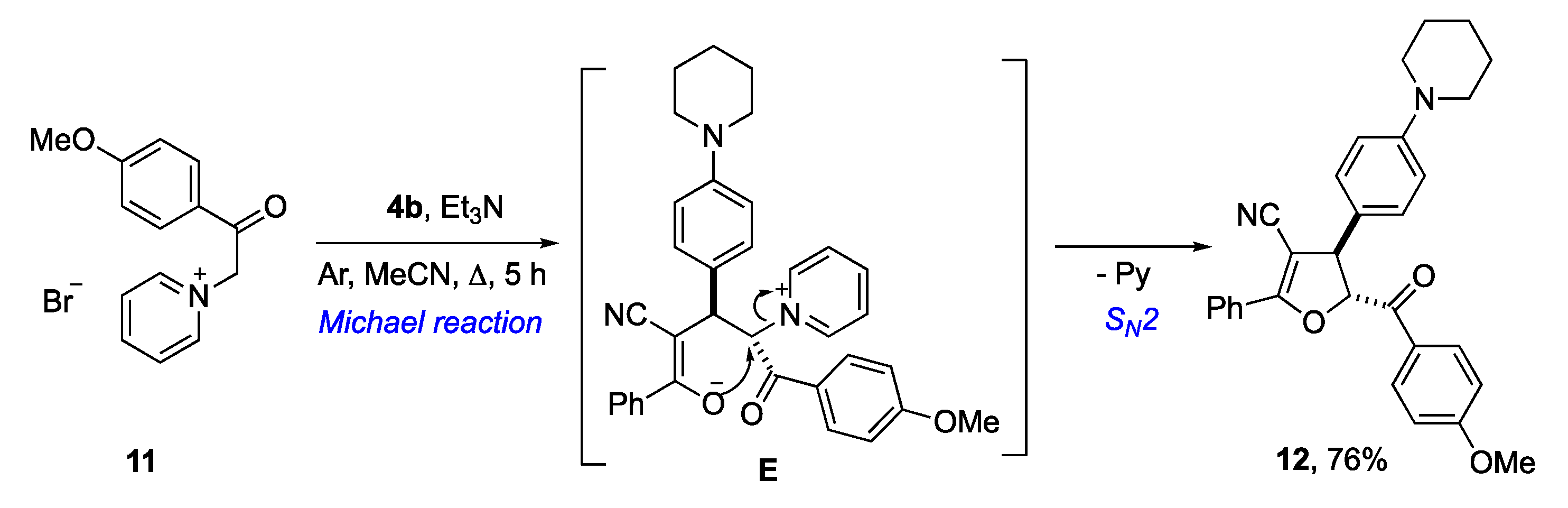
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- John, S.E.; Gulati, S.; Shankaraiah, N. Recent advances in multi-component reactions and their mechanistic insights: A triennium review. Org. Chem. Front. 2021, 8, 4237–4287. [Google Scholar] [CrossRef]
- Graebin, C.S.; Ribeiro, F.V.; Rogério, K.R.; Kümmerle, A.E. Multicomponent Reactions for the Synthesis of Bioactive Compounds: A Review. Curr. Org. Synth. 2018, 16, 855–899. [Google Scholar] [CrossRef]
- Solangi, M.; Khan, K.M.; Saleem, F.; Hameed, S.; Iqbal, J.; Shafique, Z.; Qureshi, U.; Ul-Haq, Z.; Taha, M.; Perveen, S.; et al. Indole acrylonitriles as potential anti-hyperglycemic agents: Synthesis, α-glucosidase inhibitory activity and molecular docking studies. Bioorg. Med. Chem. 2020, 28, 115605. [Google Scholar] [CrossRef] [PubMed]
- Janssen, G.V.; Zhang, S.; Merkx, R.; Schiesswohl, C.; Chatterjee, C.; Darwin, K.H.; Geurink, P.P.; van der Heden van Noort, G.J.; Ovaa, H. Development of Tyrphostin Analogues to Study Inhibition of the Mycobacterium tuberculosis Pup Proteasome System. ChemBioChem 2021, 22, 3082–3089. [Google Scholar] [CrossRef]
- Schrag, A. Entacapone in the treatment of Parkinson’s disease. Lancet Neurol. 2005, 4, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Blum, G.; Gazit, A.; Levitzki, A. Development of New Insulin-like Growth Factor-1 Receptor Kinase Inhibitors Using Catechol Mimics. J. Biol. Chem. 2003, 278, 40442–40454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarleton, M.; Dyson, L.; Gilbert, J.; Sakoff, J.A.; McCluskey, A. Focused library development of 2-phenylacrylamides as broad spectrum cytotoxic agents. Bioorg. Med. Chem. 2013, 21, 333–347. [Google Scholar] [CrossRef]
- Ke, S.; Yang, Z.; Zhang, Z.; Liang, Y.; Wang, K.; Liu, M.; Shi, L. Multisubstituted indole–acrylonitrile hybrids as potential cytotoxic agents. Bioorg. Med. Chem. Lett. 2014, 24, 1907–1911. [Google Scholar] [CrossRef]
- Amancha, P.K.; Lai, Y.-C.; Chen, I.-C.; Liu, H.-J.; Zhu, J.-L. Diels–Alder reactions of acyclic α-cyano α,β-alkenones: A new approach to highly substituted cyclohexene system. Tetrahedron 2010, 66, 871–877. [Google Scholar] [CrossRef]
- Duan, C.-Q.; He, X.-L.; Du, W.; Chen, Y.-C. Asymmetric [4 + 2] cycloadditions with 3-furfural derivatives and α-cyano-α,β-unsaturated ketones. Org. Chem. Front. 2018, 5, 2057–2060. [Google Scholar] [CrossRef]
- Inokuma, T.; Sakamoto, S.; Takemoto, Y. Enantioselective Nitrocyclopropanation of α,β-Unsaturated α-Cyanoimides Catalyzed by Bifunctional Thiourea. Synlett 2009, 10, 1627–1630. [Google Scholar] [CrossRef]
- Elgazwy, A.-S.S.H.; Refaee, M.R.M. The Chemistry of Alkenyl Nitriles and its Utility in Heterocyclic Synthesis. Org. Chem. Curr. Res. 2013, 2, 1000117. [Google Scholar] [CrossRef]
- Han, G.-F.; Zhao, L.-J.; Chen, L.-Z.; Du, J.-W.; Wang, Z.-X. The Convenient Synthesis of 11-Methyl-3,8-disubstituted-12-aryl-3,4,7,8,9,12-hexahydro-1H-chromeno[2,3-b]quinoline-1,10(2H)-dione Derivatives. J. Heterocycl. Chem. 2015, 52, 1219–1225. [Google Scholar] [CrossRef]
- Elnagdi, M.H.; Elghandour, A.H.H.; Ibrahim, M.K.A.; Hafiz, I.S.A. Studies with Polyfunctionally Substituted Heterocycles: Synthesis of New Pyridines, Naphtho[l,2-b]pyrans, Pyrazolo[3,4-b]pyridines and Pyrazolo[l,5-a]pyrimidines. Z. Naturforsch. 1992, 47, 572–578. [Google Scholar] [CrossRef]
- Sun, J.; Zhu, D.; Gong, H.; Yan, C.-G. The molecular diversity of three-component reactions of 4-dimethylamino- or 4-methoxypyridine with acetylenedicarboxylates and arylidene cyanoacetates. Tetrahedron 2013, 69, 10565–10572. [Google Scholar] [CrossRef]
- Liu, W.; Zhou, J.; Zheng, C.; Chen, X.; Xiao, H.; Yang, Y.; Guo, Y.; Zhao, G. Tandem cross-Rauhute–Currier/cyclization reactions of activated alkenes to give densely functionalized 3,4-dihydropyrans. Tetrahedron 2011, 67, 1768–1773. [Google Scholar] [CrossRef]
- Qi, W.-J.; Han, Y.; Liu, C.-Z.; Yan, C.-G. Three-component reaction of triphenylphosphine, dialkyl but-2-ynedioate and arylidene pivaloylacetonitrile for diastereoselective synthesis of densely substituted 2,3-dihydrofurans. Chin. Chem. Lett. 2017, 28, 442–445. [Google Scholar] [CrossRef]
- Gunasekaran, P.; Balamurugan, K.; Sivakumar, S.; Perumal, S.; Menéndez, J.C.; Almansour, A.I. Domino reactions in water: Diastereoselective synthesis of densely functionalized indolyldihydrofuran derivatives. Green Chem. 2012, 14, 750–757. [Google Scholar] [CrossRef]
- Chen, R.; Fan, X.; Xu, Z.; He, Z. Facile synthesis of polysubstituted furans and dihydrofurans via cyclization of bromonitromethane with oxodiene. Tetrahedron Lett. 2017, 58, 3722–3726. [Google Scholar] [CrossRef]
- Liang, L.; Li, E.; Dong, X.; Huang, Y. DABCO-Mediated [4 + 4]-Domino Annulation: Access to Functionalized Eight-Membered Cyclic Ethers. Org. Lett. 2015, 17, 4914–4917. [Google Scholar] [CrossRef]
- Pachore, S.S.; Ambhaikar, N.B.; Siddaiah, V.; Khobare, S.R.; Kumar, S.; Dahanukar, V.H.; Kumar, U.K.S. Successful utilization of β-ketonitrile in Biginelli reaction: Synthesis of 5-cyanodihydropyrimidine. J. Chem. Sci. 2018, 130, 69. [Google Scholar] [CrossRef] [Green Version]
- Galil, F.M.A.; Sallam, M.M.; Sherif, S.M.; Elnagdi, M.H. Activated Nitriles in Heterocyclic Synthesis: The Reaction of Cyanothioacetamide with Activated Double Bond Systems. Liebigs Ann. Chem. 1986, 1986, 1639–1644. [Google Scholar] [CrossRef]
- Kendre, D.B.; Toche, R.B.; Jachak, M.N. Michael Addition of Dimedone with α,β-unsaturated Ketones: Synthesis of Quinoline and Chromene Derivatives. J. Heterocycl. Chem. 2008, 45, 667–671. [Google Scholar] [CrossRef]
- Burini, E.; Fioravanti, S.; Morreale, A.; Pellacani, L.; Tardella, P.A. Efficient Synthesis of 4-Cyano 2,3-Dihydrooxazoles by Direct Amination of 2-Alkylidene 3-Oxo Nitriles. Synlett 2005, 17, 2673–2675. [Google Scholar] [CrossRef]
- Kolosov, M.A.; Orlov, V.D.; Kolos, N.N.; Shishkin, O.V.; Zubatyuk, R.I. Reactions of α-cyanochalcones with phenylhydrazine. Arkivoc 2007, xvi, 187–194. [Google Scholar] [CrossRef]
- Slätt, J.; Romero, I.; Bergman, J. Cyanoacetylation of Indoles, Pyrroles and Aromatic Amines with the Combination Cyanoacetic Acid and Acetic Anhydride. Synthesis 2004, 16, 2760–2765. [Google Scholar] [CrossRef]
- Semenova, I.A.; Osyanin, V.A.; Osipov, D.V.; Klimochkin, Y.N. A synthesis of carbonyl-substituted 4-aryl-4H-benzo[h]chromenes based on a three-component condensation of α-naphthol, aromatic aldehydes, and β-enaminones. Chem. Heterocycl. Compd. 2022, 58, 656–660. [Google Scholar] [CrossRef]
- Demidov, M.R.; Osyanin, V.A.; Osipov, D.V.; Klimochkin, Y.N. Three-Component Condensation of Pyridinium Ylides, β-Ketonitriles, and Aldehydes with Divergent Regioselectivity: Synthesis of 4,5-Dihydrofuran-3- and 2H-Pyran-5-carbonitriles. J. Org. Chem. 2021, 86, 7460–7476. [Google Scholar] [CrossRef]
- Demidov, M.R.; Osipov, D.V.; Korol’kov, K.A.; Osyanin, V.A. Two-Step Sequence Multicomponent Synthesis/Reductive Rearrangement of 2-Acyl-2,3-dihydrofurans for Modular Assembly of Annulated 4H-Pyrans. Adv. Synth. Catal. 2021, 363, 3737–3743. [Google Scholar] [CrossRef]
- Demidov, M.R.; Dobrokvashina, A.N.; Osipov, D.V.; Osyanin, V.A.; Klimochkin, Y.N. Three-component synthesis of 2-acyl-2,3-dihydro-4H-thiochromeno[4,3-b]furan-4-ones and their reductive rearrangement into 4H,5H-thiochromeno[4,3-b]pyran-5-ones. Chem. Heterocycl. Compd. 2021, 57, 568–573. [Google Scholar] [CrossRef]
- Osipov, D.V.; Demidov, M.R.; Osyanin, V.A.; Klimochkin, Y.N. Three-component condensation of cyclic 1,3-dicarbonyl compounds, N-phenacylpyridinium salts, and isatins or aromatic aldehydes as a method for the synthesis of novel condensed 2-aroyl-2,3-dihydrofurans. Chem. Heterocycl. Compd. 2021, 57, 1045–1050. [Google Scholar] [CrossRef]
- Kvetkin, E.A.; Osipov, D.V.; Krasnikov, P.E.; Osyanin, V.A.; Klimochkin, Y.N. Regio- and diastereoselective synthesis of N-substituted 2-acyl-3-aryl-3,5-dihydrofuro[3,2-c]pyridin-4(2H)-ones. Chem. Heterocycl. Compd. 2020, 56, 1423–1428. [Google Scholar] [CrossRef]
- Osipov, D.V.; Osyanin, V.A.; Klimochkin, Y.N. Synthesis of β-(o-hydroxybenzyl)pyridines by three-component condensation of ammonia, carbonyl-substituted 4H-chromenes, and CH acids. Chem. Heterocycl. Compd. 2018, 54, 1121–1126. [Google Scholar] [CrossRef]
- Robinson, C.N.; Slater, C.D.; Covington, J.S., III; Chang, C.R.; Dewey, L.S.; Franceschini, J.M.; Fritzsche, J.L.; Hamilton, J.E.; Irving, C.C., Jr.; Morris, J.M.; et al. Carbon-13 NMR Chemical Shift–Substituent Effect Correlations in Substituted Styrenes. J. Magn. Reson. 1980, 41, 293–301. [Google Scholar] [CrossRef]
- Zhu, H.; Xu, G.; Du, H.; Zhang, C.; Ma, N.; Zhang, W. Prolinamide functionalized polyacrylonitrile fiber with tunable linker length and surface microenvironment as efficient catalyst for Knoevenagel condensation and related multicomponent tandem reactions. J. Catal. 2019, 374, 217–229. [Google Scholar] [CrossRef]
- Deshpande, S.J.; Leger, P.R.; Sieck, S.R. Microwave synthesis of α-cyano chalcones. Tetrahedron Lett. 2012, 53, 1772–1775. [Google Scholar] [CrossRef]
- Marshal, J.L. Carbon–Proton Couplings. In Carbon–Carbon and Carbon–Proton NMR Couplings: Applications to Organic Stereochemistry and Conformational Analysis; VerlagChemie International: Deerfield Beech, FL, USA, 1983. [Google Scholar]
- Xu, H.; Yu, X.; Sun, L.; Liu, J.; Fan, W.; Shen, Y.; Wang, W. Microwave-assisted three-component Knoevenagel-nucleophilic aromatic substitution reactions. Tetrahedron Lett. 2008, 49, 4687–4689. [Google Scholar] [CrossRef]
- Bello, K.A.; Cheng, L.; Griffiths, J. Near-infrared absorbing methine dyes based on dicyanovinyl derivatives of indane-1,3-dione. J. Chem. Soc. Perkin Trans. 1987, 2, 815–818. [Google Scholar] [CrossRef]
- Landmesser, T.; Linden, A.; Hansen, H.-J. A Novel Route to 1-Substituted 3-(Dialkylamino)-9-oxo-9H-indeno[2,1-c]pyridine-4-carbonitriles. Helv. Chim. Acta 2008, 91, 265–284. [Google Scholar] [CrossRef]
- Osyanin, V.A.; Osipov, D.V.; Klimochkin, Y.N. Reactions of o Quinone Methides with Pyridinium Methylides: A Diastereoselective Synthesis of 1,2-Dihydronaphtho[2,1 b]furans and 2,3-Dihydrobenzofurans. J. Org. Chem. 2013, 78, 5505–5520. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osipov, D.V.; Korzhenko, K.S.; Osyanin, V.A. Three-Component Condensation of β-Ketonitriles, 4-Fluorobenzaldehyde, and Secondary Cyclic Amines. Reactions 2022, 3, 625-633. https://doi.org/10.3390/reactions3040042
Osipov DV, Korzhenko KS, Osyanin VA. Three-Component Condensation of β-Ketonitriles, 4-Fluorobenzaldehyde, and Secondary Cyclic Amines. Reactions. 2022; 3(4):625-633. https://doi.org/10.3390/reactions3040042
Chicago/Turabian StyleOsipov, Dmitry V., Kirill S. Korzhenko, and Vitaly A. Osyanin. 2022. "Three-Component Condensation of β-Ketonitriles, 4-Fluorobenzaldehyde, and Secondary Cyclic Amines" Reactions 3, no. 4: 625-633. https://doi.org/10.3390/reactions3040042
APA StyleOsipov, D. V., Korzhenko, K. S., & Osyanin, V. A. (2022). Three-Component Condensation of β-Ketonitriles, 4-Fluorobenzaldehyde, and Secondary Cyclic Amines. Reactions, 3(4), 625-633. https://doi.org/10.3390/reactions3040042





