On the Importance of the Thiazole Nitrogen in Epothilones: Semisynthesis and Microtubule-Binding Affinity of Deaza-Epothilone C
Abstract
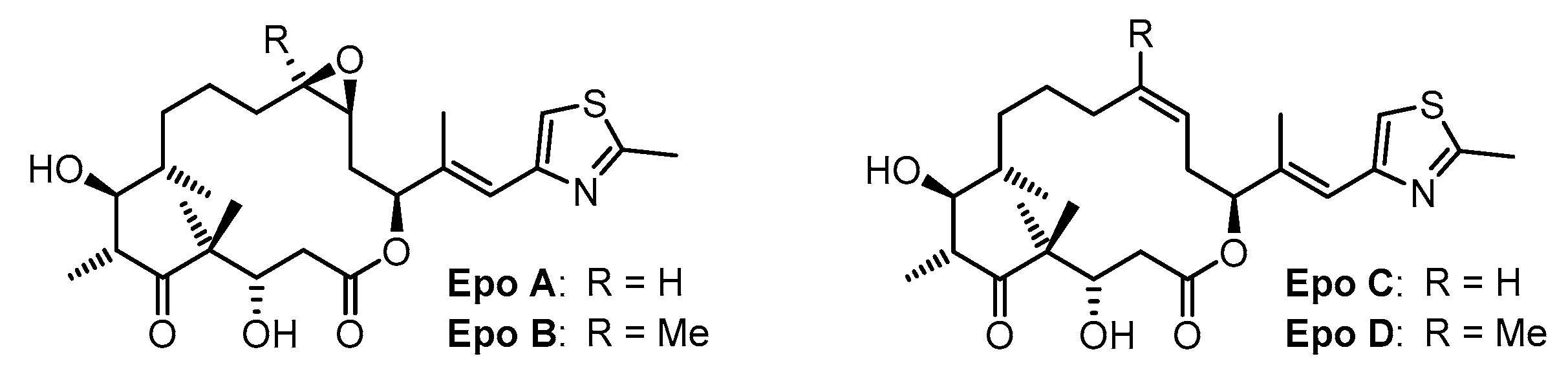
:1. Introduction
2. Materials and Methods
3. Results and Discussion
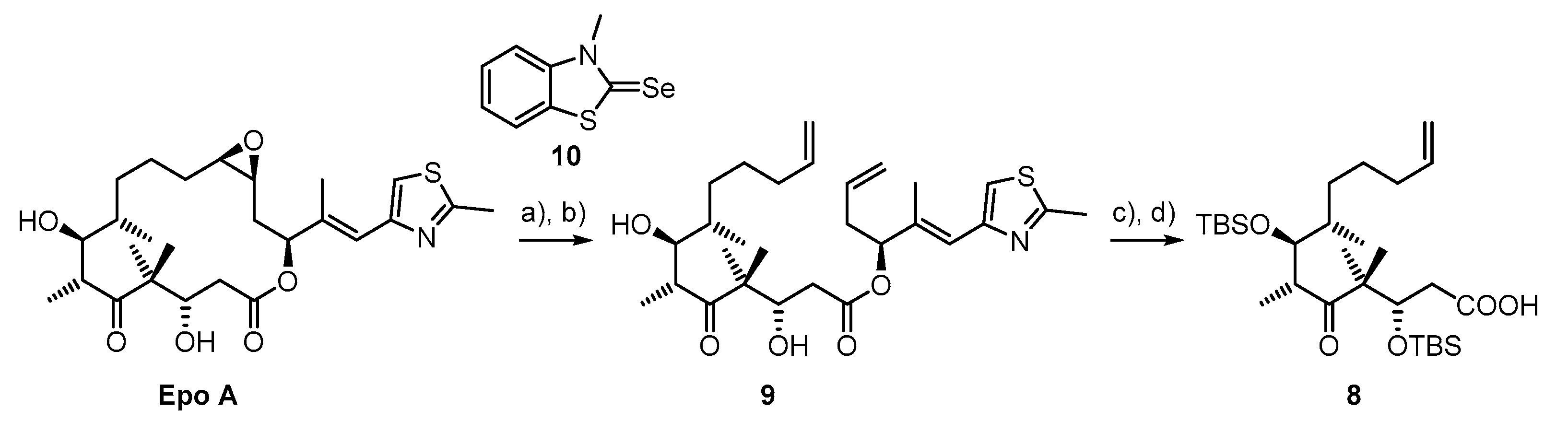
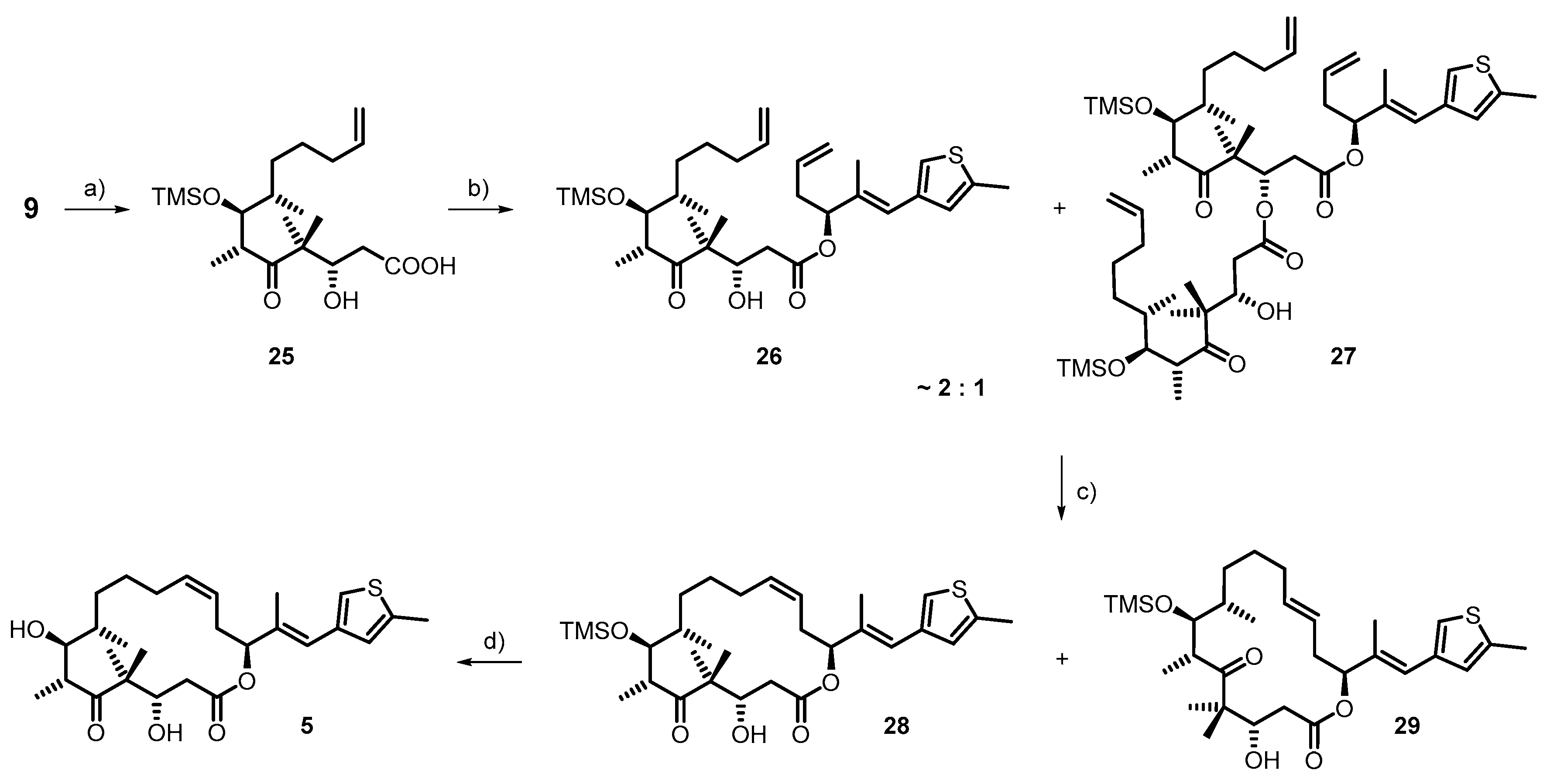
3.1. Synthesis of Deaza-Epo C (5)
3.2. Biochemical and Cellular Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References and Notes
- Gerth, K.; Bedorf, N.; Höfle, G.; Irschik, H.; Reichenbach, H. Epothilons A and B: Antifungal and Cytotoxic Compounds from Sorangium cellulosum (Myxobacteria). Production, Physico-chemical and Biological Properties. J. Antibiot. 1996, 49, 560–563. [Google Scholar] [CrossRef]
- Höfle, G.; Reichenbach, H. Epothilone, a myxobacterial metabolite with promising antitumor activity. In Anticancer Agents from Natural Products; Cragg, G.M., Kingston, D.G.I., Newman, D.J., Eds.; Taylor & Francis: Boca Raton, FL, USA, 2005; pp. 413–450. [Google Scholar] [CrossRef]
- Hardt, I.H.; Steinmetz, H.; Gerth, K.; Sasse, F.; Reichenbach, H.; Höfle, G. New Natural Epothilones from Sorangium cellulosum, Strains So ce90/B2 and So ce90/D13: Isolation, Structure Elucidation, and SAR Studies. J. Nat. Prod. 2001, 64, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Höfle, G.H.; Bedorf, N.; Steinmetz, H.; Schomburg, D.; Gerth, K.; Reichenbach, H. Antibiotics from gliding bacteria. 77. Epothilone A and B-novel 16-membered macrolides with cytotoxic activity: Isolation, crystal structure, and conformation in solution. Angew. Chem. Int. Ed. 1996, 35, 1567–1569. [Google Scholar] [CrossRef]
- Bollag, D.M.; McQueney, P.A.; Zhu, J.; Hensens, O.; Koupal, L.; Liesch, J.; Goetz, M.; Lazarides, E.; Woods, C.M. Epothilones, a new class of microtubule-stabilizing agents with a Taxol-like mechanism of action. Cancer Res. 1995, 55, 2325–2333. [Google Scholar]
- Kowalski, R.J.; Giannakakou, P.; Hamel, E. Activities of the microtubule-stabilizing agents epothilones A and B with purified tubulin and in cells resistant to paclitaxel (Taxol). J. Biol. Chem. 1997, 272, 2534–2541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altmann, K.-H.; Wartmann, M.; O’Reilly, T. Epothilones and related structures-a new class of microtubule inhibitors with potent in vivo antitumor activity. Biochim. Biophys. Acta 2000, 1470, M79–M91. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Roschangar, F.; Vourloumis, D. Chemical Biology of Epothilones. Angew. Chem. Int. Ed. 1998, 37, 2014–2045. [Google Scholar] [CrossRef]
- Harris, C.R.; Danishefsky, S.J. Complex target-oriented synthesis in the drug discovery process: A case history in the dEpoB series. J. Org. Chem. 1999, 64, 8434–8456. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Ritzén, A.; Namoto, K. Recent developments in the chemistry, biology and medicine of the epothilones. Chem. Commun. 2001, 1523–1535. [Google Scholar] [CrossRef]
- Altmann, K.-H. The merger of natural product synthesis and medicinal chemistry: On the chemistry and chemical biology of epothilones. Org. Biomol. Chem. 2004, 2, 2137–2151. [Google Scholar] [CrossRef]
- Watkins, E.B.; Chittiboyina, A.G.; Avery, M.A. Recent Developments in the Syntheses of the Epothilones and Related Analogues. Eur. J. Org. Chem. 2006, 4071–4084. [Google Scholar] [CrossRef]
- Nicolaou, K.C. The Chemistry-Biology-Medicine Continuum and the Drug Discovery and Development Process in Academia. Chem. Biol. 2014, 21, 1039–1045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altmann, K.-H.; Pfeiffer, B.; Arseniyadis, S.; Pratt, B.A.; Nicolaou, K.C. The chemistry and biology of epothilones-The wheel keeps turning. ChemMedChem 2007, 2, 397–423. [Google Scholar] [CrossRef] [PubMed]
- Altmann, K.H.; Kinghorn, A.D.; Höfle, G.; Müller, R.; Prantz, K. For a Book on Epothilones cf.: The Epothilones: An Outstanding Family of Anti-Tumor Agents (Progress Chem. Org. Nat. Prod. 90); Kinghorn, A.D., Falk, H., Kobayashi, J., Eds.; Springer Wien: New York, NY, USA, 2009; ISBN 978-3-211-78207-1. [Google Scholar] [CrossRef]
- Altmann, K.-H.; Gaugaz, F.Z.; Schiess, R. Diversity through semisynthesis: The chemistry and biological activity of semisynthetic epothilone derivatives. Mol. Divers. 2011, 15, 383–399. [Google Scholar] [CrossRef] [PubMed]
- Lechleider, R.J.; Kaminskas, E.; Jiang, X.; Aziz, R.; Bullock, J.; Kasliwal, R.; Harapanhalli, R.; Pope, S.; Sridhara, R.; Leighton, J.; et al. Ixabepilone in Combination with Capecitabine and as Monotherapy for Treatment of Advanced Breast Cancer Refractory to Previous Chemotherapies. Clin. Cancer Res. 2008, 14, 4378–4384. [Google Scholar] [CrossRef] [Green Version]
- Barten, D.M.; Fanara, P.; Andorfer, C.; Hoque, N.; Wong, P.Y.A.; Husted, K.H.; Cadelina, G.W.; DeCarr, L.B.; Yang, L.; Liu, V.; et al. Hyperdynamic microtubules, cognitive deficits, and pathology are improved in tau transgenic mice with low doses of the microtubule-stabilizing agent BMS-241027. J. Neurosci. 2012, 32, 7137–7145. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; Sun, T.; Zhang, Q.; Yuan, Z.; Jiang, Z.; Wang, X.J.; Cui, S.; Teng, Y.; Hu, X.-C.; Yang, J.; et al. Utidelone plus capecitabine versus capecitabine alone for heavily pretreated metastatic breast cancer refractory to anthracyclines and taxanes: A multicentre, open-label, superiority, phase 3, randomised controlled trial. Lancet Oncol. 2017, 18, 371–383. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Scarpelli, R.; Bollbuck, B.; Werschkun, B.; Pereira, M.M.A.; Wartmann, M.; Altmann, K.-H.; Zaharevitz, D.; Gussio, R.; Giannakakou, P. Chemical synthesis and biological properties of pyridine epothilones. Chem. Biol. 2000, 7, 593–599. [Google Scholar] [CrossRef] [Green Version]
- Su, D.-S.; Meng, D.; Bertinato, P.; Balog, A.; Sorensen, E.J.; Danishefsky, S.J.; Zheng, Y.-H.; Chou, T.C.; He, L.; Horwitz, S.B. Total synthesis of (-)-epothilone B: An extension of the Suzuki coupling method and insights into structure-activity relationships of the epothilones. Angew. Chem. Int. Ed. 1997, 37, 757–759. [Google Scholar] [CrossRef]
- Dietrich, S.; Lindauer, R.; Stierlin, C.; Gertsch, J.; Matesanz, R.; Notararigo, S.; Díaz, J.F.; Altmann, K.-H. Epothilone Analogs with Benzimidazole and Quinoline Side Chains: Chemical Synthesis, Antiproliferative Activity, and Interactions with Tubulin. Chem. Eur. J. 2009, 15, 10144–10157. [Google Scholar] [CrossRef] [Green Version]
- Prota, A.E.; Bargsten, K.; Zurwerra, D.; Field, J.J.; Díaz, J.F.; Altmann, K.-H.; Steinmetz, M.O. Molecular Mechanism of Action of Microtubule-Stabilizing Anticancer Agents. Science 2013, 339, 587–590. [Google Scholar] [CrossRef] [Green Version]
- Sefkow, M.; Kiffe, M.; Schummer, D.; Höfle, G. Oxidative and reductive transformations of epothilone A. Bioorg. Med. Chem. Lett. 1998, 8, 3025–3030. [Google Scholar] [CrossRef]
- Höfle, G.; Glaser, N.; Leibold, T.; Karama, U.; Sasse, F.; Steinmetz, H. Semisynthesis and degradation of the tubulin inhibitors epothilone and tubulysin. Pure Appl. Chem. 2003, 75, 167–178. [Google Scholar] [CrossRef]
- Erdélyi, M.; Navarro-Vázquez, A.; Pfeiffer, B.; Kuzniewski, C.N.; Felser, A.; Widmer, T.; Gertsch, J.; Pera, B.; Díaz, J.F.; Altmann, K.-H.; et al. The binding mode of side chain- and C3-modified epothilones to tubulin. ChemMedChem 2010, 5, 911–920. [Google Scholar] [CrossRef] [Green Version]
- Schiess, R.; Gertsch, J.; Schweizer, W.B.; Altmann, K.-H. Stereoselective Synthesis of 12,13-Cyclopropyl-Epothilone B and Side-Chain-Modified Variants. Org. Lett. 2011, 13, 1436–1439. [Google Scholar] [CrossRef]
- Karama, U.; Höfle, G. Synthesis of epothilone 16,17-alkyne analogs by replacement of the C13-C15(O)-ring segment of natural epothilone C. Eur. J. Org. Chem. 2003, 1042–1049. [Google Scholar] [CrossRef]
- Schinzer, D.; Limberg, A.; Bauer, A.; Böhm, O.M.; Cordes, M. Total synthesis of (-)-epothilone A. Angew. Chem. Int. Ed. Engl. 1997, 36, 523–524. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; He, Y.; Vourloumis, D.; Vallberg, H.; Zang, Z. Total synthesis of epothilone A: The olefin metathesis approach. Angew. Chem. Int. Ed. Engl. 1997, 36, 166–168. [Google Scholar] [CrossRef]
- Balog, A.; Meng, D.; Kamenecka, T.; Bertinato, P.; Su, D.S.; Sorensen, E.J.; Danishefsky, S.J. Total synthesis of (-)-epothilone A. Angew. Chem. Int. Ed. Engl. 1996, 35, 2801–2803. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; He, Y.; Vourloumis, D.; Vallberg, H.; Roschangar, F.; Sarabia, F.; Ninkovic, S.; Yang, Z.; Trujillo, J.I. The Olefin Metathesis Approach to Epothilone A and Its Analogs. J. Am. Chem. Soc. 1997, 119, 7960–7973. [Google Scholar] [CrossRef]
- Altmann, K.-H.; Flörsheimer, A.; Bold, G.; Caravatti, G.; End, N.; Wartmann, M. Natural product-based drug discovery-Epothilones as lead structures for the development of new anticancer agents. Chimia 2004, 58, 686–690. [Google Scholar] [CrossRef]
- Calo, V.; Lopez, L.; Mincuzzi, A.; Pesce, G. 3-Methyl-2-selenoxobenzothiazole, a New Reagent for the Stereospecific Deoxygenation of Epoxides and the Desulfurization of Episulfides into Olefins. Synthesis 1976, 200–201. [Google Scholar] [CrossRef]
- Bond, S.; Perlmutter, P. N-Acetylbornane-10,2-sultam: A Useful, Enantiomerically Pure Acetate Synthon for Asymmetric Aldol Reactions. J. Org. Chem. 1997, 62, 6397–6400. [Google Scholar] [CrossRef]
- The (S)-configuration of the major isomer is inferred from the known stereochemical outcome of acetate aldol reactions with N-acetylbornane-10,2-sultam 18 related (see refs. [22,35]).
- Inanaga, J.; Hirata, K.; Saeki, H.; Katsuki, T.; Yamaguchi, M. A Rapid Esterification by Means of Mixed Anhydride and Its Application to Large-ring Lactonization. Bull. Chem. Soc. Jpn. 1979, 52, 1989–1993. [Google Scholar] [CrossRef] [Green Version]
- Lecourt, C.; Dhambri, S.; Allievi, L.; Sanogo, Y.; Zeghbib, N.; Ben Othman, R.; Lannou, M.-I.; Sorin, G.; Ardisson, J. Natural products and ring-closing metathesis: Synthesis of sterically congested olefins. Nat. Prod. Rep. 2018, 35, 105–124. [Google Scholar] [CrossRef]
- Niggemann, J.; Michaelis, K.; Frank, R.; Zander, N.; Höfle, G. Natural product-derived building blocks for combinatorial synthesis. Part 1. Fragmentation of natural products from myxobacteria. J. Chem. Soc. Perkin Trans. 1 2002, 2490–2503. [Google Scholar] [CrossRef]
- The structural assignment of 27 is tentatively based on 1H-NMR analysis and MS.
- Buey, R.M.; Díaz, J.F.; Andreu, J.M.; O’Brate, A.; Giannakakou, P.; Nicolaou, K.C.; Sasmal, P.K.; Ritzén, A.; Namoto, K. Interaction of Epothilone Analogs with the Paclitaxel Binding Site: Relationship between Binding Affinity, Microtubule Stabilization, and Cytotoxicity. Chem. Biol. 2004, 11, 225–236. [Google Scholar] [CrossRef]
- Matesanz, R.; Barasoain, I.; Yang, C.; Wang, L.; Li, X.; De Ines, C.; Coderch, C.; Gago, F.; Jiménez-Barbero, J.; Andreu, J.M.; et al. Optimization of taxane binding to microtubules. Binding affinity dissection and incremental construction of a high-affinity analogue of paclitaxel. Chem. Biol. 2008, 15, 573–585. [Google Scholar] [CrossRef] [Green Version]







| Compound | Kb [107 M−1] | ||
|---|---|---|---|
| 26 °C | 30 °C | 35 °C | |
| 5 | 0.39 ± 0.03 | 0.33 ± 0.02 | 0.37 ± 0.05 |
| Epo C | 1.46 ± 0.33 | 1.19 ± 0.15 | 1.93 ± 0.27 |
| Epo A2 | 7.48 ± 1.00 | 5.81 ± 1.08 | 3.63 ± 0.51 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Edenharter, A.; Ryckewaert, L.; Cintulová, D.; Estévez-Gallego, J.; Díaz, J.F.; Altmann, K.-H. On the Importance of the Thiazole Nitrogen in Epothilones: Semisynthesis and Microtubule-Binding Affinity of Deaza-Epothilone C. Chemistry 2020, 2, 499-509. https://doi.org/10.3390/chemistry2020030
Edenharter A, Ryckewaert L, Cintulová D, Estévez-Gallego J, Díaz JF, Altmann K-H. On the Importance of the Thiazole Nitrogen in Epothilones: Semisynthesis and Microtubule-Binding Affinity of Deaza-Epothilone C. Chemistry. 2020; 2(2):499-509. https://doi.org/10.3390/chemistry2020030
Chicago/Turabian StyleEdenharter, Adriana, Lucie Ryckewaert, Daniela Cintulová, Juan Estévez-Gallego, José Fernando Díaz, and Karl-Heinz Altmann. 2020. "On the Importance of the Thiazole Nitrogen in Epothilones: Semisynthesis and Microtubule-Binding Affinity of Deaza-Epothilone C" Chemistry 2, no. 2: 499-509. https://doi.org/10.3390/chemistry2020030





