Non-Contacting Plant Health Monitoring via Ultrasound in Ambient Air
Abstract
:1. Introduction
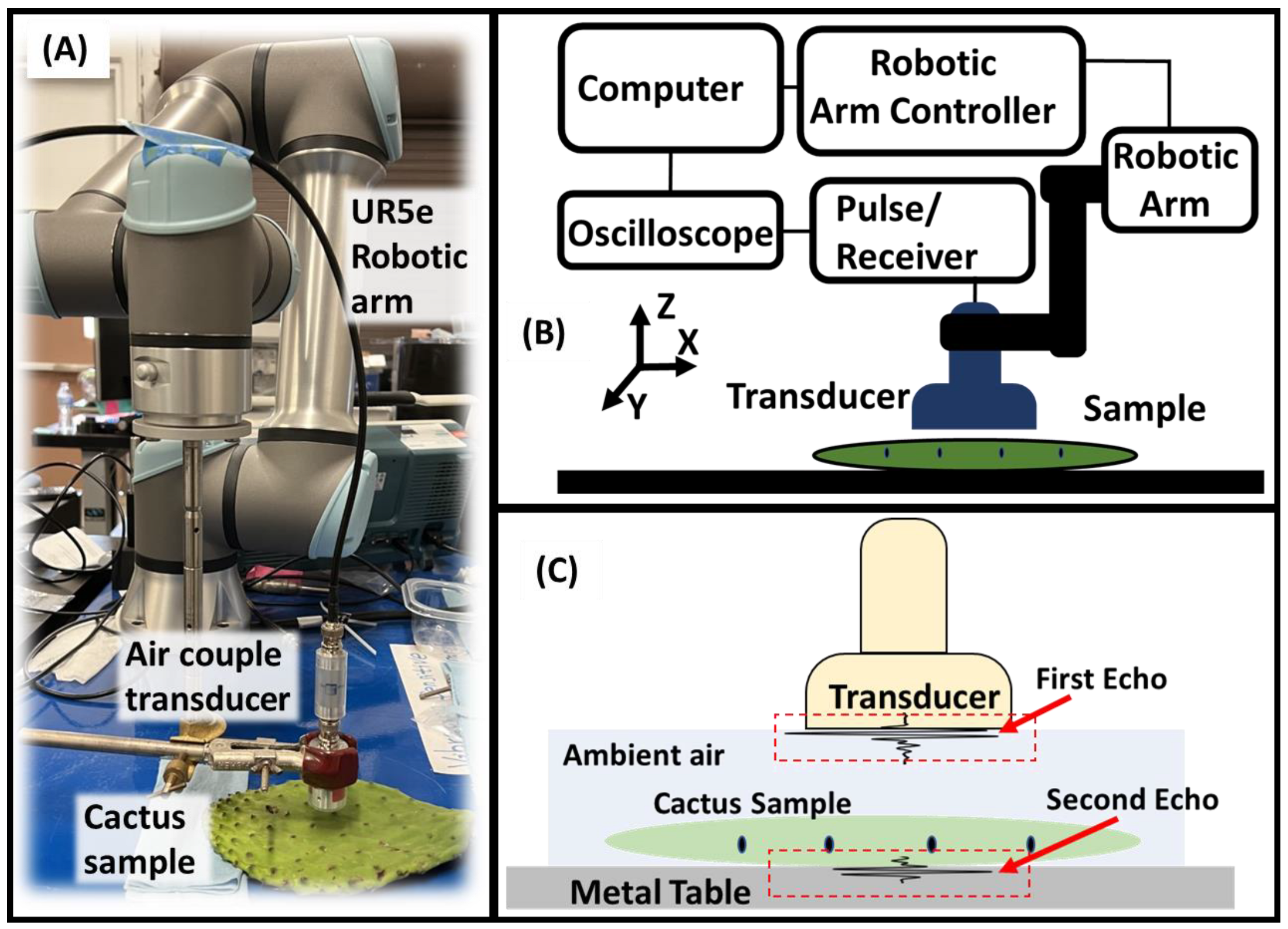
2. Materials and Methods
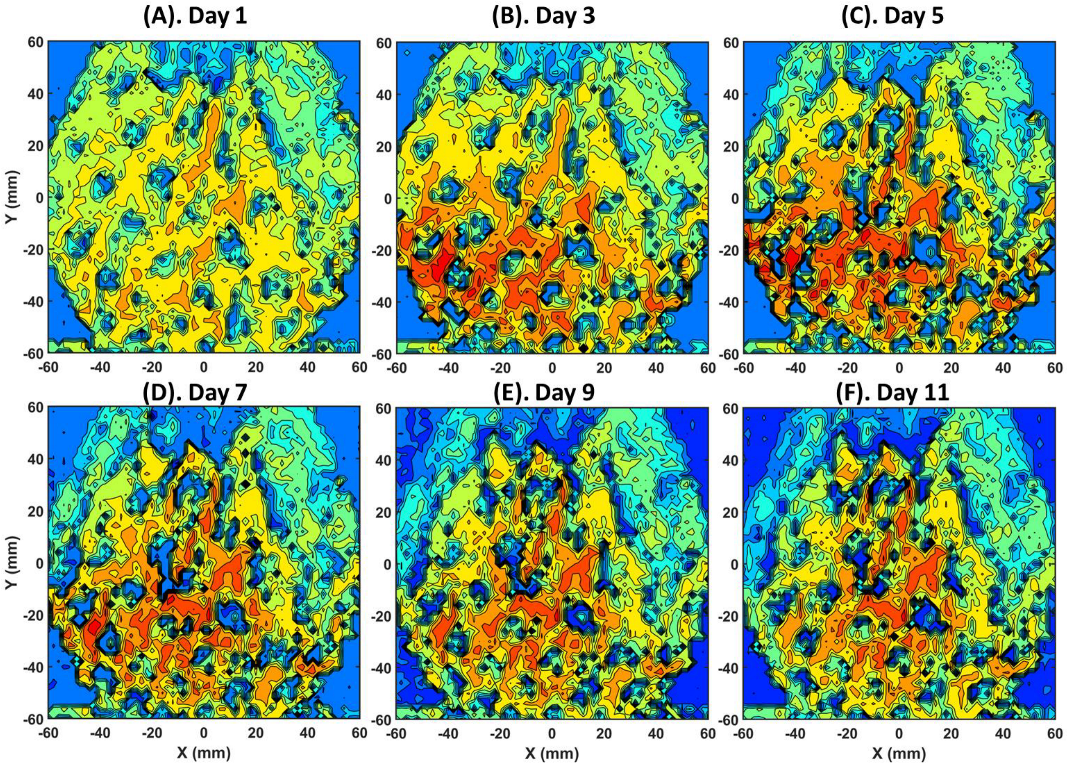
3. Results
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Model number | NCT 1.5-D13-P25 |
| Active area | 13 mm diameter |
| Nominal frequency | 1.5 MHz |
| Bandwidth | 650–1.35 MHz |
| Focal length in air | 25 mm with sphere focal point |
| Amplitude | 0.85 V @ receiver |
| Pulse duration | 4.0 μs |
| Low-pass filter | 5.0 MHz |
| High-pass filter | 0 MHz |
| Gain | 40 dB |
| Voltage | 400 V |
| Damping | 94.0 Ohms |
References
- Sweet, S.K.; Wolfe, D.W.; DeGaetano, A.; Benner, R. Anatomy of the 2016 drought in the Northeastern United States: Implications for agriculture and water resources in humid climates. Agric. For. Meteorol. 2017, 247, 571–581. [Google Scholar] [CrossRef]
- Cogato, A.; Meggio, F.; De Antoni Migliorati, M.; Marinello, F. Extreme weather events in agriculture: A systematic review. Sustainability 2019, 11, 2547. [Google Scholar] [CrossRef]
- West, H.; Quinn, N.; Horswell, M. Remote sensing for drought monitoring & impact assessment: Progress, past challenges and future opportunities. Remote Sens. Environ. 2019, 232, 111291. [Google Scholar]
- Cooley, H.; Donnelly, K.; Phurisamban, R.; Subramanian, M. Impacts of California’s Ongoing Drought: Agriculture; Pacific Institute: Oakland, CA, USA, 2015. [Google Scholar]
- Medellín-Azuara, J.; MacEwan, D.; Howitt, R.E.; Sumner, D.A.; Lund, J.R.; Scheer, J.; Gailey, R.; Hart, Q.; Alexander, N.D.; Arnold, B.; et al. Economic Analysis of the 2016 California Drought on Agriculture; Center for Watershed Sciences, University of California: Davis, CA, USA, 2016. [Google Scholar]
- Kogan, F.; Guo, W.; Yang, W. Drought and food security prediction from NOAA new generation of operational satellites. Geomat. Nat. Hazards Risk 2019, 10, 651–666. [Google Scholar] [CrossRef]
- Roper, J.M.; Garcia, J.F.; Tsutsui, H. Emerging technologies for monitoring plant health in vivo. ACS omega 2021, 6, 5101–5107. [Google Scholar] [CrossRef]
- Liu, W.; Stewart Jr, C.N. Plant synthetic biology. Trends Plant Sci. 2015, 20, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Alemu, K. Real-time PCR and its application in plant disease diagnostics. Adv. Life Sci. Technol. 2014, 27, 39–49. [Google Scholar]
- Pastorelli, A.A.; Blasi, E.; Giammarioli, S.; Silano, M.; Stacchini, P.; Boniglia, C. Presence of soy in cereals and cereal products: Validation of an ELISA technique and monitoring of products from the Italian market. J. Consum. Prot. Food Saf. 2021, 16, 315–321. [Google Scholar] [CrossRef]
- Costa, J.M.; Grant, O.M.; Chaves, M.M. Thermography to explore plant–environment interactions. J. Exp. Bot. 2013, 64, 3937–3949. [Google Scholar] [CrossRef]
- Chaerle, L.; Van Der Straeten, D. Seeing is believing: Imaging techniques to monitor plant health. Biochim. Biophys. Acta (BBA)-Gene Struct. Expr. 2001, 1519, 153–166. [Google Scholar] [CrossRef]
- Farber, C.; Mahnke, M.; Sanchez, L.; Kurouski, D. Advanced spectroscopic techniques for plant disease diagnostics. A review. TrAC Trends Anal. Chem. 2019, 118, 43–49. [Google Scholar] [CrossRef]
- Jeon, E.; Choi, S.; Yeo, K.-H.; Park, K.S.; Rathod, M.L.; Lee, J. Development of electrical conductivity measurement technology for key plant physiological information using microneedle sensor. J. Micromech. Microeng. 2017, 27, 085009. [Google Scholar] [CrossRef]
- Gallego-Juárez, J.A. Ultrasound in Food Processing: Recent Advances; Wiley: Hoboken, NJ, USA, 2017. [Google Scholar] [CrossRef]
- Petcher, P.A.; Dixon, S. Weld defect detection using PPM EMAT generated shear horizontal ultrasound. NDT E Int. 2015, 74, 58–65. [Google Scholar] [CrossRef]
- Jin, Y.; Yang, T.; Heo, H.; Krokhin, A.; Shi, S.Q.; Dahotre, N.; Choi, T.-Y.; Neogi, A. Novel 2D dynamic elasticity maps for inspection of anisotropic properties in fused deposition modeling objects. Polymers 2020, 12, 1966. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Mazumder, S.; Jin, Y.; Squires, B.; Sofield, M.; Pantawane, M.V.; Dahotre, N.B.; Neogi, A. A review of diagnostics methodologies for metal additive manufacturing processes and products. Materials 2021, 14, 4929. [Google Scholar] [CrossRef] [PubMed]
- Pantawane, M.V.; Yang, T.; Jin, Y.; Joshi, S.S.; Dasari, S.; Sharma, A.; Krokhin, A.E.A. Crystallographic texture dependent bulk anisotropic elastic response of additively manufactured Ti6Al4V. Sci. Rep. 2021, 11, 633. [Google Scholar] [CrossRef] [PubMed]
- Ritter, T.A.; Shrout, T.R.; Tutwiler, R.; Shung, K.K. A 30-MHz piezo-composite ultrasound array for medical imaging applications. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2002, 49, 217–230. [Google Scholar] [CrossRef] [PubMed]
- Saini, K.; Dewal, M.L.; Rohit, M. Ultrasound imaging and image segmentation in the area of ultrasound: A review. Int. J. Adv. Sci. Technol. 2010, 24. [Google Scholar]
- Blomme, E.; Bulcaen, D.; Declercq, F. Air-coupled ultrasonic NDE: Experiments in the frequency range 750 kHz–2 MHz. NDT E Int. 2002, 35, 417–426. [Google Scholar] [CrossRef]
- Chimenti, D.E. Review of air-coupled ultrasonic materials characterization. Ultrasonics 2014, 54, 1804–1816. [Google Scholar] [CrossRef]
- Jin, Y.; Walker, E.; Krokhin, A.; Heo, H.; Choi, T.-Y.; Neogi, A. Enhanced Instantaneous Elastography in Tissues and Hard Materials Using Bulk Modulus and Density Determined without Externally Applied Material Deformation. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2019, 67, 624–634. [Google Scholar] [CrossRef] [PubMed]
- Neogi, A.; Jin, Y.; Yang, T. Ltrasonic Sensor Based In-Situ Diagnostics for at Least One of Additive Manufacturing and 3D Printers. U.S. Patent 17/677,592, 25 August 2022. [Google Scholar]
- Yang, T.; Jin, Y.; Squires, B.; Choi, T.-Y.; Dahotre, N.B.; Neogi, A. In-situ monitoring and ex-situ elasticity mapping of laser induced metal melting pool using ultrasound: Numerical and experimental approaches. J. Manuf. Process. 2021, 71, 178. [Google Scholar] [CrossRef]
- Essig, W.; Bernhardt, Y.; Döring, D.; Solodov, I.; Gautzsch, T.; Gaal, M.; Hufschläger, D. Air-coupled Ultrasound–Emerging NDT Method. Fachbeiträge 2021, 1, 32. [Google Scholar]
- Pantawane, M.V.; Yang, T.; Jin, Y.; Mazumder, S.; Pole, M.; Dasari, S.; Krokhin, A.; Neogi, A.; Mukherjee, S.; Banerjee, R.; et al. Thermomechanically Influenced Dynamic Elastic Constants of Laser Powder Bed Fusion Additively Manufactured Ti6Al4V. Mater. Sci. Eng. A 2021, 811, 140990. [Google Scholar] [CrossRef]
- Tevet, O.; Svetlizky, D.; Harel, D.; Barkay, Z.; Geva, D.; Eliaz, N. Measurement of the anisotropic dynamic elastic constants of additive manufactured and wrought Ti6Al4V alloys. Materials 2022, 15, 638. [Google Scholar] [CrossRef] [PubMed]
- Xia, N.; Zhao, P.; Zhang, J.; Xie, J.; Zhang, C.; Fu, J. Investigation of ultrasound velocity measurements of polymeric parts with different surface roughness. Polym. Test. 2020, 81, 106231. [Google Scholar] [CrossRef]
- Jin, Y.; Heo, H.; Walker, E.; Krokhin, A.; Choi, T.Y.; Neogi, A. The effects of temperature and frequency dispersion on sound speed in bulk poly (Vinyl Alcohol) poly (N-isopropylacrylamide) hydrogels caused by the phase transition. Ultrasonics 2020, 104, 105931. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Yang, T.; Ju, S.; Zhang, H.; Choi, T.-Y.; Neogi, A. Thermally Tunable Dynamic and Static Elastic Properties of Hydrogel Due to Volumetric Phase Transition. Polymers 2020, 12, 1462. [Google Scholar] [CrossRef] [PubMed]
- Kirschner, G.K.; Xiao, T.T.; Blilou, I. Rooting in the desert: A developmental overview on desert plants. Genes 2021, 12, 709. [Google Scholar] [CrossRef]
- Gibson, A.C. Photosynthetic organs of desert plants. Bioscience 1998, 48, 911–920. [Google Scholar] [CrossRef]
- Lüttge, U. Ability of crassulacean acid metabolism plants to overcome interacting stresses in tropical environments. AoB Plants 2010, 2010, plq005. [Google Scholar] [CrossRef] [PubMed]
- López, R.A.; Ita, D.; Vaca, M. Drying of prickly pear cactus cladodes ((Opuntia ficus indica) in a forced convection tunnel. Energy Convers. Manag. 2009, 50, 2119–2126. [Google Scholar] [CrossRef]
- Gibson, A.C.; Nobel, P.S. The Cactus Primer; Harvard University Press: Cambridge, MA, USA, 1986. [Google Scholar]
- Mylo, M.D.; Hesse, L.; Masselter, T.; Leupold, J.; Drozella, K.; Speck, T.; Speck, O. Morphology and anatomy of branch–branch junctions in Opuntia ficus-indica and Cylindropuntia bigelovii: A comparative study supported by mechanical tissue quantification. Plants 2021, 10, 2313. [Google Scholar] [CrossRef] [PubMed]
- Kamal, T.; Ul-Islam, M.; Khan, S.B.; Bakhsh, E.M.; Chani, M.T.S. Preparation, Characterization, and Biological Features of Cactus Coated Bacterial Cellulose Hydrogels. Gels 2022, 8, 88. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, S.; dos Santos, D.P.; Machado Enes, A.; Sobrinha, M.N.A.B.; Gazzola, J.; Dal Fabbro, I.M. Mechanical Properties Characterization of Spineless Cactus (Opuntia FiCus-Indica). Int. J. Sci. Eng. Investig. 2015, 4, 1. [Google Scholar]
- Yang, T.; Jin, Y.; Choi, T.-Y.; Dahotre, N.; Neogi, A. Mechanically tunable ultrasonic metamaterial lens with a subwavelength resolution at long working distances for bioimaging. Smart Mater. Struct. 2020, 30, 015022. [Google Scholar] [CrossRef]
- Walker, E.L.; Jin, Y.; Reyes, D.; Neogi, A. Sub-wavelength lateral detection of tissue-approximating masses using an ultrasonic metamaterial lens. Nat. Commun. 2020, 11, 5967. [Google Scholar] [CrossRef]
- Jin, Y.; Wang, X.; Fox, E.A.; Xie, Z.; Neogi, A.; Mishra, R.S.; Wang, T. Numerically Trained Ultrasound AI for Monitoring Tool Degradation. Adv. Intell. Syst. 2022, 4, 2100215. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, T.; Jin, Y.; Dahotre, N.B.; Neogi, A. Non-Contacting Plant Health Monitoring via Ultrasound in Ambient Air. Biophysica 2022, 2, 315-323. https://doi.org/10.3390/biophysica2040029
Yang T, Jin Y, Dahotre NB, Neogi A. Non-Contacting Plant Health Monitoring via Ultrasound in Ambient Air. Biophysica. 2022; 2(4):315-323. https://doi.org/10.3390/biophysica2040029
Chicago/Turabian StyleYang, Teng, Yuqi Jin, Narendra B. Dahotre, and Arup Neogi. 2022. "Non-Contacting Plant Health Monitoring via Ultrasound in Ambient Air" Biophysica 2, no. 4: 315-323. https://doi.org/10.3390/biophysica2040029






