Phenotypic and Biomechanical Characteristics of Human Fetal Neural Progenitor Cells Exposed to Pesticide Compounds
Abstract
:1. Introduction
2. Materials & Methods
2.1. Materials
2.2. Cell Culture and Compound Concentrations
2.3. Cell Viability Assay
2.4. DNA Content Assay
2.5. Cell Survival Quantification
2.6. AFM Analysis
2.7. Wound Healing Assay
2.8. Statistical Analysis
3. Results and Discussion
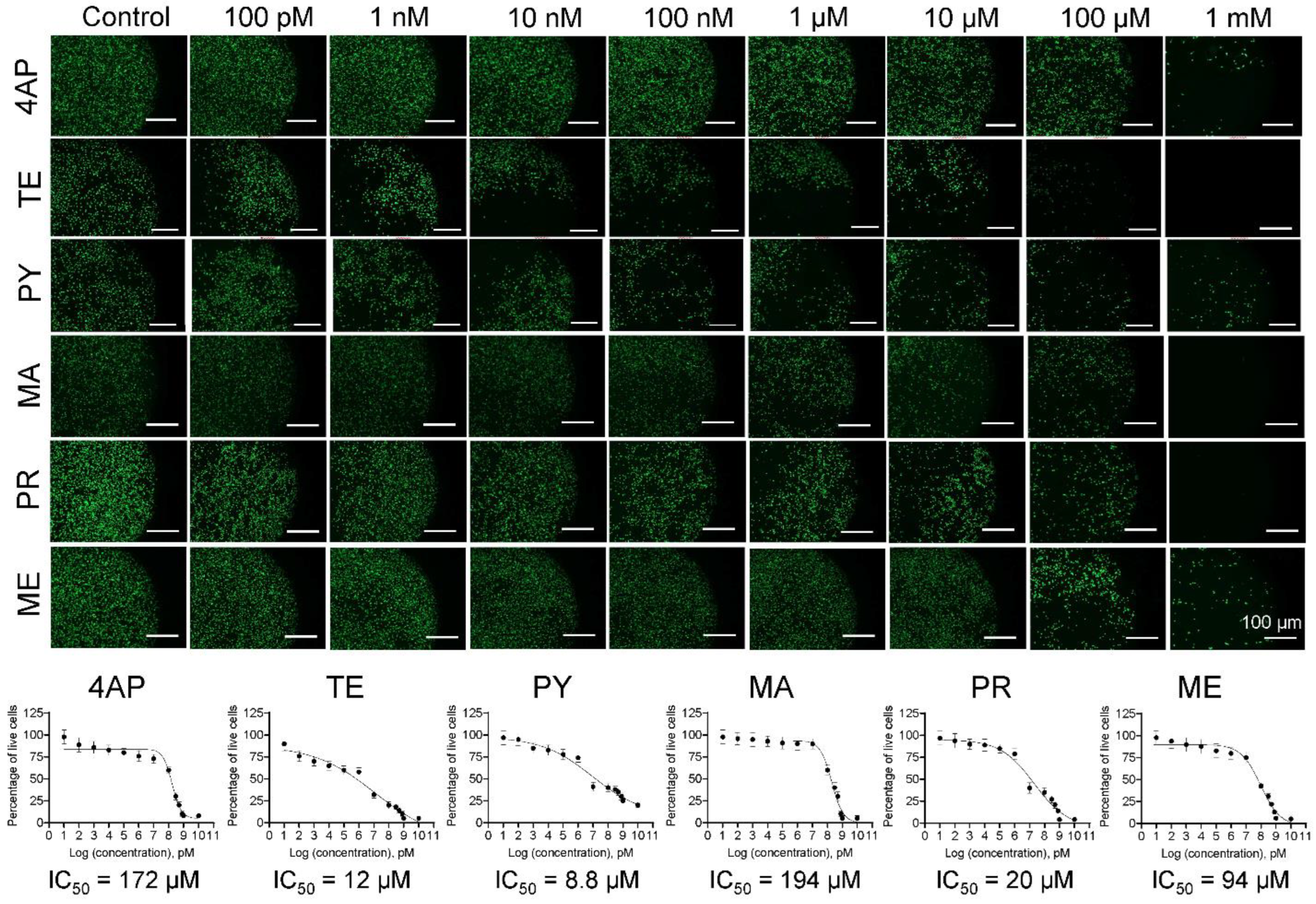
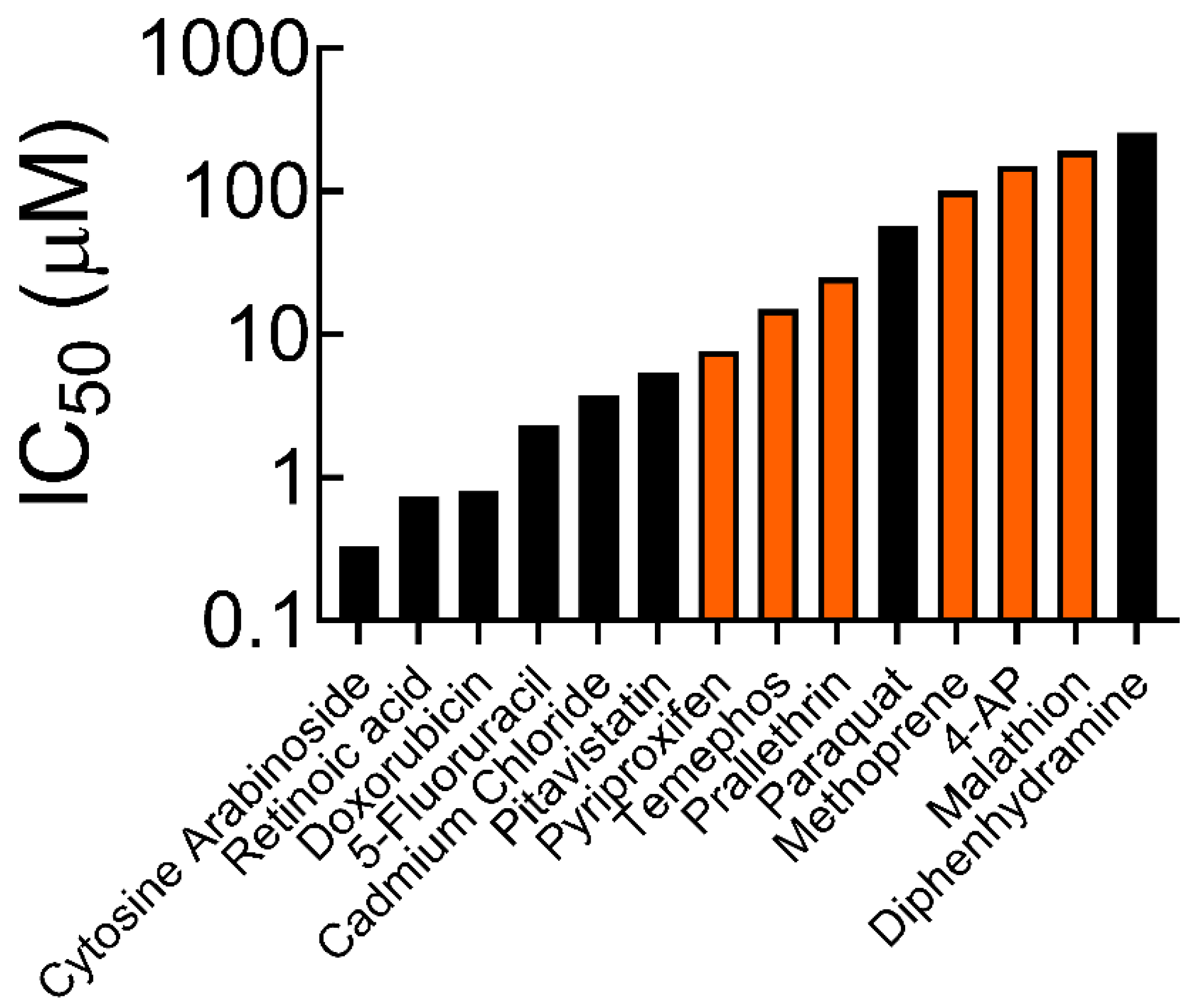
3.1. Cell Survival and IC50 Values
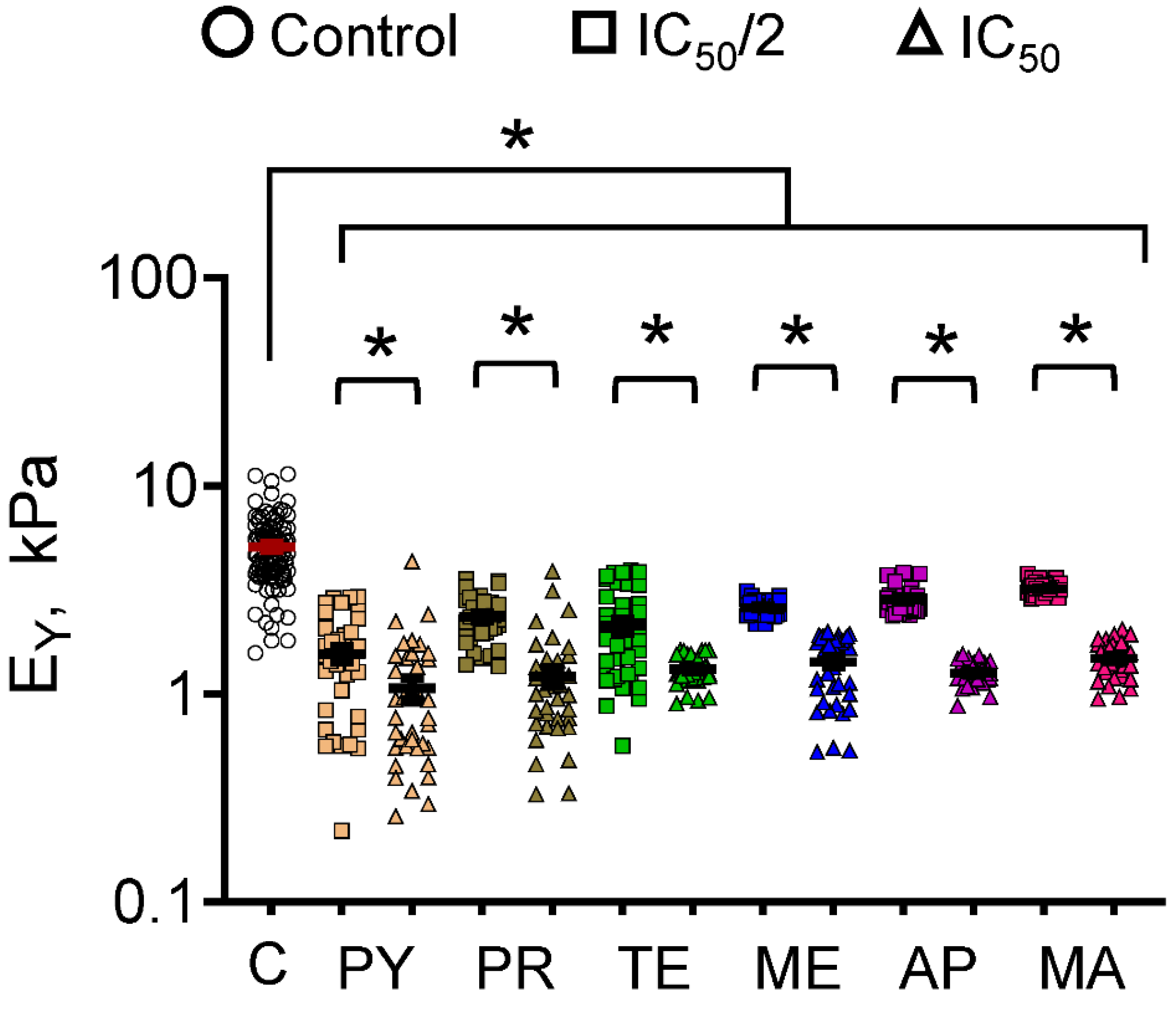
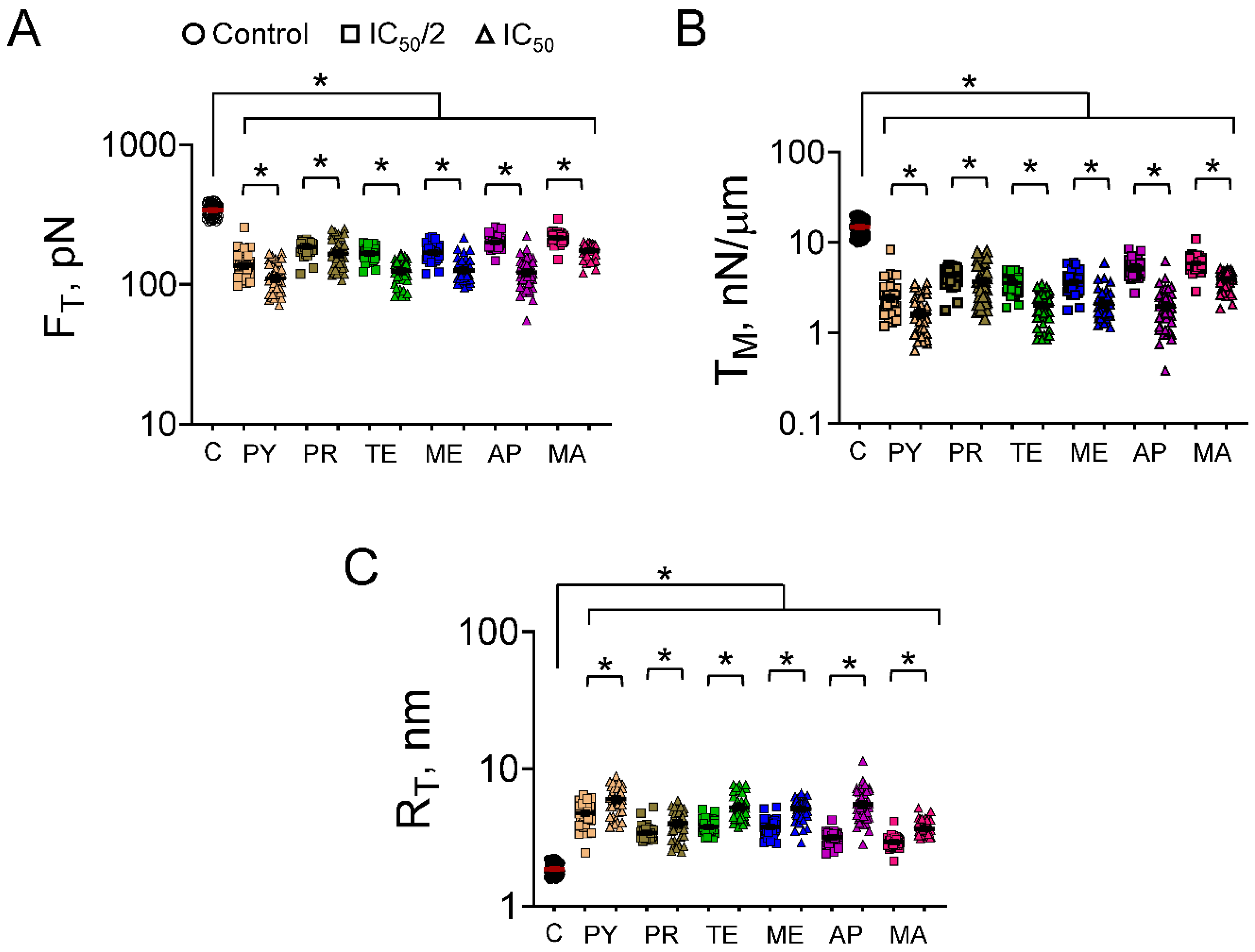
3.2. Cell Mechanics
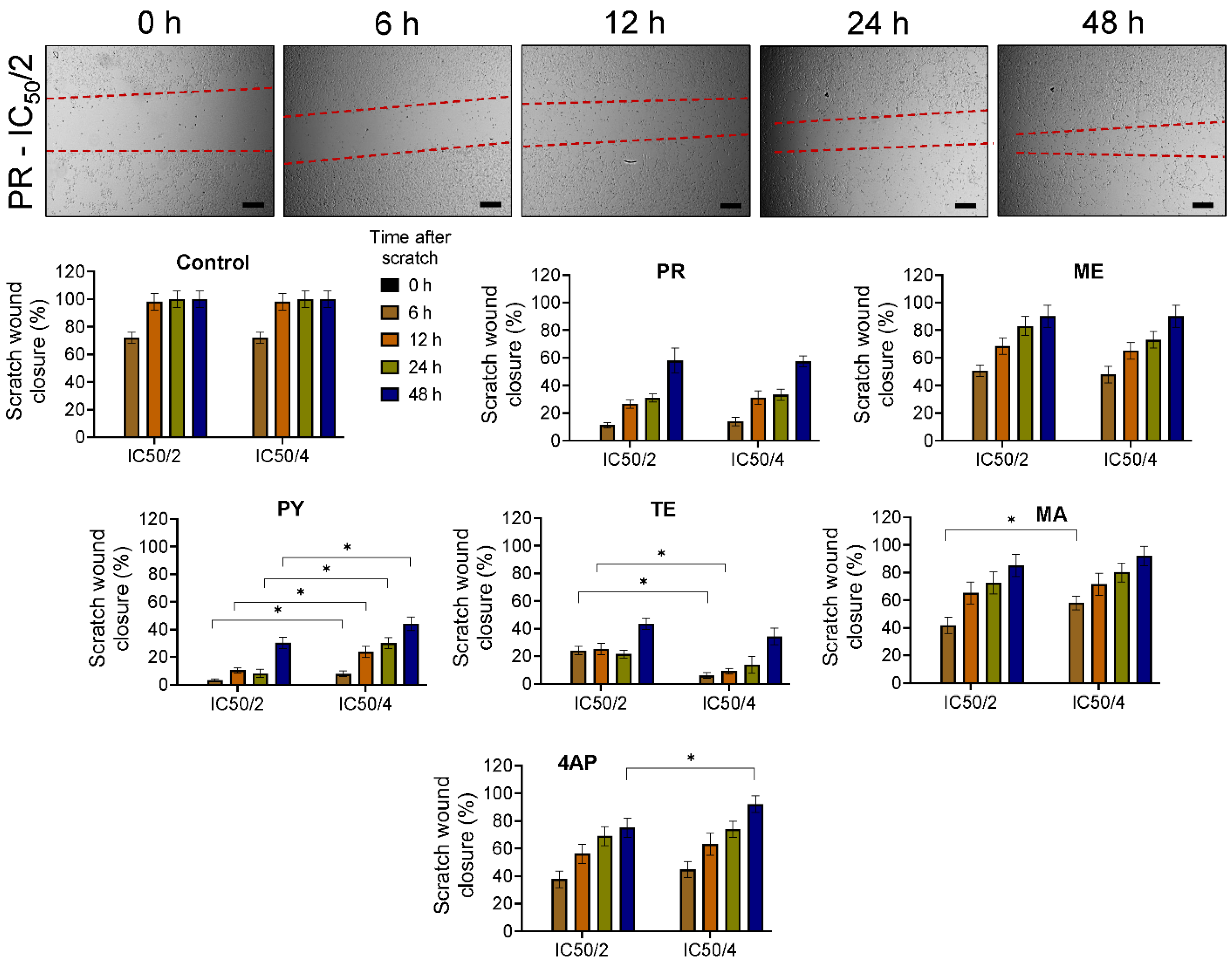
3.3. Cell Migration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, W. Global pesticide use: Profile, trend, cost/benefit and more. Proc. Int. Acad. Ecol. Environ. Sci. 2018, 8, 1–27. [Google Scholar]
- Food and Agriculture Organization. FAOSTAT: Database Collection of the Food and Agriculture Organization of the United Nations; FAO: Rome, Italy, 2019. [Google Scholar]
- Sharma, A.; Kumar, V.; Shahzad, B.; Tanveer, M.; Sidhu, G.P.; Handa, N.; Kohli, S.K.; Yadav, P.; Bali, A.S.; Parihar, R.D.; et al. Worldwide pesticide usage and its impacts on ecosystem. SN Appl. Sci. 2019, 1, 1446. [Google Scholar] [CrossRef]
- Boedeker, W.; Watts, M.; Clausing, P.; Marquez, E. The global distribution of acute unintentional pesticide poisoning: Estimations based on a systematic review. BMC Public Health 2020, 20, 1875. [Google Scholar] [CrossRef]
- Tang, F.H.; Lenzen, M.; McBratney, A.; Maggi, F. Risk of pesticide pollution at the global scale. Nat. Geosci. 2021, 14, 206–210. [Google Scholar] [CrossRef]
- Mnif, W.; Hassine, A.I.H.; Bouaziz, A.; Bartegi, A.; Thomas, O.; Roig, B. Effect of Endocrine Disruptor Pesticides: A Review. Int J. Environ. Res. Public Health 2011, 8, 2265–2303. [Google Scholar] [CrossRef]
- Gilden, R.C.; Huffling, K.; Sattler, B. Pesticides and Health Risks. J. Obstet. Gynecol. Neonatal Nurs. 2010, 39, 103–110. [Google Scholar] [CrossRef]
- Mehrpour, O.; Karrari, P.; Zamani, N.; Tsatsakis, A.M.; Abdollahi, M. Occupational exposure to pesticides and consequences on male semen and fertility: A review. Toxicol. Lett. 2014, 230, 146–156. [Google Scholar] [CrossRef]
- Kallioraab, C.; Mamoulakis, C.; Vasilopoulos, E.; Stamatiades, G.A.; Kalafati, L.; Barouni, R.; Karakousi, T.; Abdollahi, M.; Tsatsakis, A. Association of pesticide exposure with human congenital abnormalities. Toxicol. Appl. Pharmacol. 2018, 346, 58–75. [Google Scholar] [CrossRef] [PubMed]
- Alavanja, M.C.R. Pesticides Use and Exposure Extensive Worldwide. Rev. Environ. Health 2009, 24, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-H.; Kabir, E.; Jahan, S.A. Exposure to Pesticides and The Associated Human Health Effects. Sci. Total Environ. 2017, 575, 525–535. [Google Scholar] [CrossRef]
- Tudi, M.; Li, H.; Li, H.; Wang, L.; Lyu, J.; Yang, L.; Tong, S.; Yu, Q.J.; Ruan, H.D.; Atabila, A.; et al. Exposure Routes and Health Risks Associated with Pesticide Application. Toxics 2022, 10, 335. [Google Scholar] [CrossRef]
- Aktar, M.W.; Sengupta, D.; Chowdhury, A. Impact of Pesticides Use in Agriculture: Their Benefits and Hazards. Interdiscip. Toxicol. 2009, 2, 1–12. [Google Scholar] [CrossRef]
- Carvalho, F.P. Pesticides, Environment, and Food Safety. Food Energy Secur. 2017, 6, 48–60. [Google Scholar] [CrossRef]
- Monteiro, J.P.; Jurado, A.S. Methoprene. In Encyclopedia of Toxicology, 3rd ed.; Academic Press/Elsevier: London, UK, 2014; pp. 246–249. [Google Scholar]
- Ramaseshadri, P.; Farkaš, R.; Palli, S.R. Recent Progress in Juvenile Hormone Analogs (JHA) Research. Adv. Insect Physiol. 2012, 43, 353–436. [Google Scholar]
- Devillers, J. Fate of Pyriproxyfen in Soils and Plants. Toxics 2020, 8, 20. [Google Scholar] [CrossRef]
- Kumar, S.S.; Ghosh, P.; Malyan, S.K.; Sharma, J. A comprehensive review on enzymatic degradation of the organophosphate pesticide malathion in the environment. J. Environ. Sci. Health 2019, 37, 288–329. [Google Scholar] [CrossRef] [PubMed]
- Casida, J.E.; Quistad, G.B. Organophosphate toxicology: Safety aspects of nonacetylcholinesterase secondary targets. Chem. Res. Toxicol. 2004, 17, 983–998. [Google Scholar] [CrossRef] [PubMed]
- Katsuda, Y. Progress and Future of Pyrethroids. In Pyrethroids. Topics in Current Chemistry; Matsuo, N., Mori, T., Eds.; Springer: Berlin/Heidelberg, Germay, 2011; Volume 314. [Google Scholar]
- Georgiadis, N.; Tsarouhas, K.; Tsitsimpikou, C.; Vardavas, A.; Rezaee, R.; Germanakis, I.; Tsatsakis, A.; Stagos, D.; Kouretas, D. Pesticides and cardiotoxicity. Where do we stand? Toxicol. Appl. Pharmacol. 2018, 353, 1–14. [Google Scholar] [CrossRef]
- Schafer, E.W.; Brunton, R.B.; Cunningham, D.J. A summary of the acute toxicity of 4-aminopyridine to birds and mammals. Toxicol. Appl. Pharmacol. 1978, 26, 532–538. [Google Scholar] [CrossRef] [PubMed]
- London, L.; Beseler, C.; Bouchard, M.F.; Bellinger, D.C.; Colosio, C.; Grandjean, P.; Harari, R.; Kootbodien, T.; Kromhout, H.; Little, F.; et al. Neurobehavioral and neurodevelopmental effects of pesticide exposures. Neurotoxicology 2012, 33, 887–896. [Google Scholar] [CrossRef]
- Burns, C.J.; McIntosh, L.J.; Mink, P.J.; Jurek, A.M.; Li, A.A. Pesticide exposure and neurodevelopmental outcomes: Review of the epidemiologic and animal studies. J. Toxicol. Environ. Health B Crit. Rev. 2013, 16, 127–283. [Google Scholar] [CrossRef]
- Sapbamrer, R.; Hongsibsong, S. Effects of prenatal and postnatal exposure to organophosphate pesticides on child neurodevelopment in different age groups: A systematic review. Environ. Sci. Pollut. Res. Int. 2019, 26, 18267–18290. [Google Scholar] [CrossRef]
- Muñoz-Quezada, M.T.; Lucero, B.A.; Barr, D.B.; Steenland, K.; Levy, K.; Ryan, P.B.; Iglesias, V.; Alvarado, S.; Concha, C.; Rojas, E.; et al. Neurodevelopmental effects in children associated with exposure to organophosphate pesticides: A systematic review. Neurotoxicology 2013, 39, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, G.K.; Singh, S.; Kumar, V.; Dhanjal, D.S.; Datta, S.; Singh, J. Toxicity, monitoring and biodegradation of organophosphate pesticides: A review. Crit. Rev. Environ. Sci. Technol. 2019, 49, 1135–1187. [Google Scholar] [CrossRef]
- Franze, K. The mechanical control of nervous system development. Development 2013, 140, 3069–3077. [Google Scholar] [CrossRef] [PubMed]
- Barnes, J.M.; Przybyla, L.; Weaver, V.M. Tissue mechanics regulate brain development, homeostasis and disease. J. Cell Sci. 2017, 130, 71–82. [Google Scholar] [CrossRef]
- Donato, R.; Miljan, E.A.; Hines, S.J.; Aouabdi, S.; Pollock, K.; Patel, S.; Edwards, F.A.; Sinden, J.D. Differential development of neuronal physiological responsiveness in two human neural stem cell lines. BMC Neurosci. 2007, 8, 36. [Google Scholar] [CrossRef]
- Mahajan, G.; Lee, M.Y.; Kothapalli, C.R. Biophysical and biomechanical properties of neural progenitor cells as indicators of developmental neurotoxicity. Arch. Toxicol. 2019, 93, 2979–2992. [Google Scholar] [CrossRef]
- Diz-Munoz, A.; Fletcher, D.A.; Weiner, O.D. Use the force: Membrane tension as an organizer of cell shape and motility. Trends Cell Biol. 2013, 23, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Diz-Munoz, A.; Krieg, M.; Bergert, M.; Ibarlucea-Benitez, I.; Muller, D.J.; Paluch, E.; Heisenberg, C.P. Control of directed cell migration in vivo by membrane-to-cortex attachment. PLoS Biol. 2010, 8, e1000544. [Google Scholar] [CrossRef] [PubMed]
- Hochmuth, R.M.; Shao, J.Y.; Dai, J.; Sheetz, M.P. Deformation and Flow of Membrane into Tethers Extracted from Neuronal Growth Cones. Biophys. J. 1996, 70, 358–369. [Google Scholar] [CrossRef]
- Martinotti, S.; Ranzato, E. Scratch Wound Healing Assay. Methods Mol. Biol. 2020, 2109, 225–229. [Google Scholar]
- Suarez-Arnedo, A.; Figueroa, F.T.; Clavijo, C.; Arbeláez, P.; Cruz, J.C.; Muñoz-Camargo, C. An image J plugin for the high throughput image analysis of in vitro scratch wound healing assays. PLoS ONE 2020, 15, e0232565. [Google Scholar] [CrossRef] [PubMed]
- Nierode, G.J.; Perea, B.C.; McFarland, S.K.; Pascoal, J.F.; Clark, D.S.; Schaffer, D.V.; Dordick, J.S. High-Throughput Toxicity and Phenotypic Screening of 3D Human Neural Progenitor Cell Cultures on a Microarray Chip Platform. Stem Cell Rep. 2016, 7, 970–982. [Google Scholar] [CrossRef] [PubMed]
- Chiba, S.; Ikawa, T.; Takeshita, H.; Kanno, S.; Nagai, T.; Takada, M.; Mukai, T.; Wempe, M.F. Human organic cation transporter 2 (hOCT2): Inhibitor studies using S2-hOCT2 cells. Toxicology 2013, 310, 98–103. [Google Scholar] [CrossRef]
- Masoud, L.; Vijayasarathy, C.; Fernandez-Cabezudo, M.; Petroianu, G.; Saleh, A.M. Effect of malathion on apoptosis of murine L929 fibroblasts: A possible mechanism for toxicity in low dose exposure. Toxicology 2003, 185, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Karami-Mohajeri, S.; Najafi, A.; Behnam, B.; Sadeghi-Meymandi, M.; Kashitarash-Ifahani, Z.; Jafari, E.; Heidari, M.; Mohamadi, N.; Sharififar, F. Protective effect of Zataria multiflora Boiss. and its main compound, rosmarinic acid, against malathion induced oxidative stress and apoptosis in HepG2 cells. J. Environ. Sci. Health B 2021, 56, 297–306. [Google Scholar] [CrossRef]
- Samimi, A.; Last, J.A. Inhibition of lysyl hydroxylase by malathion and malaoxon. Toxicol. Appl. Pharmacol. 2001, 172, 203–209. [Google Scholar] [CrossRef]
- Elmorsy, E.; Al-Ghafari, A.; Al Doghaither, H.; Salama, M.; Carter, W.G. An Investigation of the Neurotoxic Effects of Malathion, Chlorpyrifos, and Paraquat to Different Brain Regions. Brain Sci. 2022, 12, 975. [Google Scholar] [CrossRef]
- Shieh, P.; Jan, C.R.; Liang, W.Z. The protective effects of the antioxidant N-acetylcysteine (NAC) against oxidative stress-associated apoptosis evoked by the organophosphorus insecticide malathion in normal human astrocytes. Toxicology 2019, 417, 1–14. [Google Scholar] [CrossRef]
- Liu, L.; Koo, Y.; Russell, T.; Gay, E.; Li, Y.; Yun, Y. Three-dimensional brain-on-chip model using human iPSC-derived GABAergic neurons and astrocytes: Butyrylcholinesterase post-treatment for acute malathion exposure. PLoS ONE 2020, 15, e0230335. [Google Scholar] [CrossRef]
- Lumsden, E.W.; McCowan, L.; Pescrille, J.D.; Fawcett, W.P.; Chen, H.; Albuquerque, E.X.; Mamczarz, J.; Pereira, E.F.R. Learning and memory retention deficits in prepubertal guinea pigs prenatally exposed to low levels of the organophosphorus insecticide malathion. Neurotoxicol. Teratol. 2020, 81, 106914. [Google Scholar] [CrossRef] [PubMed]
- Pham, B.; Miranda, A.; Allinson, G.; Nugegoda, D. Assessing interactive mixture toxicity of carbamate and organophosphorus insecticides in the yabby (Cherax destructor). Ecotoxicology 2018, 27, 1217–1224. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Balotra, S.; Pandey, G.; Kumar, A. Binary combinations of organophosphorus and synthetic pyrethroids are more potent acetylcholinesterase inhibitors than organophosphorus and carbamate mixtures: An in vitro assessment. Toxicol. Lett. 2017, 268, 8–16. [Google Scholar] [CrossRef]
- Audouze, K.; Taboureau, O.; Grandjean, P. A systems biology approach to predictive developmental neurotoxicity of a larvicide used in the prevention of Zika virus transmission. Toxicol. Appl. Pharmacol. 2018, 354, 56–63. [Google Scholar] [CrossRef]
- Díaz-Coránguez, M.; Segovia, J.; López-Ornelas, A.; Puerta-Guardo, H.; Ludert, J.; Chávez, B.; Meraz-Cruz, N.; González-Mariscal, L. Transmigration of Neural Stem Cells across the Blood Brain Barrier Induced by Glioma Cells. PLoS ONE 2013, 8, e60655. [Google Scholar] [CrossRef] [PubMed]
- Farrell, K.; Mahajan, G.; Srinivasan, P.; Lee, M.Y.; Kothapalli, C.R. Pediatric glioblastoma cells inhibit neurogenesis and promote astrogenesis, phenotypic transformation and migration of human neural progenitor cells within cocultures. Exp. Cell Res. 2018, 362, 159–171. [Google Scholar] [CrossRef]
- Li, X.; Liu, X.; Zhao, W.; Wen, X.; Zhang, N. Manipulating neural-stem-cell mobilization and migration in vitro. Acta Biomater. 2012, 8, 2087–2095. [Google Scholar] [CrossRef]
- Schmitz, A.; Dempewolf, S.; Tan, S.; Bicker, G.; Stern, M. Developmental Neurotoxicity of Fipronil and Rotenone on a Human Neuronal In Vitro Test System. Neurotox. Res. 2021, 39, 1189–1202. [Google Scholar] [CrossRef]
- Ishido, M.; Suzuki, J. Inhibition by rotenone of mesencephalic neural stem-cell migration in a neurosphere assay in vitro. Toxicol. In Vitro 2010, 24, 552–557. [Google Scholar] [CrossRef]
- Lee, H.S.; Song, H.J.; Park, Y.; Smolensky, D.; Lee, S.H. Permethrin inhibits tube formation and viability of endothelial cells. J Sci. Food Agric. 2022, 102, 4079–4085. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarsfield, M.C.; Vasu, J.; Abuoun, S.M.; Allena, N.; Kothapalli, C.R. Phenotypic and Biomechanical Characteristics of Human Fetal Neural Progenitor Cells Exposed to Pesticide Compounds. Biophysica 2023, 3, 348-361. https://doi.org/10.3390/biophysica3020023
Sarsfield MC, Vasu J, Abuoun SM, Allena N, Kothapalli CR. Phenotypic and Biomechanical Characteristics of Human Fetal Neural Progenitor Cells Exposed to Pesticide Compounds. Biophysica. 2023; 3(2):348-361. https://doi.org/10.3390/biophysica3020023
Chicago/Turabian StyleSarsfield, Marissa C., Jennifer Vasu, Sabreen M. Abuoun, Nischal Allena, and Chandrasekhar R. Kothapalli. 2023. "Phenotypic and Biomechanical Characteristics of Human Fetal Neural Progenitor Cells Exposed to Pesticide Compounds" Biophysica 3, no. 2: 348-361. https://doi.org/10.3390/biophysica3020023
APA StyleSarsfield, M. C., Vasu, J., Abuoun, S. M., Allena, N., & Kothapalli, C. R. (2023). Phenotypic and Biomechanical Characteristics of Human Fetal Neural Progenitor Cells Exposed to Pesticide Compounds. Biophysica, 3(2), 348-361. https://doi.org/10.3390/biophysica3020023






