Synthetic Chemicals as Potential Tracers of Impacts of Fracturing Fluids on Groundwater
Abstract
:1. Introduction
1.1. Previous Ideas on Fracturing Fluid Indicators
1.2. Detections of Chemicals Derived from Fracturing Fluids in Groundwater
1.3. Focus of This Study
2. Methods
2.1. Selection of FF Chemicals
2.2. Collection of Field Samples
- Shallow (~1.5 m depth) “pristine” groundwater and sediment (aquifer) samples collected from a site near Duck Lake, Saskatchewan.
- Sediment samples (17–34 m depth) from a borehole near Saint-Édouard, Quebec, and groundwater from the same borehole.
- Shallow sediment (aquifer) samples (0.3–0.4 m depth) from a site near Alliston, Ontario, which were mixed in a 1:1 ratio by volume with silica sand and groundwater (10–15 m depth) sampled at Burlington, Ontario.
2.3. Preparation of Microcosms (Batch Tests)
2.4. Microcosm Sampling
2.5. Analytical Methods
2.6. Error Bars for Analyses
2.7. Calculations of Distribution (Sorption) Coefficients
3. Results and Discussion
3.1. Validation of Analytical Methods
3.2. Evidence for Sorption
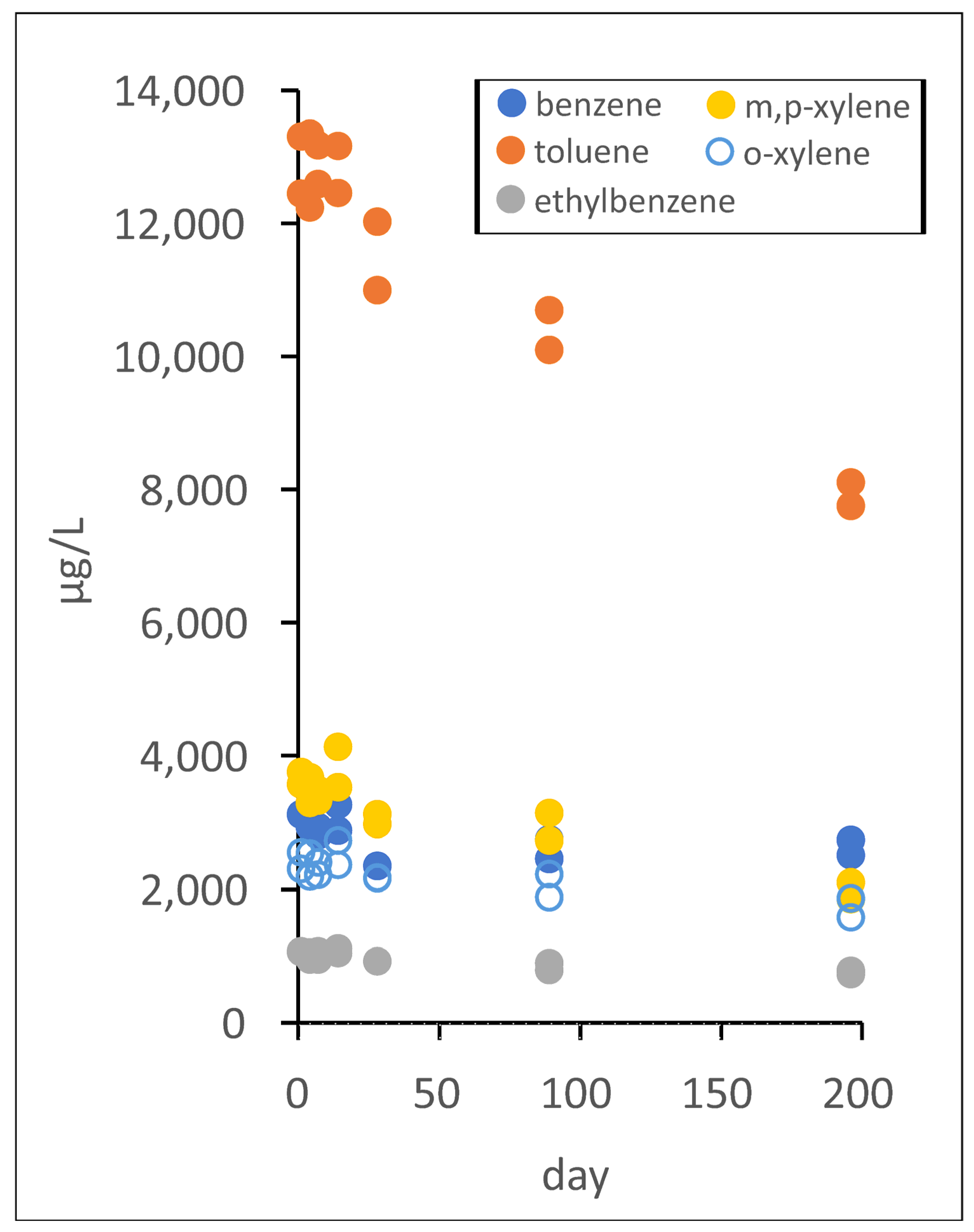
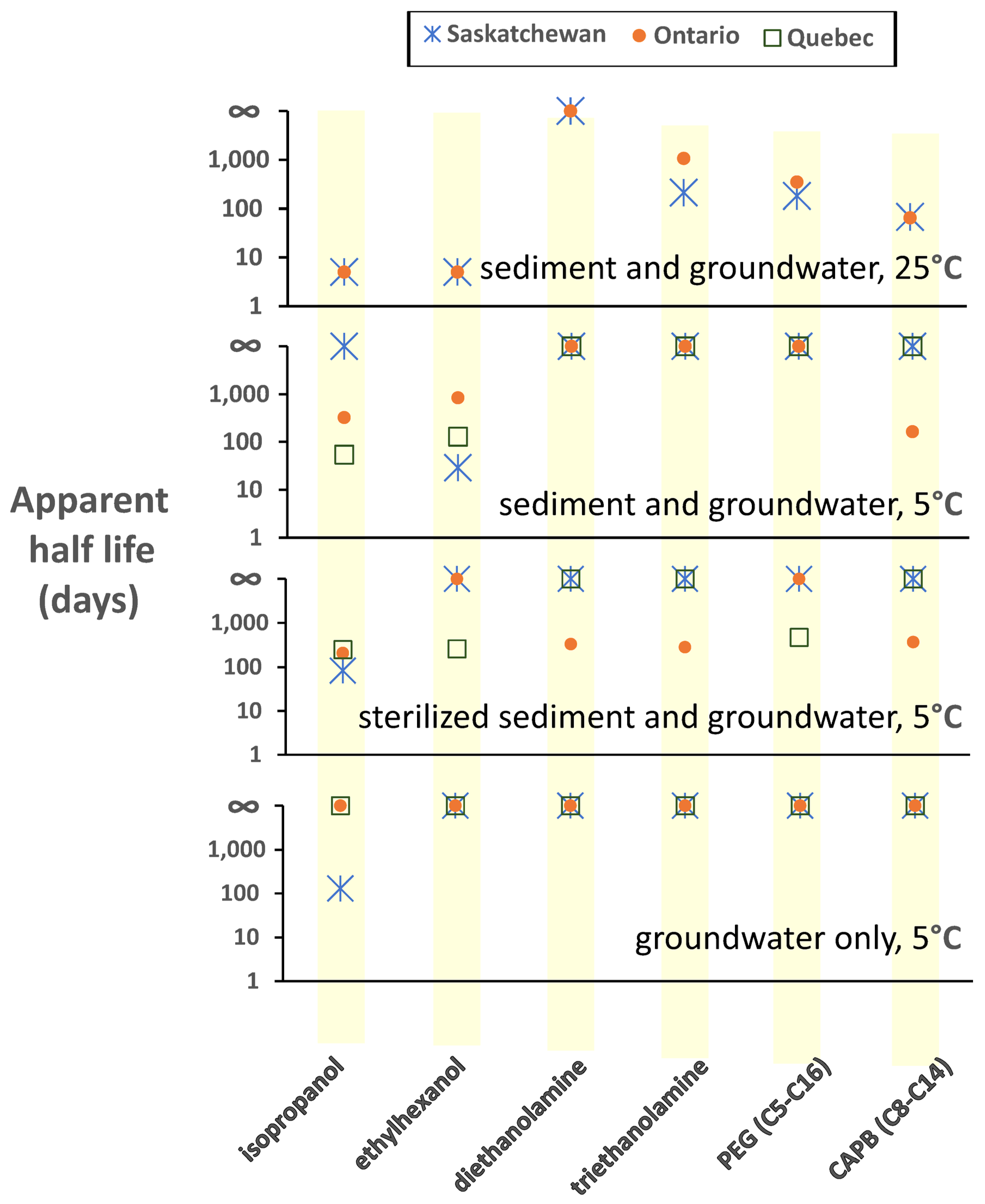
3.3. Evidence for Biodegradation of FF Chemicals
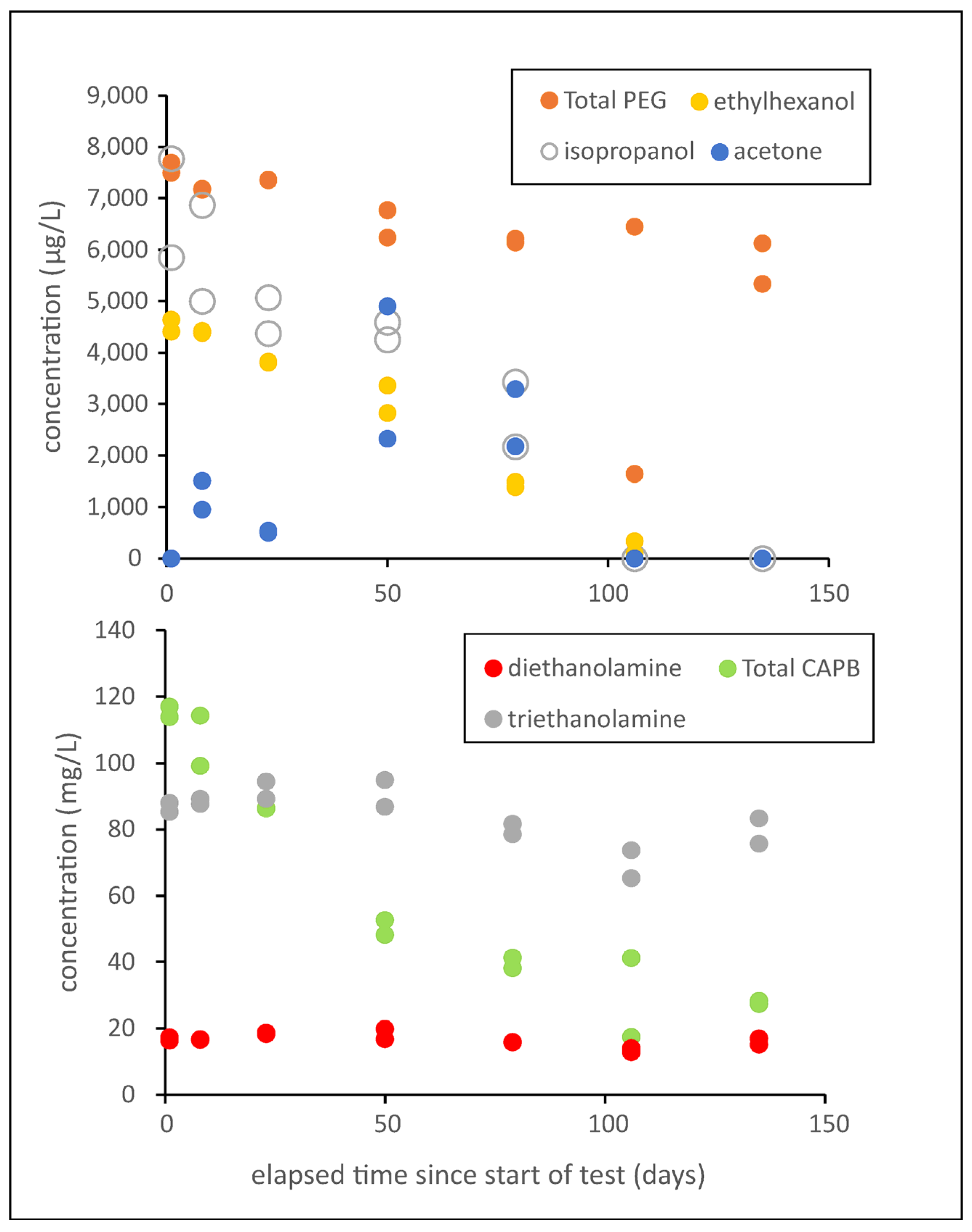
3.4. Biodegradation of Alcohols
3.5. Biodegradation of Ethanolamines
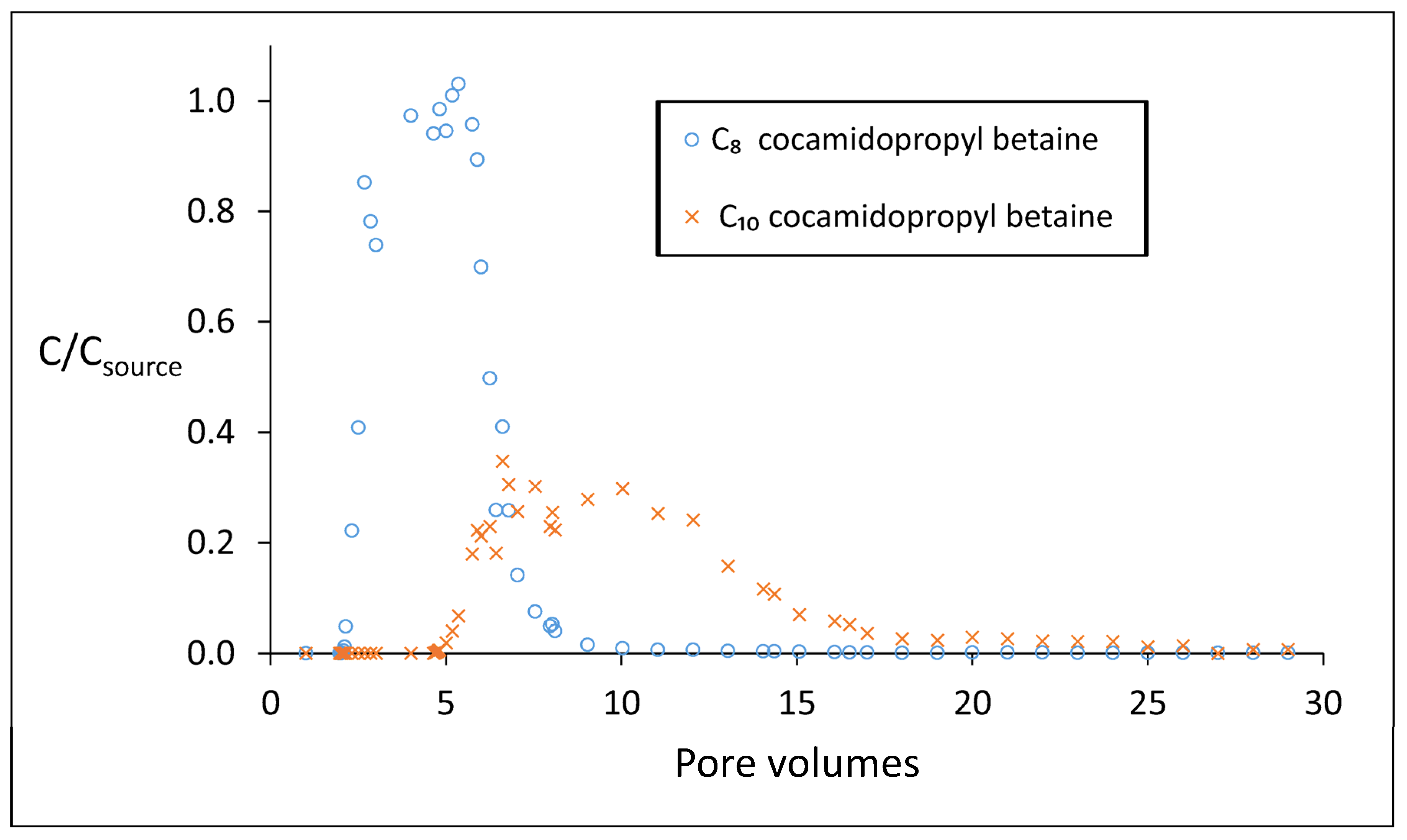
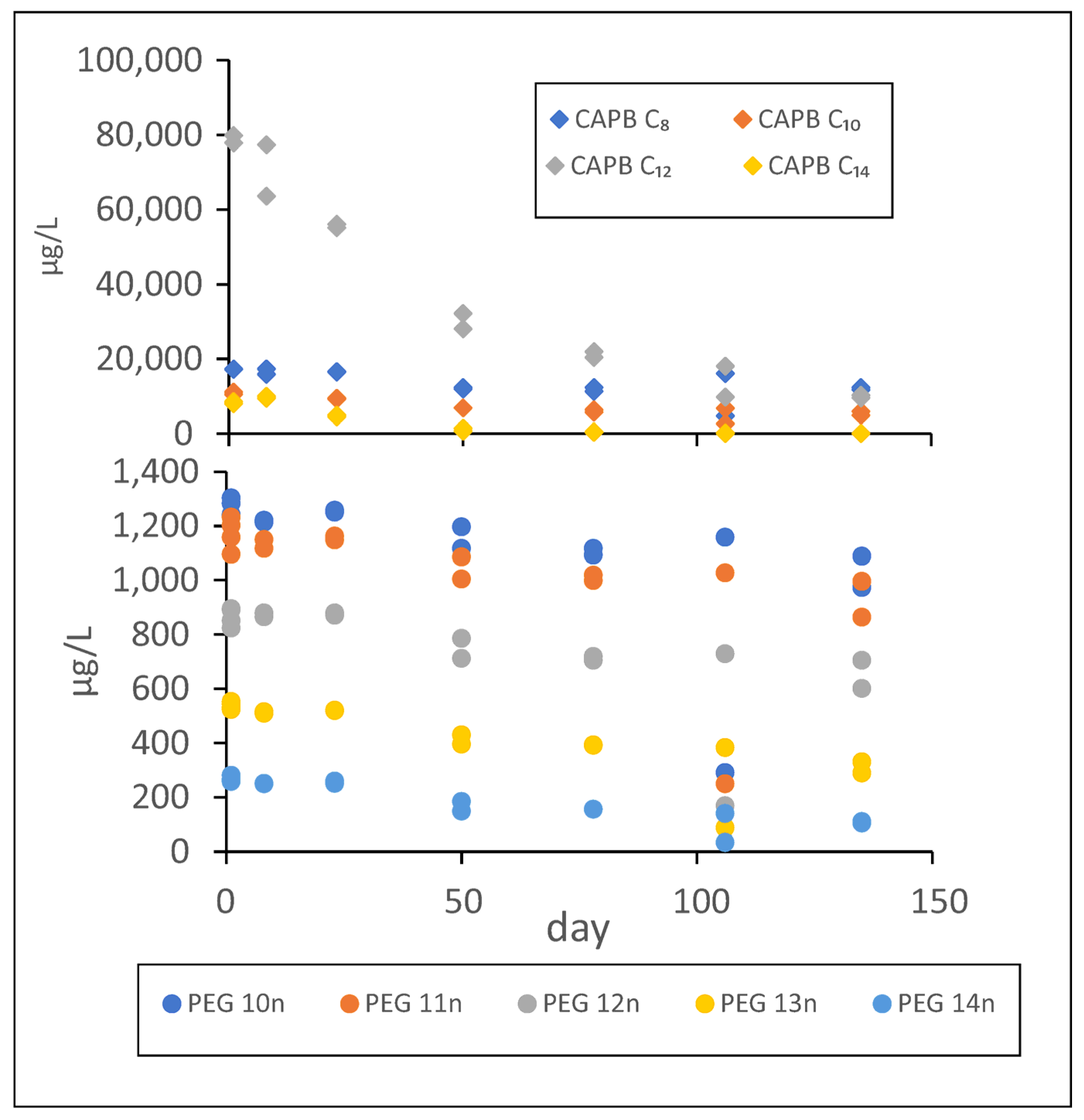
3.6. Biodegradation of Cocamidopropyl Betaine
3.7. Biodegradation of Polyethylene Glycols
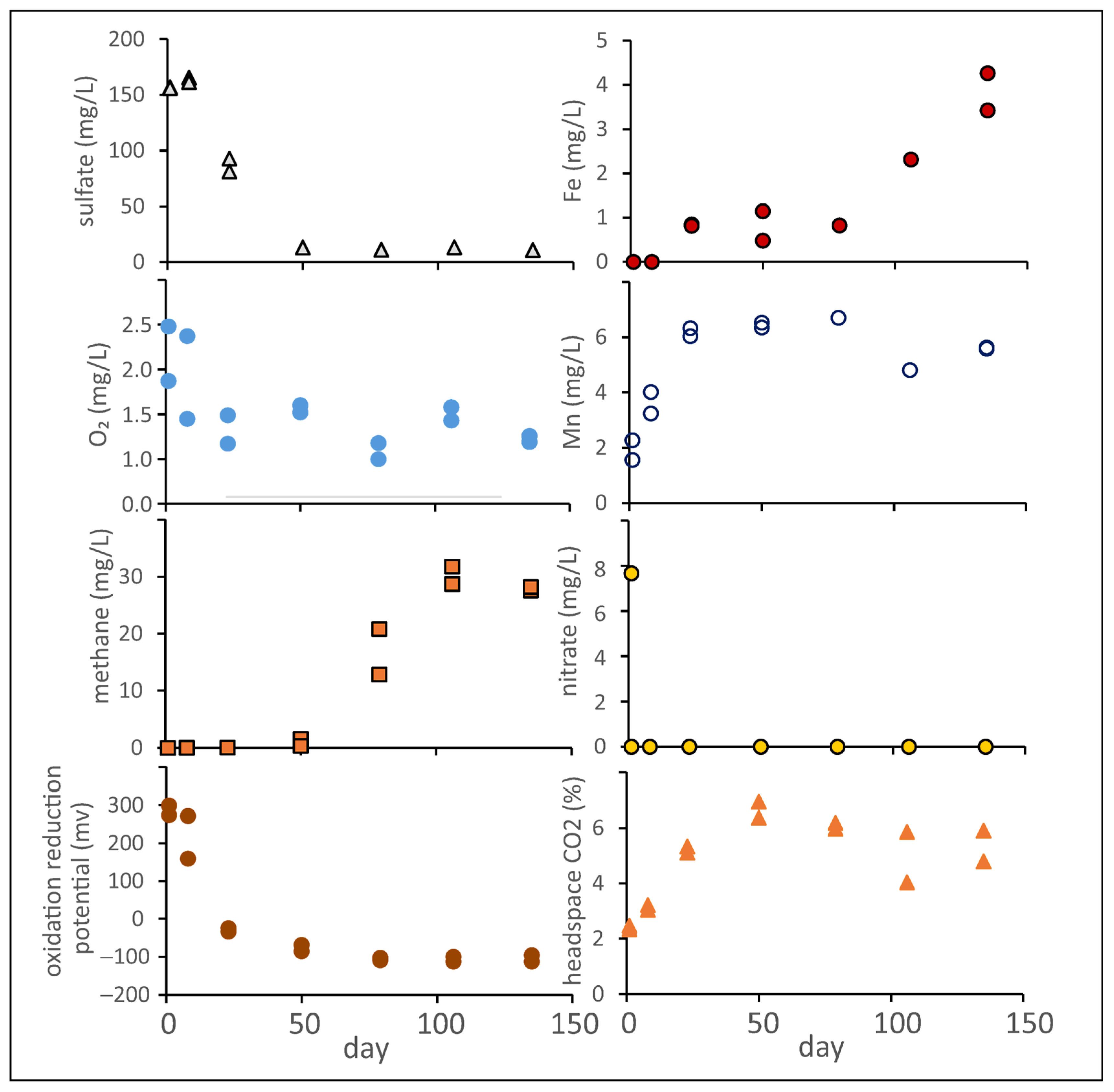
3.8. Trends in Dissolved Oxygen, Nitrate, Sulfate, Methane, Iron, and Manganese
4. Discussion
Suggested Future Studies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- King, G.E. Thirty Years of Gas Shale Fracturing: What Have We Learned? In Proceedings of the SPE Annual Technical Conference and Exhibition (SPE 133456), Florence, Italy, 19–22 September 2010. [Google Scholar]
- Stevens, S.; Kuuskraa, V. Special Report: Gas Shale-1: Seven Plays Dominate North America Activity. Oil Gas J. 2009, 107, 36–41. [Google Scholar]
- Canada Energy Regulator. Canada Energy Future 2023: Energy Supply and Demand Projections to 2050. Available online: https://www.cer-rec.gc.ca/en/data-analysis/canada-energy-future/2023/canada-energy-futures-2023.pdf (accessed on 15 July 2024).
- Shaheen, S.; Wen, T.; Herman, A.; Brantley, S.L. Geochemical evidence of potential groundwater contamination with human health risks where hydraulic fracturing overlaps with extensive legacy hydrocarbon extraction. Environ. Sci. Technol. 2022, 56, 10010–10019. [Google Scholar] [CrossRef] [PubMed]
- Gordalla, B.C.; Ewers, U.; Frimmel, F.H. Hydraulic fracturing: A toxicological threat for groundwater and drinking-water? Environ. Earth Sci. 2013, 70, 3875–3893. [Google Scholar] [CrossRef]
- Birdsell, D.T.; Rajaram, H.; Dempsey, D.; Viswanathan, H.S. Hydraulic fracturing fluid migration in the subsurface: A review and expanded modeling results. Water Resour. Res. 2015, 51, 7159–7188. [Google Scholar] [CrossRef]
- Jasechko, S.; Perrone, D. Hydraulic fracturing near domestic groundwater wells. Proc. Natl. Acad. Sci. USA 2017, 114, 13138–13143. [Google Scholar] [CrossRef] [PubMed]
- Taherdangkoo, R.; Tatomir, A.; Anighoro, T.; Sauter, M. Modeling fate and transport of hydraulic fracturing fluid in the presence of abandoned wells. J. Contam. Hydrol. 2019, 221, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Taherdangkoo, R.; Tatomir, A.; Taherdangkoo, M.; Qiu, P.; Sauter, M. Nonlinear autoregressive neural networks to predict hydraulic fracturing fluid leakage into shallow groundwater. Water 2020, 12, 841. [Google Scholar] [CrossRef]
- Mallants, D.; Bekele, E.; Schmid, W.; Miotlinski, K.; Taylor, A.; Gerke, K.; Gray, B. A generic method for predicting environmental concentrations of hydraulic fracturing chemicals in soil and shallow groundwater. Water 2020, 12, 941. [Google Scholar] [CrossRef]
- Jackson, R.E.; Gorody, A.W.; Mayer, B.; Roy, J.W.; Ryan, M.C.; Van Stempvoort, D.R. Groundwater protection and unconventional gas extraction: The critical need for field-based hydrogeological research. Groundwater 2013, 51, 488–510. [Google Scholar] [CrossRef]
- Vidic, R.D.; Brantley, S.L.; Vandenbossche, J.M.; Yoxtheimer, D.; Abad, J.D. Impact of shale gas development on regional water quality. Science 2013, 340, 1235009. [Google Scholar] [CrossRef]
- Lacoursière, J.-P.; Lacoursière, S. Étude De Risques Technologiques Associés À L’extraction Du Gaz De Schiste. Étude E3-4, Préparé pour: L’Évaluation Environnementale stratégique sur le gaz de schiste; Département de Génie Chimique, Université de Sherbrooke, JP Lacoursière Inc.: Repentigny, QC, Canada, 2013. [Google Scholar]
- Vengosh, A.; Jackson, R.B.; Warner, N.; Darrah, T.H.; Kondash, A. Critical review of the risks to water resources from unconventional shale gas development and hydraulic fracturing in the United States. Environ. Sci. Technol. 2014, 48, 8334–8348. [Google Scholar] [CrossRef] [PubMed]
- Adgate, J.L.; Goldstein, B.D.; McKenzie, L.M. Potential public health hazards, exposures and health effects from unconventional natural gas development. Environ. Sci. Technol. 2014, 48, 8307–8320. [Google Scholar] [CrossRef] [PubMed]
- U.S. Environmental Protection Agency. Assessment of the Potential Impacts of Hydraulic Fracturing for Oil and Gas on Drinking Water Resources; EPA/600/R-15/047a External Review Draft; Office of Research and Development: Washington, DC, USA, 2015. [Google Scholar]
- Elsner, M.; Hoelzer, K. Quantitative survey and structural classification of hydraulic fracturing chemicals reported in unconventional gas production. Environ. Sci. Technol. 2016, 50, 3290–3314. [Google Scholar] [CrossRef] [PubMed]
- Luek, J.L.; Michael Gonsior, M. Organic compounds in hydraulic fracturing fluids and wastewaters: A review. Water Res. 2017, 123, 536–548. [Google Scholar] [CrossRef] [PubMed]
- Blake, U. Designing a water quality program for shale exploration. In Proceedings of the Technical Workshops for the Hydraulic Fracturing Study: Chemical & Analytical Methods, Arlington, VA, USA, 24–25 February 2011. Publication No. EPA 600-R-11-066. [Google Scholar]
- McGrath, A.E. Fate and transport of select compounds of potential concern (COPC) in fracing fluids. In Proceedings of the Technical Workshops for the Hydraulic Fracturing Study: Fate & Transport, Arlington, VA, USA, 24–25 February 2011. Publication No. EPA 600-R-11-047. [Google Scholar]
- Aminto, A.; Stone Olson, M. Four-compartment partition model of hazardous components in hydraulic fracturing fluid additives. J. Nat. Gas Sci. Eng. 2012, 7, 16–21. [Google Scholar] [CrossRef]
- Bibby, K.J.; Brantley, S.L.; Reible, D.D.; Linden, K.G.; Mouser, P.J.; Gregory, K.B.; Ellis, B.R.; Vidic, R.D. Suggested reporting parameters for investigations of wastewater from unconventional shale gas extraction. Environ. Sci. Technol. 2013, 47, 13220–13221. [Google Scholar] [CrossRef] [PubMed]
- Rosenblum, J.; Thurman, E.M.; Ferrer, I.; Aiken, G.; Linden, K.G. Organic Chemical Characterization and Mass Balance of a Hydraulically Fractured Well: From Fracturing Fluid to Produced Water over 405 Days. Environ. Sci. Technol. 2017, 51, 14006–14015. [Google Scholar] [CrossRef] [PubMed]
- Thurman, E.M.; Ferrer, I.; Blotevogel, J.; Borch, T. Analysis of hydraulic fracturing flowback and produced waters using accurate mass: Identification of ethoxylated surfactants. Anal. Chem. 2014, 86, 9653–9661. [Google Scholar] [CrossRef] [PubMed]
- Thurman, E.M.; Ferrer, I.; Rosenblum, J.; Linden, K.; Ryan, J.N. Identification of polypropylene glycols and polyethylene glycol carboxylates in flowback and produced water from hydraulic fracturing. J. Hazard. Mater. 2017, 323, 11–17. [Google Scholar] [CrossRef]
- Llewellyn, G.T.; Dorman, F.; Westland, J.L.; Yoxtheimer, D.; Grieve, P.; Sowers, T.; Humston-Fulmer, E.; Brantley, S.L. Evaluating a groundwater supply contamination incident attributed to Marcellus Shale gas development. Proc. Natl. Acad. Sci. USA 2015, 112, 6325–6330. [Google Scholar] [CrossRef]
- McAdams, B.C.; Carter, K.E.; Blotevogel, J.; Borch, T.; Hakala, J.A. In situ transformation of hydraulic fracturing surfactants from well injection to produced water. Environ. Sci. Process. Impacts 2019, 21, 1777–1786. [Google Scholar] [CrossRef] [PubMed]
- Sitterley, K.A.; Silverstein, J.; Rosenblum, J.; Linden, K.G. Aerobic biological degradation of organic matter and fracturing fluid additives in high salinity hydraulic fracturing wastewaters. Sci. Total Environ. 2021, 758, 143622. [Google Scholar] [CrossRef] [PubMed]
- Asadi, M.; Woodroof, R.A.; Himes, R.E. Comparative Study of Flowback Analysis Using Polymer Concentrations and Fracturing-Fluid Tracer Methods: A Field Study. SPE Prod. Oper. 2008, 23, 147–157. [Google Scholar] [CrossRef]
- Li, G. Oilfield Tracer Analysis and Application for Hydraulic Fracture Diagnostics. Master’s Thesis, Texas A&M University, College Station, TX, USA, May 2021. [Google Scholar]
- Gross, S.A.; Avens, H.J.; Banducci, A.M.; Sahmel, J.; Panko, J.M.; Tvermoes, B.E. Analysis of BTEX groundwater concentrations from surface spills associated with hydraulic fracturing operations. J. Air Waste Manag. Assoc. 2013, 63, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Fontenot, B.E.; Hunt, L.R.; Hildenbrand, Z.L.; Carlton, D.D.; Oka, H.; Walton, J.L.; Hopkins, D.; Osorio, A.; Bjorndal, B.; Hu, Q.H.; et al. An evaluation of water quality in private drinking water wells near natural gas extraction sites in the Barnett shale formation. Environ. Sci. Technol. 2013, 47, 10032–10040. [Google Scholar] [CrossRef] [PubMed]
- Hildenbrand, Z.L.; Carlton, D.D.; Fontenot, B.E.; Meik, J.M.; Walton, J.L.; Thacker, J.B.; Korlie, S.; Shelor, C.P.; Kadjo, A.F.; Clark, A.; et al. Temporal variation in groundwater quality in the Permian Basin of Texas, a region of increasing unconventional oil and gas development. Sci. Total Environ. 2016, 562, 906–913. [Google Scholar] [CrossRef] [PubMed]
- McMahon, P.B.; Barlow, J.R.; Engle, M.A.; Belitz, K.; Ging, P.B.; Hunt, A.G.; Jurgens, B.C.; Kharaka, Y.K.; Tollett, R.W.; Kresse, T.M. Methane and benzene in drinking-water wells overlying the Eagle Ford, Fayetteville, and Haynesville shale hydrocarbon production areas. Environ. Sci. Technol. 2017, 51, 6727–6734. [Google Scholar] [CrossRef]
- Rodriguez, J.; Heo, J.; Kim, K.H. The impact of hydraulic fracturing on groundwater quality in the Permian Basin, West Texas, USA. Water 2020, 12, 796. [Google Scholar] [CrossRef]
- DiGiulio, D.C.; Jackson, R.B. Impact to underground sources of drinking water and domestic wells from production well stimulation and completion practices in the Pavillion, Wyoming, field. Environ. Sci. Technol. 2016, 50, 4524–4536. [Google Scholar] [CrossRef]
- Xiong, B.; Soriano, M.A., Jr.; Gutchess, K.M.; Hoffman, N.; Clark, C.J.; Siegel, H.G.; De Vera, G.A.D.; Li, Y.; Brenneis, R.J.; Cox, A.J.; et al. Groundwaters in Northeastern Pennsylvania near intense hydraulic fracturing activities exhibit few organic chemical impacts. Environ. Sci. Process. Impacts 2022, 24, 252–264. [Google Scholar] [CrossRef]
- BC Oil & Gas Commission, 2014–2016 (Years Accessed). FracFocus, a Website with an Online Database Listing the Chemicals Used in Fracturing Fluids in Canada, on a Well by Well Basis (Primarily British Columbia, Alberta, Saskatchewan). This Project Is Intended “to Provide Objective Information on Hydraulic Fracturing. Fracturing Fluids, Groundwater and Surface Water Protection and Related Oil and Gas Activities in Canada”. Available online: http://fracfocus.ca/ (accessed on 2 June 2016).
- Fiorenza, S.; Duston, K.L.; Ward, C.H. Decision making—Is bioremediation a viable option? J. Hazard. Mater. 1991, 28, 171–183. [Google Scholar] [CrossRef]
- Wang, L.J.; Schnute, W.C. Quantitative RSLC-MS/MS Analysis of Nitrogen Mustard Hydrolysis Products as Ethanolamines in Water Samples; Dionex Corporation: Sunnyvale, CA, USA, 2011. [Google Scholar]
- Doucette, W.J. Quantitative structure-activity relationships for predicting soil-sediment sorption coefficients for organic chemicals. Environ. Toxicol. Chem. 2003, 22, 1771–1788. [Google Scholar] [CrossRef]
- Grathwohl, P.; Farrell, J.; Reinhard, M. Desorption kinetics of volatile organic contaminants from aquifer materials. In Contaminated Soil ‘90, Third KfK/TNO Conference; Arendt, F., Ed.; Kluwer: Karlsruhe, Germany, 1990; Volume I, pp. 343–350. [Google Scholar]
- Eichhorn, P.; Knepper, T.P. Electrospray ionization mass spectrometric studies on the amphoteric surfactant cocamidopropylbetaine. J. Mass Spectrom. 2001, 36, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.W. Physico-chemical factors influencing ethyleneamine sorption to soil. Environ. Toxicol. Chem. 1993, 12, 27–35. [Google Scholar]
- Hawthorne, S.B.; Kubátová, A.; Gallagher, J.R.; Sorensen, J.A.; Miller, D.J. Persistence and biodegradation of monoethanolamine and 2-propanolamine at an abandoned industrial site. Environ. Sci. Technol. 2005, 39, 3639–3645. [Google Scholar] [CrossRef]
- Schrap, S.M.; Opperhuizen, A. On the contradictions between experimental sorption data and the sorption partitioning model. Chemosphere 1992, 24, 1259–1282. [Google Scholar] [CrossRef]
- Kekacs, D.; Drollette, B.D.; Brooker, M.; Plata, D.L.; Mouser, P.J. Aerobic biodegradation of organic compounds in hydraulic fracturing fluids. Biodegradation 2015, 26, 271–287. [Google Scholar] [CrossRef] [PubMed]
- Heyob, K.M.; Blotevogel, J.; Brooker, M.; Evans, M.V.; Lenhart, J.J.; Wright, J.; Lamendella, R.; Borch, T.; Mouser, P.J. Natural Attenuation of Nonionic Surfactants Used in Hydraulic Fracturing Fluids: Degradation Rates, Pathways, and Mechanisms. Environ. Sci. Technol. 2017, 51, 13985–13994. [Google Scholar] [CrossRef] [PubMed]
- Bustard, M.T.; McEvoy, E.M.; Goodwin, J.A.S.; Burgess, J.G.; Wright, P.C. Biodegradation of propanol and isopropanol by a mixed microbial consortium. Appl. Microbiol. Biotechnol. 2000, 54, 424–431. [Google Scholar] [CrossRef]
- Rogers, J.D.; Ferrer, I.; Tummings, S.S.; Bielefeldt, A.R.; Ryan, J.N. Inhibition of Biodegradation of Hydraulic Fracturing Compounds by Glutaraldehyde: Groundwater Column and Microcosm Experiments. Environ. Sci. Technol. 2017, 51, 10251–10261. [Google Scholar] [CrossRef]
- Akyon, B.; Lipus, D.; Bibby, K. Glutaraldehyde inhibits biological treatment of organic additives in hydraulic fracturing produced water. Sci. Total Environ. 2019, 666, 1161–1168. [Google Scholar] [CrossRef]
- Knapp, J.S.; Jenkey, N.D.; Townsley, C.C. The anaerobic biodegradation of diethanolamine by a nitrate reducing bacterium. Biodegradation 1996, 7, 183–189. [Google Scholar] [CrossRef] [PubMed]
- West, R.J.; Gonsior, S.J. Biodegradation of triethanolamine. Environ. Toxicol. Chem. 1996, 15, 472–480. [Google Scholar] [CrossRef]
- Mrklas, O.; Chu, A.; Lunn, S.; Bentley, L.R. Biodegradation of monoethanolamine, ethylene glycol and triethylene glycol in laboratory bioreactors. Water Air Soil Pollut. 2004, 159, 249–263. [Google Scholar] [CrossRef]
- Speranza, G.; Morelli, C.F.; Cairoli, P.; Müller, B.; Schink, B. Mechanism of anaerobic degradation of triethanolamine by a homoacetogenic bacterium. Biochem. Biophys. Res. Commun. 2006, 349, 480–484. [Google Scholar] [CrossRef]
- Kookana, R.S.; Williams, M.; Gregg, A.; Semmler, A.; Du, J.; Apte, S.C. Sorption, degradation and microbial toxicity of chemicals associated with hydraulic fracturing fluid and produced water in soils. Environ. Pollut. 2022, 309, 119754. [Google Scholar] [CrossRef]
- Garcia, M.T.; Campos, E.; Marsal, A.; Ribosa, I. Fate and effects of amphoteric surfactants in the aquatic environment. Environ. Int. 2008, 34, 1001–1005. [Google Scholar] [CrossRef] [PubMed]
- Garland, J.L.; Levine, L.H.; Yorio, N.C.; Hummerick, M.E. Response of graywater recycling systems based on hydroponic plant growth to three classes of surfactants. Water Res. 2004, 38, 1952–1962. [Google Scholar] [CrossRef] [PubMed]
- Vonlanthen, S.; Brown, M.T.; Turner, A. Toxicity of the amphoteric surfactant, cocamidopropyl betaine, to the marine macroalga, Ulva lactuca. Ecotoxicology 2011, 20, 202–207. [Google Scholar] [CrossRef]
- Gheorghe, S.; Lucaciu, I.; Pascu, L. Biodegradability assessment of cationic and amphoteric raw materials. J. Environ. Prot. Ecol. 2012, 13, 155–163. [Google Scholar]
- Blagojević, S.N.; Blagojević, S.M.; Pejić, N.D. Performance and Efficiency of Anionic Dishwashing Liquids with Amphoteric and Nonionic Surfactants. J. Surfactants Deterg. 2016, 19, 363–372. [Google Scholar] [CrossRef]
- Burlachenko, A.S.; Salishcheva, O.V.; Dyshlyuk, L.S.; Prosekov, A.Y. Investigation of the kinetic regularities of the process of biodegradation of betaine surfactant by bacteria of the genus pseudomonas. Appl. Sci. 2021, 11, 8939. [Google Scholar] [CrossRef]
- Kawai, F. Biodegradation of polyethylene glycols and polyethoxylated surfactants. In Polyethers and Polyethylene Glycol: Characterization, Properties and Applications; Williams, J., Ed.; Nova Science Pub Inc.: Hauppauge, NY, USA, 2015; pp. 27–50. [Google Scholar]
- McLaughlin, M.C.; Borch, T.; Blotevogel, J. Spills of hydraulic fracturing chemicals on agricultural topsoil: Biodegradation, sorption, and co-contaminant interactions. Environ. Sci. Technol. 2016, 50, 6071–6078. [Google Scholar] [CrossRef] [PubMed]
- Burgin, A.J.; Yang, W.H.; Hamilton, S.K.; Silver, W.L. Beyond carbon and nitrogen: How the microbial energy economy couples elemental cycles in diverse ecosystems. Front. Ecol. Environ. 2011, 9, 44–52. [Google Scholar] [CrossRef]
- Grbić-Galić, D. Methanogenic transformation of aromatic hydrocarbons and phenols in groundwater aquifers. Geomicrobiol. J. 1990, 8, 167–200. [Google Scholar] [CrossRef]
- Stringfellow, W.T.; Domen, J.K.; Camarillo, M.K.; Sandelin, W.L.; Borglin, S. Physical, chemical, and biological characteristics of compounds used in hydraulic fracturing. J. Hazard. Mater. 2014, 275, 37–54. [Google Scholar] [CrossRef]


| Simulated Fracturing SKB (October 2014) | Concentration g/L | Simulated Fracturing SKC (3 Batches: April, May, June, 2015) | Concentration g/L |
|---|---|---|---|
| Organics | Organics | ||
| guar gum ** | 4.0 | guar gum ** (after pretreating with ammonium persulfate) | 4.0 |
| 2-ethylhexanol | 0.054 | 2-ethylhexanol | 0.27 |
| isopropanol | 0.054 | isopropanol | 0.27 |
| diethanolamine | 0.19 | diethanolamine | 0.19 |
| triethanolamine | 0.91 | triethanolamine | 0.91 |
| cocamidopropyl betaine * | ~1.5 | cocamidopropyl betaine | 3.75 |
| polyethylene glycols | 0.063 | polyethylene glycols | 0.32 |
| ethoxylated alcohols | 0.043 | ||
| ethylene glycol ** | 0.56 | ||
| naphthalene | 8.1 × 10−5 | ||
| 1,2,4-trimethylbenzene | 0.017 | ||
| naphtha | 5.6 ## | ||
| Inorganics | Inorganics | ||
| ammonium persulfate *** | 1.9 | ammonium persulfate (pre-treatment) | 1.4 |
| sodium chloride * | 0.048 | ||
| potassium carbonate | 0.69 | ||
| boric acid # | 0.35 | ||
| potassium hydroxide | 0.36 |
| CAPB | Cocoamidopropyl Betaine |
|---|---|
| Cgw | Concentration of a dissolved fracturing fluid chemical in a “groundwater only” batch (µg/L) (sometimes reported as mg/L) |
| Cgw+sed | Concentration of a dissolved fracturing fluid chemical in a “groundwater and sediment” batch (µg/L) |
| Csed | Concentration of sorbed fracturing fluid chemical in a “groundwater and sediment” batch (µg/kg) |
| FF | Fracturing fluids |
| gw | Groundwater |
| HF | Hydraulic fracturing |
| Kd | Distribution (sorption) coefficient |
| msed | Mass of sediment in batch (kg) |
| PEG | Polyethylene glycol |
| Rf | Retardation factor, which accounts for delay in transport of a dissolved chemical through a test column due to sorption |
| sed | Sediment |
| SKB, SKC | Two synthetic fracturing fluids prepared in this study |
| t½ | Apparent half-life of synthetic chemical in a batch test |
| Vgw | Volume of groundwater in a “groundwater and sediment” batch (L) |
| ψ | an adjustment factor, taking into account the additional water in the “groundwater and sediment” batches compared to the “groundwater only” batches |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van Stempvoort, D.R.; Brown, S.; Kulasekera, P.; Collins, P. Synthetic Chemicals as Potential Tracers of Impacts of Fracturing Fluids on Groundwater. Pollutants 2024, 4, 373-392. https://doi.org/10.3390/pollutants4030026
Van Stempvoort DR, Brown S, Kulasekera P, Collins P. Synthetic Chemicals as Potential Tracers of Impacts of Fracturing Fluids on Groundwater. Pollutants. 2024; 4(3):373-392. https://doi.org/10.3390/pollutants4030026
Chicago/Turabian StyleVan Stempvoort, Dale R., Susan Brown, Priyantha Kulasekera, and Pamela Collins. 2024. "Synthetic Chemicals as Potential Tracers of Impacts of Fracturing Fluids on Groundwater" Pollutants 4, no. 3: 373-392. https://doi.org/10.3390/pollutants4030026






