Anthocyanins of Açaí Applied as a Colorimetric Indicator of Milk Spoilage: A Study Using Agar-Agar and Cellulose Acetate as Solid Support to Be Applied in Packaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Açaí (Euterpe oleraceae Mart.) Fruit Acquisition
2.3. The Acquisition of the Crude Phenolic Extract
2.4. Determination of Total Anthocyanins
2.5. Film Preparation
2.5.1. Cellulose Acetate Films
2.5.2. Agar-Agar Films
2.6. Scanning Electron Microscopy (SEM)
2.7. Infrared Spectroscopy (FT-IR)
2.8. Colorimetric Response of the Extract to Lactic Acid
2.9. Colorimetric Response of the Film to Lactic Acid
2.10. Colorimetric Response of Milk Samples to Lactic Acid
2.11. Acidity and pH Analysis
2.12. Statistical Analysis
3. Results and Discussion
3.1. Chromatic Changes for the Açaí Anthocyanins Extract Solution
3.2. Color Change on Cellulose Acetate (CA)
3.3. Color Change on Agar-Agar (AA)
3.4. Scanning Electron Microscopy (SEM)
3.5. Infrared Spectroscopy (FT-IR)
3.6. Thermogravimetric Analysis (TGA)
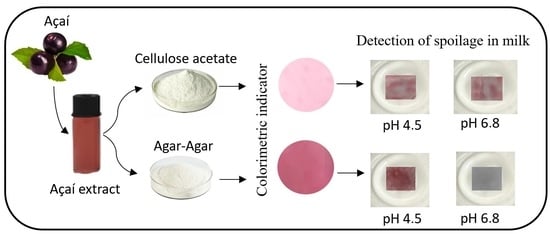
3.7. Detection of Spoilage in Milk
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- United Nations (UN). Food Waste Index Report 2021; United Nations Environment Programme: Nairobi, Kenya, 2021. [Google Scholar]
- Ghaani, M.; Cozzolino, C.A.; Castelli, G.; Farris, S. An Overview of the Intelligent Packaging Technologies in the Food Sector. Trends Food Sci. Technol. 2016, 51, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Ma, Q.; Wang, L. Preparation of a Visual PH-Sensing Film Based on Tara Gum Incorporating Cellulose and Extracts from Grape Skins. Sensors Actuators B Chem. 2016, 235, 401–407. [Google Scholar] [CrossRef]
- Teixeira, S.C.; de Oliveira, T.V.; Assis Silva, R.R.; Ribeiro, A.R.C.; Stringheta, P.C.; Rigolon, T.C.B.; Pinto, M.R.M.R.; Soares, N. de F.F. Colorimetric Indicators of Açaí Anthocyanin Extract in the Biodegradable Polymer Matrix to Indicate Fresh Shrimp. Food Biosci. 2022, 48, 101808. [Google Scholar] [CrossRef]
- Ferreira, E.; Stringheta, P.; Cano, M. Stability of Anthocyanin in Spinach Vine (Basella Rubra) Fruits. Cienc. Investig. Agrar. 2007, 34, 85–90. [Google Scholar] [CrossRef] [Green Version]
- Le, X.T.; Huynh, M.T.; Pham, T.N.; Than, V.T.; Toan, T.Q.; Bach, L.G.; Trung, N. Optimization of Total Anthocyanin Content, Stability and Antioxidant Evaluation of the Anthocyanin. Fruits Processes 2019, 7, 468. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Lu, S.; Chen, X. A Visual PH Sensing Film Using Natural Dyes from Bauhinia Blakeana Dunn. Sens. Actuators B Chem. 2014, 198, 268–273. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and Anthocyanins: Colored Pigments as Food, Pharmaceutical Ingredients, and the Potential Health Benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [Green Version]
- Curtis, P.J.; Kroon, P.A.; Hollands, W.J.; Walls, R.; Jenkins, G.; Kay, C.D.; Cassidy, A. Cardiovascular Disease Risk Biomarkers and Liver and Kidney Function Are Not Altered in Postmenopausal Women after Ingesting an Elderberry Extract Rich in Anthocyanins for 12 Weeks. J. Nutr. 2009, 139, 2266–2271. [Google Scholar] [CrossRef] [Green Version]
- Rossi, A.; Serraino, I.; Dugo, P.; Di Paola, R.; Mondello, L.; Genovese, T.; Morabito, D.; Dugo, G.; Sautebin, L.; Caputi, A.P.; et al. Protective Effects of Anthocyanins from Blackberry in a Rat Model of Acute Lung Inflammation. Free Radic. Res. 2003, 37, 891–900. [Google Scholar] [CrossRef] [Green Version]
- KANEKO, T.; TAHARA, S.; BABA, N. Inhibition of Linoleic Acid Hydroperoxide-Induced Toxicity in Cultured Human Fibroblasts by Anthocyanidins. Biosci. Biotechnol. Biochem. 2003, 67, 1391–1393. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, H.-J.; Choung, M.-G. Anthocyanin Compositions and Biological Activities from the Red Petals of Korean Edible Rose (Rosa Hybrida Cv. Noblered). Food Chem. 2011, 129, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Wu, X. Anthocyanins: Structural Characteristics That Result in Unique Metabolic Patterns and Biological Activities. Free Radic. Res. 2006, 40, 1014–1028. [Google Scholar] [CrossRef] [PubMed]
- Uranga, J.; Etxabide, A.; Guerrero, P.; de la Caba, K. Development of Active Fish Gelatin Films with Anthocyanins by Compression Molding. Food Hydrocoll. 2018, 84, 313–320. [Google Scholar] [CrossRef]
- Moazami Goodarzi, M.; Moradi, M.; Tajik, H.; Forough, M.; Ezati, P.; Kuswandi, B. Development of an Easy-to-Use Colorimetric PH Label with Starch and Carrot Anthocyanins for Milk Shelf Life Assessment. Int. J. Biol. Macromol. 2020, 153, 240–247. [Google Scholar] [CrossRef]
- Pourjavaher, S.; Almasi, H.; Meshkini, S.; Pirsa, S.; Parandi, E. Development of a Colorimetric PH Indicator Based on Bacterial Cellulose Nanofibers and Red Cabbage (Brassica Oleraceae) Extract. Carbohydr. Polym. 2017, 156, 193–201. [Google Scholar] [CrossRef]
- Freitas, P.A.V.; Silva, R.R.A.; de Oliveira, T.V.; Soares, R.R.A.; Junior, N.S.; Moraes, A.R.F.; Pires, A.C. dos S.; Soares, N.F.F. Development and Characterization of Intelligent Cellulose Acetate-Based Films Using Red Cabbage Extract for Visual Detection of Volatile Bases. LWT 2020, 132, 109780. [Google Scholar] [CrossRef]
- Rocha, F.S.; Gomes, A.J.; Lunardi, C.N.; Kaliaguine, S.; Patience, G.S. Experimental Methods in Chemical Engineering: Ultraviolet Visible Spectroscopy-UV-Vis. Can. J. Chem. Eng. 2018, 96, 2512–2517. [Google Scholar] [CrossRef]
- Fuleki, T.; Francis, F.J. Quantitative Methods for Anthocyanins. 2. Determination of Total Anthocyanin and Degradation Index for Cranberry Juice. J. Food Sci. 1968, 33, 78–83. [Google Scholar] [CrossRef]
- Weston, M.; Phan, M.A.T.; Arcot, J.; Chandrawati, R. Anthocyanin-Based Sensors Derived from Food Waste as an Active Use-by Date Indicator for Milk. Food Chem. 2020, 326, 127017. [Google Scholar] [CrossRef]
- Franceschinis, L.; Salvatori, D.M.; Sosa, N.; Schebor, C.; Franceschinis, L.; Salvatori, D.M.; Sosa, N.; Schebor, C.; Franceschinis, L.; Salvatori, D.M.; et al. Physical and Functional Properties of Blackberry Freeze- and Spray-Dried Powders Physical and Functional Properties of Blackberry Freeze- and Spray-Dried Powders. Dry. Technol. 2013, 32, 197–207. [Google Scholar] [CrossRef]
- Gallori, S.; Bilia, A.R.; Bergonzi, M.C.; Barbosa, W.L.R.; Vincieri, F.F. Polyphenolic Constituents of Fruit Pulp of Euterpe Oleracea Mart. (Açai Palm). Chromatographia 2004, 59, 739–743. [Google Scholar] [CrossRef]
- Pola, C.C.; Medeiros, E.A.A.; Pereira, O.L.; Souza, V.G.L.; Otoni, C.G.; Camilloto, G.P.; Soares, N.F.F. Cellulose Acetate Active Fi Lms Incorporated with Oregano ( Origanum Vulgare ) Essential Oil and Organophilic Montmorillonite Clay Control the Growth of Phytopathogenic Fungi. Food Packag. Shelf Life 2016, 9, 69–78. [Google Scholar] [CrossRef]
- Freitas, P.A.V.; de Oliveira, T.V.; Silva, R.R.A.; Fialho e Moraes, A.R.; Pires, A.C. dos S.; Soares, R.R.A.; Junior, N.S.; Soares, N.F.F. Effect of PH on the Intelligent Film-Forming Solutions Produced with Red Cabbage Extract and Hydroxypropylmethylcellulose. Food Packag. Shelf Life 2020, 26, 100604. [Google Scholar] [CrossRef]
- Gontijo de Melo, P.; Fornazier Borges, M.; Afonso Ferreira, J.; Vicente Barbosa Silva, M.; Ruggiero, R. Bio-Based Cellulose Acetate Films Reinforced with Lignin and Glycerol. Int. J. Mol. Sci. 2018, 19, 1143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vos, K.D.; Burris, F.O.; Riley, R.L. Kinetic Study of the Hydrolysis of Cellulose Acetate in the PH Range of 2–10. J. Appl. Polym. Sci. 1966, 10, 825–832. [Google Scholar] [CrossRef]
- Dangles, O.; Fenger, J.-A. The Chemical Reactivity of Anthocyanins and Its Consequences in Food Science and Nutrition. Molecules 2018, 23, 1970. [Google Scholar] [CrossRef] [Green Version]
- Lewis, C. Effect of Polysaccharides on the Colour of Anthocyanins. Food Chem. 1995, 54, 315–319. [Google Scholar] [CrossRef]
- Martínez-Sanz, M.; Gómez-Mascaraque, L.G.; Ballester, A.R.; Martínez-Abad, A.; Brodkorb, A.; López-Rubio, A. Production of Unpurified Agar-Based Extracts from Red Seaweed Gelidium Sesquipedale by Means of Simplified Extraction Protocols. Algal Res. 2019, 38, 101420. [Google Scholar] [CrossRef]
- Teixeira, S.C.; Silva, R.R.A.; de Oliveira, T.V.; Stringheta, P.C.; Pinto, M.R.M.R.; Soares, N. de F.F. Glycerol and Triethyl Citrate Plasticizer Effects on Molecular, Thermal, Mechanical, and Barrier Properties of Cellulose Acetate Films. Food Biosci. 2021, 42, 101202. [Google Scholar] [CrossRef]
- El-hefian, E.A.; Nasef, M.M.; Yahaya, A.H. Preparation and Characterization of Chitosan/Agar Blended Films: Part 1. Chemical Structure and Morphology. E-J. Chem. 2012, 9, 1431–1439. [Google Scholar] [CrossRef]
- Rodrigues Filho, G.; Monteiro, D.S.; Meireles, C. da S.; de Assunção, R.M.N.; Cerqueira, D.A.; Barud, H.S.; Ribeiro, S.J.L.; Messadeq, Y. Synthesis and Characterization of Cellulose Acetate Produced from Recycled Newspaper. Carbohydr. Polym. 2008, 73, 74–82. [Google Scholar] [CrossRef]
- SCHMIDT, V.C.R.; BLANCO-PASCUAL, N.; TRIBUZI, G.; LAURINDO, J.B. Effect of the Degree of Acetylation, Plasticizer Concentration and Relative Humidity on Cassava Starch Films Properties. Food Sci. Technol. 2019, 39, 491–499. [Google Scholar] [CrossRef] [Green Version]
- Barbosa, L.C.A. Infrared Spectroscopy: In the Characterization of Organic Compounds, 1st ed.; UFV Publisher: Viçosa, MG, Brazil, 2007; ISBN 9788572692809. [Google Scholar]
- Rodríguez, F.J.; Cortés, L.A.; Guarda, A.; Galotto, M.J.; Bruna, J.E. Characterization of Cetylpyridinium Bromide-Modified Montmorillonite Incorporated Cellulose Acetate Nanocomposite Films. J. Mater. Sci. 2015, 50, 3772–3780. [Google Scholar] [CrossRef]
- Silva-Pereira, M.C.; Teixeira, J.A.; Pereira-Júnior, V.A.; Stefani, R. Chitosan/Corn Starch Blend Films with Extract from Brassica Oleraceae (Red Cabbage) as a Visual Indicator of Fish Deterioration. LWT Food Sci. Technol. 2015, 61, 258–262. [Google Scholar] [CrossRef] [Green Version]
- Hong, J.; Chen, R.; Zeng, X.-A.; Han, Z. Effect of Pulsed Electric Fields Assisted Acetylation on Morphological, Structural and Functional Characteristics of Potato Starch. Food Chem. 2016, 192, 15–24. [Google Scholar] [CrossRef]
- Wu, Y.; Geng, F.; Chang, P.R.; Yu, J.; Ma, X. Effect of Agar on the Microstructure and Performance of Potato Starch Film. Carbohydr. Polym. 2009, 76, 299–304. [Google Scholar] [CrossRef]
- Kanmani, P.; Rhim, J.-W. Antimicrobial and Physical-Mechanical Properties of Agar-Based Films Incorporated with Grapefruit Seed Extract. Carbohydr. Polym. 2014, 102, 708–716. [Google Scholar] [CrossRef]
- Pereira, L.; Gheda, S.F.; Ribeiro-Claro, P.J.A. Analysis by Vibrational Spectroscopy of Seaweed Polysaccharides with Potential Use in Food, Pharmaceutical, and Cosmetic Industries. Int. J. Carbohydr. Chem. 2013, 2013, 1–7. [Google Scholar] [CrossRef]
- Basha, M.A.; Morsi, R.M.M. Spectroscopic, Electrical, and Relaxor-like Properties of Cellulose Acetate—Erbium ( III ) Chloride Composite Films. J. Appl. Polym. Sci. 2017, 45220, 1–10. [Google Scholar] [CrossRef]
- Prietto, L.; Mirapalhete, T.C.; Pinto, V.Z.; Hoffmann, J.F.; Vanier, N.L.; Lim, L.-T.; Guerra Dias, A.R.; da Rosa Zavareze, E. PH-Sensitive Films Containing Anthocyanins Extracted from Black Bean Seed Coat and Red Cabbage. LWT 2017, 80, 492–500. [Google Scholar] [CrossRef]
- Basumatary, K.; Daimary, P.; Das, S.K.; Thapa, M.; Singh, M.; Mukherjee, A.; Kumar, S. Lagerstroemia Speciosa Fruit-Mediated Synthesis of Silver Nanoparticles and Its Application as Filler in Agar Based Nanocomposite Films for Antimicrobial Food Packaging. Food Packag. Shelf Life 2018, 17, 99–106. [Google Scholar] [CrossRef]
- BRASIL. Manual de Métodos Oficiais Para Análise de Alimentos de Origem Animal, 2nd ed.; MAPA: Brasília, Brazil, 2019; ISBN 9788579911347. [Google Scholar]








| pH | L* | a* | b* |
|---|---|---|---|
| 2.0 | 46.16 ± 0.42 a | 49.91 ± 0.40 a | 31.28 ± 0.10 a |
| 3.0 | 52.80 ± 0.10 b | 39.81± 0.53 b | 32.59 ± 0.06 b |
| 4.0 | 61.38 ± 0.26 c | 19.10 ± 0.32 c | 26.70 ± 0.48 c |
| 5.0 | 64.66 ± 0.79 d | 14.38 ± 0.19 d | 7.49 ± 0.28 d |
| 6.0 | 49.35 ± 0.16 e | 4.07 ± 0.08 e | 19.73 ± 0.42 e |
| 7.0 | 26.94 ± 0.26 f | 2.93 ± 0.02 f | 2.97 ± 0.27 f |
| Sample | pH | L* | a* | b* | ΔE |
|---|---|---|---|---|---|
| AC | 6.8 | 74.81± 0.08 a | 16.37 ± 0.42 c | −1.77 ± 0.47 b | 0.00 ± 0.00 b |
| 6.0 | 77.89 ± 0.54 a | 17.07 ± 0.06 c | −2.05 ± 0.41 b | 8.47 ± 0.42 a | |
| 5.5 | 75.09 ± 0.81 a | 21.01 ± 0.15 a | −0.58 ± 0.20 a | 9.37 ± 1.63 a | |
| 5.0 | 76.73 ± 1.16 a | 19.1 ± 0.46 b | −0.56 ± 0.33 a | 10.14 ± 1.01 a | |
| 4.5 | 75.32 ± 4.13 a | 20.55 ± 0.36 a | −0.71 ± 0.05 a | 10.99 ± 1.27 a |
| Sample | pH | L* | a* | b* | ΔE |
|---|---|---|---|---|---|
| AA | 6.8 | 40.40 ± 2.25 a | 0.16 ± 0.27 d | −0.04 ± 0.04 c | 0.00 ± 0.00 d |
| 6.0 | 34.78 ± 0.76 b | 9.33 ± 0.32 c | 2.72 ± 0.43 b | 11.47 ± 1.20 c | |
| 5.5 | 34.91 ± 0.80 b | 9.17 ± 0.28 c | 2.74 ± 0.41 b | 11.54 ± 1.03 c | |
| 5.0 | 36.36 ± 1.03 b | 11.64 ± 0.37 b | 6.64 ± 0.25 a | 13.56 ± 1.00 b | |
| 4.5 | 38.88 ± 0.44 a | 9 ± 0.39 a | 7.28 ± 0.37 a | 16.31 ± 0.89 a |
| pH | Grams of Lactic Acid/100 mL |
|---|---|
| 4.5 | 0.69768 |
| 5 | 0.54162 |
| 5.5 | 0.3672 |
| 6 | 0.30753 |
| 6.8 | 0.17442 |
| Sample | pH | L* | a* | b* | ΔE |
|---|---|---|---|---|---|
| AC | 6.8 | 46.96 ± 1.17 a | 20.80 ± 1.06 a | 5.86 ± 0.26 a | 0.00 ± 0.00 b |
| 6.0 | 46.68 ± 0.84 a | 21.50 ± 0.46 a | 5.62 ± 0.14 a | 5.74 ± 0.60 a | |
| 5.5 | 46.43 ± 0.70 a | 20.92 ± 0.95 a | 5.36 ± 0.24 a | 6.63 ± 0.84 a | |
| 5.0 | 46.28 ± 0.77 a | 23.29 ± 0.61 a | 5.67 ± 0.24 a | 7.22 ± 0.84 a | |
| 4.5 | 44.75 ± 0.69 a | 23.4 ± 0.89 a | 5.61 ± 0.2 a | 6.83 ± 0.6 a | |
| AA | 6.8 | 42.37 ± 0.65 ab | 3.96 ± 0.26 c | 6.53 ± 0.33 b | 0.00 ± 0.00 d |
| 6.0 | 42.91 ± 1.22 a | 15.13 ± 0.61 b | 9.22 ± 0.10 a | 11.36 ± 0.38 c | |
| 5.5 | 39.94 ± 0.78 bc | 16.82 ± 0.24 ba | 9.29 ± 0.19 a | 13.47 ± 0.25 b | |
| 5.0 | 40.57 ± 0.41 abc | 15.50 ± 0.33 ba | 9.52 ± 0.22 a | 11.71 ± 0.37 c | |
| 4.5 | 38.35 ± 1.73 c | 17.67 ± 0.74 a | 9.43 ± 0.30 a | 20.55 ± 0.37 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teixeira, S.C.; Oliveira, T.V.d.; Batista, L.F.; Silva, R.R.A.; Lopes, M.d.P.; Ribeiro, A.R.C.; Rigolon, T.C.B.; Stringheta, P.C.; Soares, N.d.F.F. Anthocyanins of Açaí Applied as a Colorimetric Indicator of Milk Spoilage: A Study Using Agar-Agar and Cellulose Acetate as Solid Support to Be Applied in Packaging. Polysaccharides 2022, 3, 715-727. https://doi.org/10.3390/polysaccharides3040041
Teixeira SC, Oliveira TVd, Batista LF, Silva RRA, Lopes MdP, Ribeiro ARC, Rigolon TCB, Stringheta PC, Soares NdFF. Anthocyanins of Açaí Applied as a Colorimetric Indicator of Milk Spoilage: A Study Using Agar-Agar and Cellulose Acetate as Solid Support to Be Applied in Packaging. Polysaccharides. 2022; 3(4):715-727. https://doi.org/10.3390/polysaccharides3040041
Chicago/Turabian StyleTeixeira, Samiris Côcco, Taila Veloso de Oliveira, Lais Fernanda Batista, Rafael Resende Assis Silva, Matheus de Paula Lopes, Alane Rafaela Costa Ribeiro, Thaís Caroline Buttow Rigolon, Paulo César Stringheta, and Nilda de Fátima Ferreira Soares. 2022. "Anthocyanins of Açaí Applied as a Colorimetric Indicator of Milk Spoilage: A Study Using Agar-Agar and Cellulose Acetate as Solid Support to Be Applied in Packaging" Polysaccharides 3, no. 4: 715-727. https://doi.org/10.3390/polysaccharides3040041
APA StyleTeixeira, S. C., Oliveira, T. V. d., Batista, L. F., Silva, R. R. A., Lopes, M. d. P., Ribeiro, A. R. C., Rigolon, T. C. B., Stringheta, P. C., & Soares, N. d. F. F. (2022). Anthocyanins of Açaí Applied as a Colorimetric Indicator of Milk Spoilage: A Study Using Agar-Agar and Cellulose Acetate as Solid Support to Be Applied in Packaging. Polysaccharides, 3(4), 715-727. https://doi.org/10.3390/polysaccharides3040041