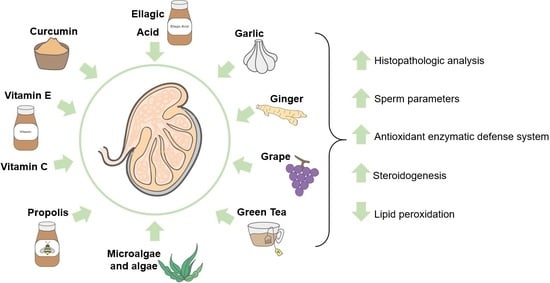
Natural Products as Protective Agents for Male Fertility
Abstract
:1. Introduction
2. Bioactive Compounds
2.1. Curcumin
2.2. Ellagic Acid
2.3. Vitamin C
2.4. Vitamin E
| Natural Product | Dosage of Natural Product | Toxic Stimulus | Dosage of Toxic Stimulus | Exposure Time | Animal Model | Reference |
|---|---|---|---|---|---|---|
| Curcumin | 30 mg/kg/day in dimethyl sulfoxide (4% DMSO + PBS), intraperitoneally | Aging | - | 21 days | Male Wistar albino rats | [15] |
| 100 mg/kg/day dissolved in 0.5 mL of olive oil, given orally | Chronic variable stress | - | 15 days | Male Sprague Dawley rats | [16] | |
| 200 mg/kg, intraperitoneally | Testicular torsion | - | 4 h/90 days | Male Sprague Dawley rats | [17] | |
| 100 mg/kg/day dissolved in dimethyl sulfoxide (DMSO), given orally | Cisplatin | 5 mg/kg, intraperitoneally | 7 days | Male Sprague Dawley rats | [31] | |
| 100 mg/kg, intraperitoneally | Cadmium chloride | 5 mg/kg, subcutaneously | 24 h | Male NMRI mice | [32] | |
| 300 mg/kg/day | Artesunate | 150 mg/kg/day (low dose) 300 mg/kg/day (high-dose) dissolved in double-distilled water, given orally | 45 days | Male Swiss albino mice (Mus musculus) | [33] | |
| 50 mg/kg/day, intraperitoneally | Cadmium chloride | 2 mg/kg/day, intraperitoneally | 10 days | Male Kunming mice | [34] | |
| 200 mg/kg/day dissolved in corn oil, given orally | Cisplatin | 7 mg/kg, intraperitoneally | 10 days | Male Wistar albino rats | [35] | |
| 25–200 mg/mL | Aflatoxins | 2–10 mg/mL | - | Male Swiss albino mice (Mus musculus) | [36] | |
| 2 mg dissolved in 0.2 mL of olive oil, given orally daily | Aflatoxins | 25 mg (low dose) and 50 mg (high dose)/0.2 mL olive oil, given orally daily | 45 days | Male Swiss albino mice (Mus musculus) | [37] | |
| 20, 40 and 80 mg/kg/day dissolved in olive oil, given by intragastric intubation | Scrotal heat stress | - | 14 days | Male ICR mice | [38] | |
| Ellagic acid | 10 mg/kg/day dissolved in alkaline solution (0.01 N NaOH), subcutaneous | Cyclosporine A | 15 mg/kg/day dissolved in 1 mL olive, given orally | 30 days | Male albino rats(Rattus norvegicus) | [43] |
| 10 mg/kg/day dissolved in olive oil, given orally | Cisplatin | 7 mg/kg, intraperitoneally | 10 days | Male Sprague Dawley rats | [54] | |
| 10 mg/kg, given orally from the 21st week to 26th week | Cisplatin | 5 mg/kg once a week in normal saline, intraperitoneally from the 23rd to 26th week | 26 weeks | Male laca mice | [55] | |
| 2 mg/kg/day dissolved in carboxy methyl cellulose, given orally | Phthalates | 500 mg/kg/day dissolved in carboxy methyl cellulose, intraperitoneally | 4 weeks | Male Wistar Albino rats | [56] | |
| 50 mg/kg/day dissolved in saline water (0.9% NaCl), given orally | Sodium arsenate | 200 ppm/day dissolved in saline water (0.9% NaCl), given orally | 40 days | Male Swiss albino mice | [57] | |
| 2 mg/kg/every other day dissolved in alkaline solution (0.01 N NaOH), given orally | Polychlorinated biphenyl (Aroclor 1254) | 2 mg/kg/day, intraperitoneally | 8 weeks | Male Sprague Dawley rats | [58] | |
| 20 mg/kg/day dissolved in 1% DMSO, given orally | Monosodium glutamate | 17.5 mg/kg/day (low dose) or 60 mg/kg/day (high dose) dissolved in 1% DMSO, given orally | 30 days | Male albino rats | [59] | |
| Vitamin C | 100 mg/day diluted in water, given orally | Ethanol | 2 g/kg/day (25% v/v), given orally | 63 days | Male Wistar rats | [63] |
| 5 mg/kg/day, given orally | Aluminum chloride | 100 mg/kg/day dissolved in 1 mL water, given orally | 4 weeks | Male Wistar rats | [65] | |
| 200 mg/kg/day, given orally | Mercuric chloride | 0.15 mg/kg/day dissolved in distilled water, intraperitoneally | 30 days | Male Sprague Dawley rats | [66] | |
| 250 mg/kg/day, given orally | Carbon tetrachloride | 2 mL/kg/day dissolved in olive oil, given orally | 10 weeks | Male Wistar albino rats | [67] | |
| 100 mg/kg/day, given orally | Lead | 25 mg/kg/daydissolved in distilled water, given orally | 90 days | Male Wistar albino rats | [68] | |
| 50 mg/kg/day, gastric gavage | Tetracycline | 30 mg/kg/day, gastric gavage | 28 days | Male Sprague Dawley rats | [69] | |
| 200 mg/kg/day, given orally | Tetracycline | 28.6 mg/kg/day dissolved in saline, given orally | 14 days | Male Wistar rats | [71] | |
| 50 mg/kg/day, given orally | Cisplatin | 8 mg/kg, intraperitoneally | 14 days | Sprague Dawley rats | [70] | |
| 100 mg/kg, intraperitoneally | Testicular torsion | - | 4 h | Male Sprague Dawley rats | [72] | |
| Vitamin E | 100 mg/kg/day, given orally | Sodium arsenite | 8 mg/kg/day, given orally | 8 weeks | Male Wistar rats | [81] |
| 100 mg/kg/day dissolved in corn oil, given orally | Para-nonylphenol | 250 mg/kg/day dissolved in corn oil, given orally | Day 7 of pregnancy to day 21 of postnatal/90 days | Wistar rats | [82] | |
| 20 mg/kg/day, given orally | 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) | 1, 10 or 100 ng/kg/day dissolved in olive oil and acetone (19:1), given orally | 45 days | Male Wistar rats | [83] | |
| 200 mg/kg, given orally | Diazinon | 60 mg/kg dissolved in olive oil, intraperitoneally | 6 weeks | Male Wistar rats | [84] | |
| 20 mg/kg/day, given orally | Carbendazim | 25 mg/kg/day dissolved in corn oil, given orally | 48 days | Male Wistar albino rats (Rattus norvegicus) | [85] | |
| 250 mg/kg/day, intraperitoneally | Methotrexate | 20 mg/kg on days 3 and 10, intraperitoneally | 17 days | Male Wistar albino rats | [86] |
3. Natural Products with Bioactive Compounds
3.1. Garlic
3.2. Ginger
3.3. Grape
3.4. Green Tea
3.5. Microalgae and Algae
3.6. Propolis
| Natural Product | Dosage of Natural Product | Toxic Stimulus | Dosage of Toxic Stimulus | Exposure Time | Animal Model | Reference |
|---|---|---|---|---|---|---|
| Garlic | 0.8 g/100 g/day, given orally | Casein-based diet (High Protein Diet) | 40, 25 or 10 g/100 g/day, given orally | 28 days | Male Sprague Dawley rats | [93] |
| 1.0 mL/100 g/day, given orally | Cadmium | 1.5 mg/100 g/day, given orally | 4 weeks | Male albino Wistar rats | [94] | |
| 80 mg/kg/day for 5 days per week, given orally | Furan | 4 mg/kg/day for 5 days per week dissolved in corn oil, given orally | 90 days | Male Sprague Dawley rats | [95] | |
| 250 mg/kg/day given orally | Adriamycin | 10 mg/kg, intraperitoneally | 14 days | Male Wistar rats | [96] | |
| Ginger | 100 mg/kg/day, given orally | Gentamicin | 50 mg/kg, intraperitoneally | 30 days | Male Wistar rats | [98] |
| 250 mg/kg/day (hydro-alcoholic extract of ginger), given orally | Carbon tetrachloride | 1 mL/kg dissolved in olive oil, intraperitoneally | 14 days | Male Wistar rats | [99] | |
| 1 g/kg/day, given orally | Ethanol | 4 g/kg/day, given orally | 28 days | Male Sprague Dawley rats | [112] | |
| 500 mg/kg/day, given orally | Sulfite salts (sodium metabisulfite) | 260 mg/kg/day dissolved in distilled water, given orally | 28 days | Male Wistar rats | [113] | |
| 1 g/kg/day, given orally | Ethanol | 4 g/kg/day, given orally | 28 days | Male Sprague Dawley rats | [115] | |
| 50, 100 and 150 mg/kg/day, given orally | Busulfan | 5mg/kg, intraperitoneally | 48 days | Male Sprague Dawley rats | [116] | |
| 300 mg/kg/day or 600 mg/kg/days, given orally | Cyclophosphamide | 100 mg/kg, intraperitoneally | 6 weeks | Male Wistar albino rats | [117] | |
| 1 g/kg/day, given orally | Cisplatin | 10 mg/kg dissolved in normal saline, intraperitoneally | 26 days | Male Wistar albino rats | [118] | |
| 500 mg/kg/day, given orally | Fructose | Fed a diet containing 60% fructose | 8 weeks | Male Sprague Dawley rats | [119] | |
| 500 mg/kg/day, given orally | Diabetes | 60 mg/kg of STZ dissolved in 0.01 M sodium citrate buffer, intraperitoneally | 6 weeks | Male Sprague Dawley rats | [120] | |
| fed a diet supplemented with ginger roots at 3% | Diabetes | 120 mg/kg of alloxan saline solution, intraperitoneally | 30 days | Male Wistar rats | [121] | |
| Grape | 75 mg/kg/day, given orally | Ethanol | 10 mL/kg/day ate 25% v/v, given orally | 10 weeks | Male albino rats | [114] |
| 100 mg/kg/day, given orally 6 days a week | Plant growth regulators | 75 ppm/L or 100 ppm/L dissolved in drinking water, given orally 6 days a week | 60 days | Male albino rats (Rattus rattus) | [122] | |
| 2 g/kg/day, given orally | Cadmium chlorine | 1.2 mg/kg diluted in 0.5 mL of distilled water, intraperitoneally | 86 days | Male Wistar rats | [125] | |
| 100 mg/kg/day dissolved in normal saline, given orally | Cadmium chloride | 5 mg/kg/day dissolved in normal saline, given orally | 30 days | Male albino rats | [132] | |
| 120 mg/kg, given orally | Cadmium chlorine | 5 mg/kg in saline, given orally | 4 weaks | Wistar albino male rats | [133] | |
| 400 mg/kg/day dissolved in water, given orally | Cadmium chlorine | 5 mg/kg/day dissolved in water, given orally | 90 days | Male Wistar rats | [134] | |
| 100 mg/kg/day, given orally | Cadmium | 2.5 mg/kg, intraperitoneally | 10 days | Male Wistar rats | [135] | |
| 1.8 g/kg/day or 2.36 g/kg/day, given orally | Cadmium chlorine | 1.2 mg/kg, intraperitoneally | 56 days | Male Wistar rats | [136] | |
| 2 g/kg/day, given orally | Cadmium chlorine | 1.2 mg/kg diluted in 0.5 mL of distilled water, intraperitoneally | 30 days | Male Wistar rats | [137] | |
| 200 or 400 mg/kg three times per week, given orally | Dexamethasone | 0.1 mg/kg three times per week, subcutaneously | 30 days | Male Wistar rats | [138] | |
| 250 mg/kg/day, given orally | Varicocele | - | 8 weeks | Male Wistar rats | [139] | |
| 100 mg/kg/day dissolved in saline, given orally | Testicular torsion | - | 7 days | Male Wistar albino rats | [140] | |
| 20 mg/kg/day of trans-resveratrol dissolved in 10 g/L of carboxymethylcellulose | - | - | 90 days | Male Sprague Dawley rats | [141] | |
| Green tea | 150 mg/kg/day, given orally | Deltamethrin | 0.6 mg/kg/day dissolved in corn oil, given orally | 28 days | Male mice | [156] |
| 2% or 5% dally, given orally | - | - | 52 days | Male Wistar rats | [158] | |
| 200 mg/kg/day dissolved in water, given orally | Para-nonylphenol | 200 mg/kg/day dissolved in corn oil, given orally | 56 days | Male Wistar rats | [159] | |
| 1.5% w/v dally, given orally | Cadmium chloride | 1.5 mg/kg dissolved in water, intraperitoneally | 13, 25 and 49 days | Male Wistar rats | [160] | |
| 70 mg/kg/day, given orally | Cadmium chloride | 3 mg/kg/day, given orally | 63 days | Male Wistar rats | [161] | |
| 2% w/v dally, given orally | Nicotine | 1 mg/kg/day dissolved in water, intraperitoneally | 60 days | Male Wistar rats | [162] | |
| 200 mg/kg/day or 500 mg/kg/day, given orally | Doxorubicin | 0.15 mg/kg, intraperitoneally 2 days per week for 5 weeks | 14 weeks | Male ICR mice | [163] | |
| Microalgae/algae | 50 mg/kg/day, dissolved in 1 mL saline given orally | Deltamethrin | 3 mg/kg/day dissolved in 1 mL saline, given orally | 8 weeks | Male albino rats | [174] |
| 70 mg/kg/day, dissolved in 0.9% sodium chloride given orally | Sodium nitrite | 80 mg/kg/day dissolved in distilled water, given orally | 90 days | Male Wistar Albino rats | [175] | |
| 150 mg/kg/day, in drinking weather given orally | Cadmium chloride | 2 mg/kg/day, subcutaneously | 10 days/30 days | Male Sprague–Dawley rats | [178] | |
| 300 mg/kg/day, via gastric tube | Furan | 16 mg/kg/day, given orally | 4 weeks | Male Sprague–Dawley rats | [179] | |
| 300 mg/kg/day, given orally | Mercuric chloride | 5 mg/kg dissolved in water, subcutaneously 3 days per week | 60 days | Male Wistar albino rats (Rattus norvegicus) | [180] | |
| 300 mg/kg/day, given orally | Bifenthrin | 5 mg/kg/day, given orally | 35 days | Male white Swiss mice | [181] | |
| 300 mg/kg, given orally | Lead acetate | 30 mg/kg, given orally | 8 weeks | Male Wistar rats | [182] | |
| 200 mg/kg/day, given orally | Diabetes | 50 mg/kg of STZ dissolved in 0.1 M citrate buffer, intraperitoneally | 28 days | Male Wistar rats | [183] | |
| 200 mg/kg, 400 mg/kg, 800 mg/kg, given by gastric intubation | Benzo[alpha]pyrene | 125 mg/kg/day dissolved in corn oil, intraperitoneally | 3 weeks | CF1 mice | [184] | |
| 200 mg/kg, 400 mg/kg, 800 mg/kg, given orally | Cyclophosphamide | 40 mg/kg dissolved in 0.9% saline, intraperitoneally | 3 weeks | CF1 mice | [185] | |
| 900 mg/kg/day, given orally | Cadmium chloride | 2.0 mg/kg/day, given orally | 21 days | BALB/c mice | [186] | |
| 13 mg/kg, 26 mg/kg or 65 mg/kg, given orally | Diabetes | 65 mg/kg of STZ and 230 mg/kg of NA, intraperitoneally | 4 weeks | Male Sprague Dawley rats | [187] | |
| 100 mg/kg/day, 200 mg/kg/day, 500 mg/kg/day dissolved in olive oil, given orally | Cisplatin | 7 mg/kg, intraperitoneally | 5 days | Male Syrian hamsters | [188] | |
| Propolis | 100 mg/kg/day dissolved in ethanol 70%, given orally | Copper sulphate | 128 mg/kg/day dissolved in water, given orally | 21 days | Male Sprague Dawley rats | [191] |
| 300 mg/kg/day dissolved in distilled water, given orally | Diabetes | 60 mg/kg of STZ dissolved in ice-cold saline, intraperitoneally | 4 weeks | Male Sprague Dawley rats | [192,206] | |
| 50 mg/kg/day, given orally | Aluminum chloride | 34 mg/kg/day, given orally | 70 days | Male Wistar Albino rats | [199] | |
| 50 mg/kg/day dissolved in DMSO, given orally | Cadmium | 1 mg/kg/day, intraperitoneally starting in day 8th | 17 days | Male Wistar rats | [205] | |
| 100 mg/kg/day dissolved in DMSO, given orally | Methotrexate | 20 mg/kg, intraperitoneally in day 8th | 15 days | Male Wistar rats | [207] | |
| 200 mg/kg 5 days per week, given orally | Doxorubicin | 3 mg/kg, intraperitoneally in day 8th, 10th, 12th, 15th, 17th and 19th | 21 days | Male Wistar albino rats | [208] | |
| 50 mg/kg/day dissolved in 1 mL distilled water, given orally | Paclitaxel | 5 mg/kg diluted in 1 mL normal saline once a week, intraperitoneally | 4 weeks | Male Sprague Dawley rats | [209] |
4. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hess, R.A.; de Franca, L.R. Spermatogenesis and Cycle of the Seminiferous Epithelium. In Molecular Mechanisms in Spermatogenesis; Cheng, C.Y., Ed.; Springer: New York, NY, USA, 2008; pp. 1–15. [Google Scholar] [CrossRef]
- Clermont, Y. Kinetics of spermatogenesis in mammals: Seminiferous epithelium cycle and spermatogonial renewal. Physiol. Rev. 1972, 52, 198–236. [Google Scholar] [CrossRef] [PubMed]
- Correia, S.; Vaz, C.V.; Silva, A.M.; Cavaco, J.E.; Socorro, S. Regucalcin counteracts tert-butyl hydroperoxide and cadmium-induced oxidative stress in rat testis. J. Appl. Toxicol. 2017, 37, 159–166. [Google Scholar] [CrossRef]
- Cocuzza, M.; Athayde, K.S.; Agarwal, A.; Pagani, R.; Sikka, S.C.; Lucon, A.M.; Srougi, M.; Hallak, J. Impact of clinical varicocele and testis size on seminal reactive oxygen species levels in a fertile population: A prospective controlled study. Fertil. Steril. 2008, 90, 1103–1108. [Google Scholar] [CrossRef] [PubMed]
- Fatemi, N.; Sanati, M.H.; Jamali Zavarehei, M.; Ayat, H.; Esmaeili, V.; Golkar-Narenji, A.; Zarabi, M.; Gourabi, H. Effect of tertiary-butyl hydroperoxide (TBHP)-induced oxidative stress on mice sperm quality and testis histopathology. Andrologia 2013, 45, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Kaur, G.; Bansal, M.P. Tertiary-butyl hydroperoxide induced oxidative stress and male reproductive activity in mice: Role of transcription factor NF-kappaB and testicular antioxidant enzymes. Reprod. Toxicol. 2006, 22, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Roman, S.D. Antioxidant systems and oxidative stress in the testes. Oxid. Med. Cell. Longev. 2008, 1, 15–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agarwal, A.; Rana, M.; Qiu, E.; AlBunni, H.; Bui, A.D.; Henkel, R. Role of oxidative stress, infection and inflammation in male infertility. Andrologia 2018, 50, e13126. [Google Scholar] [CrossRef]
- Semet, M.; Paci, M.; Saïas-Magnan, J.; Metzler-Guillemain, C.; Boissier, R.; Lejeune, H.; Perrin, J. The impact of drugs on male fertility: A review. Andrology 2017, 5, 640–663. [Google Scholar] [CrossRef]
- Dias, T.R.; Alves, M.G.; Oliveira, P.F.; Silva, B.M. Natural products as modulators of spermatogenesis: The search for a male contraceptive. Curr. Mol. Pharmacol. 2014, 7, 154–166. [Google Scholar] [CrossRef]
- Jia, Z.; Anandh Babu, P.V.; Chen, W. Natural Products Targeting on Oxidative Stress and Inflammation: Mechanisms, Therapies, and Safety Assessment. Oxid. Med. Cell. Longev. 2018, 2018, 6576093. [Google Scholar] [CrossRef] [Green Version]
- Amin, A.; Hamza, A.A.; Kambal, A.; Daoud, S. Herbal extracts counteract cisplatin-mediated cell death in rat testis. Asian J. Androl. 2008, 10, 291–297. [Google Scholar] [CrossRef]
- Mbongue, G.Y.; Kamtchouing, P.; Dimo, T. Effects of the aqueous extract of dry seeds of Aframomum melegueta on some parameters of the reproductive function of mature male rats. Andrologia 2012, 44, 53–58. [Google Scholar] [CrossRef]
- Soliman, G.A.; Donia Ael, R.; Awaad, A.S.; Alqasoumi, S.I.; Yusufoglu, H. Effect of Emex spinosa, Leptadenia pyrotechnica, Haloxylon salicornicum and Ochradenus baccatus extracts on the reproductive organs of adult male rats. Pharm. Biol. 2012, 50, 105–112. [Google Scholar] [CrossRef] [Green Version]
- Muratoğlu, S.; Akarca Dizakar, O.S. The protective role of melatonin and curcumin in the testis of young and aged rats. Andrologia 2019, 51, e13203. [Google Scholar] [CrossRef]
- Mohamadpour, M.; Noorafshan, A.; Karbalay-Doust, S.; Talaei-Khozani, T.; Aliabadi, E. Protective effects of curcumin co-treatment in rats with establishing chronic variable stress on testis and reproductive hormones. Int. J. Reprod. Biomed. 2017, 15, 447–452. [Google Scholar] [CrossRef]
- Wei, S.M.; Yan, Z.Z.; Zhou, J. Curcumin attenuates ischemia-reperfusion injury in rat testis. Fertil. Steril. 2009, 91, 271–277. [Google Scholar] [CrossRef]
- Ammon, H.P.; Wahl, M.A. Pharmacology of Curcuma longa. Planta Med. 1991, 57, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Inano, H.; Onoda, M.; Inafuku, N.; Kubota, M.; Kamada, Y.; Osawa, T.; Kobayashi, H.; Wakabayashi, K. Chemoprevention by curcumin during the promotion stage of tumorigenesis of mammary gland in rats irradiated with gamma-rays. Carcinogenesis 1999, 20, 1011–1018. [Google Scholar] [CrossRef] [Green Version]
- Mohebbati, R.; Anaeigoudari, A.; Khazdair, M.R. The effects of Curcuma longa and curcumin on reproductive systems. Endocr. Regul. 2017, 51, 220–228. [Google Scholar] [CrossRef] [Green Version]
- Moniruzzaman, M.; Min, T. Curcumin, Curcumin Nanoparticles and Curcumin Nanospheres: A Review on Their Pharmacodynamics Based on Monogastric Farm Animal, Poultry and Fish Nutrition. Pharmaceutics 2020, 12, 447. [Google Scholar] [CrossRef]
- Maheshwari, R.K.; Singh, A.K.; Gaddipati, J.; Srimal, R.C. Multiple biological activities of curcumin: A short review. Life Sci. 2006, 78, 2081–2087. [Google Scholar] [CrossRef] [PubMed]
- Bala, K.; Tripathy, B.C.; Sharma, D. Neuroprotective and anti-ageing effects of curcumin in aged rat brain regions. Biogerontology 2006, 7, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.-Y.; Shien, J.-H.; Tiley, L.; Chiou, S.-S.; Wang, S.-Y.; Chang, T.-J.; Lee, Y.-J.; Chan, K.-W.; Hsu, W.-L. Curcumin inhibits influenza virus infection and haemagglutination activity. Food Chem. 2010, 119, 1346–1351. [Google Scholar] [CrossRef]
- Niu, Y.; He, J.; Zhao, Y.; Shen, M.; Zhang, L.; Zhong, X. Effect of Curcumin on Growth Performance, Inflammation, Insulin level, and Lipid Metabolism in Weaned Piglets with IUGR. Animals 2019, 9, 1098. [Google Scholar] [CrossRef] [Green Version]
- Chainani-Wu, N. Safety and anti-inflammatory activity of curcumin: A component of tumeric (Curcuma longa). J. Altern. Complement. Med. 2003, 9, 161–168. [Google Scholar] [CrossRef] [Green Version]
- Ozaki, K.; Kawata, Y.; Amano, S.; Hanazawa, S. Stimulatory effect of curcumin on osteoclast apoptosis. Biochem. Pharmacol. 2000, 59, 1577–1581. [Google Scholar] [CrossRef]
- Larasati, Y.A.; Yoneda-Kato, N.; Nakamae, I.; Yokoyama, T.; Meiyanto, E.; Kato, J.Y. Curcumin targets multiple enzymes involved in the ROS metabolic pathway to suppress tumor cell growth. Sci. Rep. 2018, 8, 2039. [Google Scholar] [CrossRef]
- Kim, B.H.; Lee, E.S.; Choi, R.; Nawaboot, J.; Lee, M.Y.; Lee, E.Y.; Kim, H.S.; Chung, C.H. Protective Effects of Curcumin on Renal Oxidative Stress and Lipid Metabolism in a Rat Model of Type 2 Diabetic Nephropathy. Yonsei Med. J. 2016, 57, 664–673. [Google Scholar] [CrossRef] [Green Version]
- Balasubramanyam, M.; Koteswari, A.A.; Kumar, R.S.; Monickaraj, S.F.; Maheswari, J.U.; Mohan, V. Curcumin-induced inhibition of cellular reactive oxygen species generation: Novel therapeutic implications. J. Biosci. 2003, 28, 715–721. [Google Scholar] [CrossRef] [Green Version]
- Mercantepe, T.; Unal, D.; Tümkaya, L.; Yazici, Z.A. Protective effects of amifostine, curcumin and caffeic acid phenethyl ester against cisplatin-induced testis tissue damage in rats. Exp. Ther. Med. 2018, 15, 3404–3412. [Google Scholar] [CrossRef] [Green Version]
- Momeni, H.R.; Eskandari, N. Curcumin protects the testis against cadmium-induced histopathological damages and oxidative stress in mice. Hum. Exp. Toxicol. 2020, 39, 653–661. [Google Scholar] [CrossRef]
- Desai, K.R.; Rajput, D.K.; Patel, P.B.; Highland, H.N. Ameliorative Effects of Curcumin on Artesunate-Induced Subchronic Toxicity in Testis of Swiss Albino Male Mice. Dose-Response 2015, 13, 1559325815592393. [Google Scholar] [CrossRef]
- Yang, S.-H.; He, J.-B.; Yu, L.-H.; Li, L.; Long, M.; Liu, M.-D.; Li, P. Protective role of curcumin in cadmium-induced testicular injury in mice by attenuating oxidative stress via Nrf2/ARE pathway. Environ. Sci. Pollut. Res. 2019, 26, 34575–34583. [Google Scholar] [CrossRef]
- Ilbey, Y.O.; Ozbek, E.; Cekmen, M.; Simsek, A.; Otunctemur, A.; Somay, A. Protective effect of curcumin in cisplatin-induced oxidative injury in rat testis: Mitogen-activated protein kinase and nuclear factor-kappa B signaling pathways. Hum. Reprod. 2009, 24, 1717–1725. [Google Scholar] [CrossRef] [Green Version]
- Mathuria, N.; Verma, R.J. Curcumin ameliorates aflatoxin-induced lipid peroxidation in liver, kidney and testis of mice—An in vitro study. Acta Pol. Pharm. 2007, 64, 413–416. [Google Scholar]
- Verma, R.J.; Mathuria, N. Effect of curcumin on aflatoxin-induced biochemical changes in testis of mice. Fertil. Steril. 2009, 91, 597–601. [Google Scholar] [CrossRef]
- Lin, C.; Shin, D.G.; Park, S.G.; Chu, S.B.; Gwon, L.W.; Lee, J.G.; Yon, J.M.; Baek, I.J.; Nam, S.Y. Curcumin dose-dependently improves spermatogenic disorders induced by scrotal heat stress in mice. Food Funct. 2015, 6, 3770–3777. [Google Scholar] [CrossRef]
- Baker, M. Deceptive curcumin offers cautionary tale for chemists. Nature 2017, 541, 144–145. [Google Scholar] [CrossRef]
- Nelson, K.M.; Dahlin, J.L.; Bisson, J.; Graham, J.; Pauli, G.F.; Walters, M.A. The Essential Medicinal Chemistry of Curcumin. J. Med. Chem. 2017, 60, 1620–1637. [Google Scholar] [CrossRef]
- Landete, J.M. Ellagitannins, ellagic acid and their derived metabolites: A review about source, metabolism, functions and health. Food Res. Int. 2011, 44, 1150–1160. [Google Scholar] [CrossRef]
- Seigler, D.S. Tannins. In Plant Secondary Metabolism; Seigler, D.S., Ed.; Springer: Boston, MA, USA, 1998; pp. 193–214. [Google Scholar] [CrossRef]
- Abdul-Hamid, M.; Abdella, E.M.; Galaly, S.R.; Ahmed, R.H. Protective effect of ellagic acid against cyclosporine A-induced histopathological, ultrastructural changes, oxidative stress, and cytogenotoxicity in albino rats. Ultrastruct. Pathol. 2016, 40, 205–221. [Google Scholar] [CrossRef] [PubMed]
- Whitley, A.C.; Stoner, G.D.; Darby, M.V.; Walle, T. Intestinal epithelial cell accumulation of the cancer preventive polyphenol ellagic acid--extensive binding to protein and DNA. Biochem. Pharmacol. 2003, 66, 907–915. [Google Scholar] [CrossRef]
- Seeram, N.P.; Adams, L.S.; Henning, S.M.; Niu, Y.; Zhang, Y.; Nair, M.G.; Heber, D. In vitro antiproliferative, apoptotic and antioxidant activities of punicalagin, ellagic acid and a total pomegranate tannin extract are enhanced in combination with other polyphenols as found in pomegranate juice. J. Nutr. Biochem. 2005, 16, 360–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loarca-Piña, G.; Kuzmicky, P.A.; de Mejía, E.G.; Kado, N.Y. Inhibitory effects of ellagic acid on the direct-acting mutagenicity of aflatoxin B1 in the Salmonella microsuspension assay. Mutat. Res. 1998, 398, 183–187. [Google Scholar] [CrossRef]
- Yu, Y.M.; Chang, W.C.; Wu, C.H.; Chiang, S.Y. Reduction of oxidative stress and apoptosis in hyperlipidemic rabbits by ellagic acid. J. Nutr. Biochem. 2005, 16, 675–681. [Google Scholar] [CrossRef]
- Papoutsi, Z.; Kassi, E.; Tsiapara, A.; Fokialakis, N.; Chrousos, G.P.; Moutsatsou, P. Evaluation of estrogenic/antiestrogenic activity of ellagic acid via the estrogen receptor subtypes ERalpha and ERbeta. J. Agric. Food Chem. 2005, 53, 7715–7720. [Google Scholar] [CrossRef]
- Hassoun, E.A.; Walter, A.C.; Alsharif, N.Z.; Stohs, S.J. Modulation of TCDD-induced fetotoxicity and oxidative stress in embryonic and placental tissues of C57BL/6J mice by vitamin E succinate and ellagic acid. Toxicology 1997, 124, 27–37. [Google Scholar] [CrossRef]
- Hassoun, E.A.; Vodhanel, J.; Abushaban, A. The modulatory effects of ellagic acid and vitamin E succinate on TCDD-induced oxidative stress in different brain regions of rats after subchronic exposure. J. Biochem. Mol. Toxicol. 2004, 18, 196–203. [Google Scholar] [CrossRef]
- Dalvi, L.T.; Moreira, D.C.; Andrade, R., Jr.; Ginani, J.; Alonso, A.; Hermes-Lima, M. Ellagic acid inhibits iron-mediated free radical formation. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 173, 910–917. [Google Scholar] [CrossRef]
- Pari, L.; Sivasankari, R. Effect of ellagic acid on cyclosporine A-induced oxidative damage in the liver of rats. Fundam. Clin. Pharmacol. 2008, 22, 395–401. [Google Scholar] [CrossRef]
- Vattem, D.A.; Shetty, K. Biological functionality of ellagic acid: A review. J. Food Biochem. 2005, 29, 234–266. [Google Scholar] [CrossRef]
- Türk, G.; Ateşşahin, A.; Sönmez, M.; Ceribaşi, A.O.; Yüce, A. Improvement of cisplatin-induced injuries to sperm quality, the oxidant-antioxidant system, and the histologic structure of the rat testis by ellagic acid. Fertil. Steril. 2008, 89, 1474–1481. [Google Scholar] [CrossRef] [Green Version]
- Goyal, Y.; Koul, A.; Ranawat, P. Ellagic acid ameliorates cisplatin toxicity in chemically induced colon carcinogenesis. Mol. Cell. Biochem. 2019, 453, 205–215. [Google Scholar] [CrossRef]
- Başak Türkmen, N.; Ayhan, İ.; Taşlıdere, A.; Aydın, M.; Çiftçi, O. Investigation of protective effect of ellagic acid in phthalates-induced reproductive damage. Drug Chem. Toxicol. 2020, 1–14. [Google Scholar] [CrossRef]
- Guvvala, P.R.; Ravindra, J.P.; Selvaraju, S.; Arangasamy, A.; Venkata, K.M. Ellagic and ferulic acids protect arsenic-induced male reproductive toxicity via regulating Nfe2l2, Ppargc1a and StAR expressions in testis. Toxicology 2019, 413, 1–12. [Google Scholar] [CrossRef]
- Ateşşahin, A.; Türk, G.; Yilmaz, S.; Sönmez, M.; Sakin, F.; Ceribasi, A.O. Modulatory effects of lycopene and ellagic acid on reproductive dysfunction induced by polychlorinated biphenyl (Aroclor 1254) in male rats. Basic Clin. Pharmacol. Toxicol. 2010, 106, 479–489. [Google Scholar] [CrossRef] [Green Version]
- Hamza, R.Z.; Al-Baqami, N.M. Testicular protective effects of ellagic acid on monosodium glutamate-induced testicular structural alterations in male rats. Ultrastruct. Pathol. 2019, 43, 170–183. [Google Scholar] [CrossRef]
- Abdullah, M.; Jamil, R.T.; Attia, F.N. Vitamin C (Ascorbic Acid) StatPearls 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK499877/ (accessed on 18 March 2019).
- Castro, M.A.; Angulo, C.; Brauchi, S.; Nualart, F.; Concha, I.I. Ascorbic acid participates in a general mechanism for concerted glucose transport inhibition and lactate transport stimulation. Pflugers Arch. 2008, 457, 519–528. [Google Scholar] [CrossRef]
- Carr, A.; Frei, B. Does vitamin C act as a pro-oxidant under physiological conditions? FASEB J. 1999, 13, 1007–1024. [Google Scholar] [CrossRef] [Green Version]
- Siervo, G.E.; Vieira, H.R.; Ogo, F.M.; Fernandez, C.D.; Gonçalves, G.D.; Mesquita, S.F.; Anselmo-Franci, J.A.; Cecchini, R.; Guarnier, F.A.; Fernandes, G.S. Spermatic and testicular damages in rats exposed to ethanol: Influence of lipid peroxidation but not testosterone. Toxicology 2015, 330, 1–8. [Google Scholar] [CrossRef]
- Fernandes, G.S.; Fernandez, C.D.; Campos, K.E.; Damasceno, D.C.; Anselmo-Franci, J.A.; Kempinas, W.D. Vitamin C partially attenuates male reproductive deficits in hyperglycemic rats. Reprod. Biol. Endocrinol. 2011, 9, 100. [Google Scholar] [CrossRef] [PubMed]
- Akinola, B.K.; Olawuyi, T.S.; Ukwenya, V.O.; Daniel, L.D.; Faleye, B.C. Protective effects of aloe vera gel (Aloe baberdensis Miller) on aluminum chloride-induced reproductive toxicity in male Wistar rats. JBRA Assist. Reprod. 2021, 25, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Jahan, S.; Azad, T.; Ayub, A.; Ullah, A.; Afsar, T.; Almajwal, A.; Razak, S. Ameliorating potency of Chenopodium album Linn. and vitamin C against mercuric chloride-induced oxidative stress in testes of Sprague Dawley rats. Environ. Health Prev. Med. 2019, 24, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahmouni, F.; Daoud, S.; Rebai, T. Teucrium polium attenuates carbon tetrachloride-induced toxicity in the male reproductive system of rats. Andrologia 2019, 51, e13182. [Google Scholar] [CrossRef]
- El Shafai, A.; Zohdy, N.; El Mulla, K.; Hassan, M.; Morad, N. Light and electron microscopic study of the toxic effect of prolonged lead exposure on the seminiferous tubules of albino rats and the possible protective effect of ascorbic acid. Food Chem. Toxicol. 2011, 49, 734–743. [Google Scholar] [CrossRef]
- Adelakun, S.; Omotoso, O.; Aniah, J.; Oyewo, O. Senecio biafrae defeated Tetracycline-Induced Testicular Toxicity in Adult Male Sprague Dawley Rats. JBRA Assist. Reprod. 2018, 22, 314–322. [Google Scholar] [CrossRef]
- Adelakun, S.A.; Akinola, B.K.; Akingbade, G.T. Fertility Enhancing Activities of Bioactive Components of Cochlospermum planchonii Rhizome on Cisplatin Induced Reproductive Dysfunctions in Sprague-Dawley Rats. J. Fam. Reprod. Health 2018, 12, 148–159. [Google Scholar]
- Farombi, E.O.; Ugwuezunmba, M.C.; Ezenwadu, T.T.; Oyeyemi, M.O.; Ekor, M. Tetracycline-induced reproductive toxicity in male rats: Effects of vitamin C and N-acetylcysteine. Exp. Toxicol. Pathol. 2008, 60, 77–85. [Google Scholar] [CrossRef]
- Azizollahi, S.; Babaei, H.; Derakhshanfar, A.; Oloumi, M.M. Effects of co-administration of dopamine and vitamin C on ischaemia-reperfusion injury after experimental testicular torsion-detorsion in rats. Andrologia 2011, 43, 100–105. [Google Scholar] [CrossRef]
- Aro, A.; Kyllästinen, M.; Kostiainen, E.; Gref, C.G.; Elfving, S.; Uusitalo, U. No effect on serum lipids by moderate and high doses of vitamin C in elderly subjects with low plasma ascorbic acid levels. Ann. Nutr. Metab. 1988, 32, 133–137. [Google Scholar] [CrossRef]
- Shibata, M.A.; Tamano, S.; Kurata, Y.; Hagiwara, A.; Fukushima, S. Participation of urinary Na+, K+, pH, and L-ascorbic acid in the proliferative response of the bladder epithelium after the oral administration of various salts and/or ascorbic acid to rats. Food Chem. Toxicol. 1989, 27, 403–413. [Google Scholar] [CrossRef]
- Gershoff, S.N. Vitamin C (ascorbic acid): New roles, new requirements? Nutr. Rev. 1993, 51, 313–326. [Google Scholar] [CrossRef]
- Salama, A.F.; Kasem, S.M.; Tousson, E.; Elsisy, M.K. Protective role of L-carnitine and vitamin E on the testis of atherosclerotic rats. Toxicol. Ind. Health 2015, 31, 467–474. [Google Scholar] [CrossRef]
- Medina, J.; Gupta, V. Vitamin E. StatPearls 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557737/ (accessed on 18 March 2019).
- Colombo, M.L. An update on vitamin E, tocopherol and tocotrienol-perspectives. Molecules 2010, 15, 2103–2113. [Google Scholar] [CrossRef] [Green Version]
- Erdemli, Z.; Erdemli, M.E. The effects of acrylamide and Vitamin E administration during pregnancy on adult rats testis. Andrologia 2019, 51, e13292. [Google Scholar] [CrossRef]
- Lomnitski, L.; Bergman, M.; Schön, I.; Grossman, S. The effect of dietary vitamin E and beta-carotene on oxidation processes in the rat testis. Biochim. Biophys. Acta 1991, 1082, 101–107. [Google Scholar] [CrossRef]
- Momeni, H.R.; Oryan, S.; Eskandari, N. Effect of vitamin E on sperm number and testis histopathology of sodium arsenite-treated rats. Reprod. Biol. 2012, 12, 171–181. [Google Scholar] [CrossRef]
- Mehranjani, M.S.; Noorafshan, A.; Momeni, H.R.; Abnosi, M.H.; Mahmoodi, M.; Anvari, M.; Hoseini, S.M. Stereological study of the effects of vitamin E on testis structure in rats treated with para-nonylphenol. Asian J. Androl. 2009, 11, 508–516. [Google Scholar] [CrossRef] [Green Version]
- Latchoumycandane, C.; Mathur, P.P. Effects of vitamin E on reactive oxygen species-mediated 2,3,7,8-tetrachlorodi-benzo-p-dioxin toxicity in rat testis. J. Appl. Toxicol. 2002, 22, 345–351. [Google Scholar] [CrossRef]
- Rahimi Anbarkeh, F.; Nikravesh, M.R.; Jalali, M.; Sadeghnia, H.R.; Sargazi, Z.; Mohammdzadeh, L. Single dose effect of diazinon on biochemical parameters in testis tissue of adult rats and the protective effect of vitamin E. Iran. J. Reprod. Med. 2014, 12, 731–736. [Google Scholar]
- Rajeswary, S.; Mathew, N.; Akbarsha, M.A.; Kalyanasundram, M.; Kumaran, B. Protective effect of vitamin E against carbendazim-induced testicular toxicity-histopathological evidences and reduced residue levels in testis and serum. Arch. Toxicol. 2007, 81, 813–821. [Google Scholar] [CrossRef]
- Yüncü, M.; Bükücü, N.; Bayat, N.; Sencar, L.; Tarakçioğlu, M. The effect of vitamin E and L-carnitine against methotrexate-induced injury in rat testis. Turk. J. Med. Sci. 2015, 45, 517–525. [Google Scholar] [CrossRef]
- Owen, K.N.; Dewald, O. Vitamin E Toxicity StatPearls 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK564373/ (accessed on 18 March 2019).
- Hammami, I.; El May, M.V. Impact of garlic feeding (Allium sativum) on male fertility. Andrologia 2013, 45, 217–224. [Google Scholar] [CrossRef]
- Rivlin, R.S. Historical perspective on the use of garlic. J. Nutr. 2001, 131, 951s–954s. [Google Scholar] [CrossRef] [Green Version]
- Block, E. The chemistry of garlic and onions. Sci. Am. 1985, 252, 114–119. [Google Scholar] [CrossRef]
- Park, J.H.; Park, Y.K.; Park, E. Antioxidative and antigenotoxic effects of garlic (Allium sativum L.) prepared by different processing methods. Plant Foods Hum. Nutr. 2009, 64, 244–249. [Google Scholar] [CrossRef]
- Srinivasan, K. Antioxidant potential of spices and their active constituents. Crit. Rev. Food Sci. Nutr. 2014, 54, 352–372. [Google Scholar] [CrossRef]
- Oi, Y.; Imafuku, M.; Shishido, C.; Kominato, Y.; Nishimura, S.; Iwai, K. Garlic supplementation increases testicular testosterone and decreases plasma corticosterone in rats fed a high protein diet. J. Nutr. 2001, 131, 2150–2156. [Google Scholar] [CrossRef] [Green Version]
- Ola-Mudathir, K.F.; Suru, S.M.; Fafunso, M.A.; Obioha, U.E.; Faremi, T.Y. Protective roles of onion and garlic extracts on cadmium-induced changes in sperm characteristics and testicular oxidative damage in rats. Food Chem. Toxicol. 2008, 46, 3604–3611. [Google Scholar] [CrossRef] [PubMed]
- El-Akabawy, G.; El-Sherif, N.M. Protective role of garlic oil against oxidative damage induced by furan exposure from weaning through adulthood in adult rat testis. Acta Histochem. 2016, 118, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Nasr, A.Y. The impact of aged garlic extract on adriamycin-induced testicular changes in adult male Wistar rats. Acta Histochem. 2017, 119, 648–662. [Google Scholar] [CrossRef]
- Rana, S.V.; Pal, R.; Vaiphei, K.; Sharma, S.K.; Ola, R.P. Garlic in health and disease. Nutr. Res. Rev. 2011, 24, 60–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zahedi, A.; Fathiazad, F.; Khaki, A.; Ahmadnejad, B. Protective effect of ginger on gentamicin-induced apoptosis in testis of rats. Adv. Pharm. Bull. 2012, 2, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Mazani, M.; Ojarudi, M.; Banaei, S. The protective effect of cinnamon and ginger hydro-alcoholic extract on carbon tetrachloride-induced testicular damage in rats. Andrologia 2020, 52, e13651. [Google Scholar] [CrossRef]
- Khodaie, L.; Sadeghpoor, O. Ginger from ancient times to the new outlook. Jundishapur J. Nat. Pharm. Prod. 2015, 10, e18402. [Google Scholar] [CrossRef] [Green Version]
- Khaleghi Ghadiri, M.; Gorji, A. Natural remedies for impotence in medieval Persia. Int. J. Impot. Res. 2004, 16, 80–83. [Google Scholar] [CrossRef]
- Kamatenesi-Mugisha, M.; Oryem-Origa, H. Traditional herbal remedies used in the management of sexual impotence and erectile dysfunction in western Uganda. Afr. Health Sci. 2005, 5, 40–49. [Google Scholar]
- Ghasemzadeh, A.; Jaafar, H.Z.; Rahmat, A. Antioxidant activities, total phenolics and flavonoids content in two varieties of Malaysia young ginger (Zingiber officinale Roscoe). Molecules 2010, 15, 4324–4333. [Google Scholar] [CrossRef] [Green Version]
- Lantz, R.C.; Chen, G.J.; Sarihan, M.; Sólyom, A.M.; Jolad, S.D.; Timmermann, B.N. The effect of extracts from ginger rhizome on inflammatory mediator production. Phytomedicine 2007, 14, 123–128. [Google Scholar] [CrossRef]
- Ramachandran, C.; Lollett, I.V.; Escalon, E.; Quirin, K.W.; Melnick, S.J. Anticancer potential and mechanism of action of mango ginger (Curcuma amada Roxb.) supercritical CO2 extract in human glioblastoma cells. J. Evid. Based Complement. Altern. Med. 2015, 20, 109–119. [Google Scholar] [CrossRef]
- Shukla, Y.; Singh, M. Cancer preventive properties of ginger: A brief review. Food Chem. Toxicol. 2007, 45, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Chrubasik, S.; Pittler, M.H.; Roufogalis, B.D. Zingiberis rhizoma: A comprehensive review on the ginger effect and efficacy profiles. Phytomedicine 2005, 12, 684–701. [Google Scholar] [CrossRef]
- Al-Amin, Z.M.; Thomson, M.; Al-Qattan, K.K.; Peltonen-Shalaby, R.; Ali, M. Anti-diabetic and hypolipidaemic properties of ginger (Zingiber officinale) in streptozotocin-induced diabetic rats. Br. J. Nutr. 2006, 96, 660–666. [Google Scholar] [CrossRef] [Green Version]
- Kamtchouing, P.; Mbongue Fandio, G.Y.; Dimo, T.; Jatsa, H.B. Evaluation of androgenic activity of Zingiber officinale and Pentadiplandra brazzeana in male rats. Asian J. Androl. 2002, 4, 299–301. [Google Scholar] [PubMed]
- Morakinyo, F.; Achema, P.; Adegoke, O. Effect of Zingiber officinale (Ginger) on Sodium Arsenite–Induced Reproductive Toxicity in Male Rats. Afr. J. Biomed. Res. 2010, 13, 39–45. [Google Scholar]
- Sakr, S.; Badawy, G. Effect of ginger (Zingiber officinale R.) on metiram-inhibited spermatogenesis and induced apoptosis in albino mice. J. Appl. Pharm. Sci. 2011, 1, 131–136. [Google Scholar]
- Akbari, A.; Nasiri, K.; Heydari, M.; Mosavat, S.H.; Iraji, A. The Protective Effect of Hydroalcoholic Extract of Zingiber officinale Roscoe (Ginger) on Ethanol-Induced Reproductive Toxicity in Male Rats. J. Evid. Based Complement. Altern. Med. 2017, 22, 609–617. [Google Scholar] [CrossRef] [Green Version]
- Afkhami Fathabad, A.; Shekarforoush, S.; Hoseini, M.; Ebrahimi, Z. Attenuation of Sulfite-Induced Testicular Injury in Rats by Zingiber officinale Roscoe. J. Diet. Suppl. 2018, 15, 398–409. [Google Scholar] [CrossRef]
- El-Ashmawy, I.M.; Saleh, A.; Salama, O.M. Effects of marjoram volatile oil and grape seed extract on ethanol toxicity in male rats. Basic Clin. Pharmacol. Toxicol. 2007, 101, 320–327. [Google Scholar] [CrossRef]
- Akbari, A.; Nasiri, K.; Heydari, M.; Mosavat, S.H. The Prophylactic Effect of Hydroalcoholic Extract of Zingiber officinale (Ginger) on Ethanol-Induced Reproductive Toxicity in Male Rats. Iran. J. Med. Sci. 2016, 41, S71. [Google Scholar]
- Bordbar, H.; Esmaeilpour, T.; Dehghani, F.; Panjehshahin, M.R. Stereological study of the effect of ginger’s alcoholic extract on the testis in busulfan-induced infertility in rats. Iran. J. Reprod. Med. 2013, 11, 467–472. [Google Scholar] [PubMed]
- Mohammadi, F.; Nikzad, H.; Taghizadeh, M.; Taherian, A.; Azami-Tameh, A.; Hosseini, S.M.; Moravveji, A. Protective effect of Zingiber officinale extract on rat testis after cyclophosphamide treatment. Andrologia 2014, 46, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Amin, A.; Hamza, A.A. Effects of Roselle and Ginger on cisplatin-induced reproductive toxicity in rats. Asian J. Androl. 2006, 8, 607–612. [Google Scholar] [CrossRef] [PubMed]
- El-Mehi, A.E.; Faried, M.A. Effect of high-fructose diet-induced metabolic syndrome on the pituitary-gonadal axis from adolescence through adulthood in male albino rats and the possible protective role of ginger extract. A biochemical, histological and immunohistochemical study. Folia Morphol. 2020, 79, 690–708. [Google Scholar] [CrossRef] [Green Version]
- Al-Shathly, M.R.; Ali, S.S.; Ayuob, N.N. Zingiber officinale preserves testicular structure and the expression of androgen receptors and proliferating cell nuclear antigen in diabetic rats. Andrologia 2020, 52, e13528. [Google Scholar] [CrossRef]
- Ghlissi, Z.; Atheymen, R.; Boujbiha, M.A.; Sahnoun, Z.; Makni Ayedi, F.; Zeghal, K.; El Feki, A.; Hakim, A. Antioxidant and androgenic effects of dietary ginger on reproductive function of male diabetic rats. Int. J. Food Sci. Nutr. 2013, 64, 974–978. [Google Scholar] [CrossRef]
- Hassan, H.A.; El-Kholy, W.M.; Nour, S.E. Proanthocyanidin as a cytogenetic protective agent against adverse effects of plant growth regulators supplementation in rats. Cytotechnology 2014, 66, 585–596. [Google Scholar] [CrossRef] [Green Version]
- Gupta, M.; Dey, S.; Marbaniang, D.; Pal, P.; Ray, S.; Mazumder, B. Grape seed extract: Having a potential health benefits. J. Food Sci. Technol. 2020, 57, 1205–1215. [Google Scholar] [CrossRef]
- Matthäus, B. Virgin grape seed oil: Is it really a nutritional highlight? Eur. J. Lipid Sci. Technol. 2008, 110, 645–650. [Google Scholar] [CrossRef]
- Lamas, C.A.; Cuquetto-Leite, L.; do Nascimento da Silva, E.; Thomazini, B.F.; Cordeiro, G.D.S.; Predes, F.S.; Gollücke, A.P.B.; Dolder, H. Grape juice concentrate alleviates epididymis and sperm damage in cadmium-intoxicated rats. Int. J. Exp. Pathol. 2017, 98, 86–99. [Google Scholar] [CrossRef]
- Farzaei, M.H.; Abdollahi, M.; Rahimi, R. Role of dietary polyphenols in the management of peptic ulcer. World J. Gastroenterol. 2015, 21, 6499–6517. [Google Scholar] [CrossRef]
- Kaur, M.; Agarwal, C.; Agarwal, R. Anticancer and cancer chemopreventive potential of grape seed extract and other grape-based products. J. Nutr. 2009, 139, 1806s–1812s. [Google Scholar] [CrossRef] [Green Version]
- Terra, X.; Pallarés, V.; Ardèvol, A.; Bladé, C.; Fernández-Larrea, J.; Pujadas, G.; Salvadó, J.; Arola, L.; Blay, M. Modulatory effect of grape-seed procyanidins on local and systemic inflammation in diet-induced obesity rats. J. Nutr. Biochem. 2011, 22, 380–387. [Google Scholar] [CrossRef]
- Bagchi, D.; Garg, A.; Krohn, R.L.; Bagchi, M.; Tran, M.X.; Stohs, S.J. Oxygen free radical scavenging abilities of vitamins C and E, and a grape seed proanthocyanidin extract in vitro. Res. Commun. Mol. Pathol. Pharmacol. 1997, 95, 179–189. [Google Scholar]
- Maffei Facino, R.; Carini, M.; Aldini, G.; Bombardelli, E.; Morazzoni, P.; Morelli, R. Free radicals scavenging action and anti-enzyme activities of procyanidines from Vitis vinifera. A mechanism for their capillary protective action. Arzneimittel-Forschung 1994, 44, 592–601. [Google Scholar]
- Shi, J.; Yu, J.; Pohorly, J.E.; Kakuda, Y. Polyphenolics in grape seeds-biochemistry and functionality. J. Med. Food 2003, 6, 291–299. [Google Scholar] [CrossRef]
- Morsi, A.A.; Shawky, L.M.; El Bana, E.A. The potential gonadoprotective effects of grape seed extract against the histopathological alterations elicited in an animal model of cadmium-induced testicular toxicity. Folia Morphol. 2020, 79, 767–776. [Google Scholar] [CrossRef] [Green Version]
- Evcimen, M.; Aslan, R.; Gulay, M.S. Protective effects of polydatin and grape seed extract in rats exposed to cadmium. Drug Chem. Toxicol. 2020, 43, 225–233. [Google Scholar] [CrossRef]
- Alkhedaide, A.; Alshehri, Z.S.; Sabry, A.; Abdel-Ghaffar, T.; Soliman, M.M.; Attia, H. Protective effect of grape seed extract against cadmium-induced testicular dysfunction. Mol. Med. Rep. 2016, 13, 3101–3109. [Google Scholar] [CrossRef]
- Sönmez, M.F.; Tascioglu, S. Protective effects of grape seed extract on cadmium-induced testicular damage, apoptosis, and endothelial nitric oxide synthases expression in rats. Toxicol. Ind. Health 2016, 32, 1486–1494. [Google Scholar] [CrossRef]
- Pires, V.C.; Gollücke, A.P.; Ribeiro, D.A.; Lungato, L.; D’Almeida, V.; Aguiar, O., Jr. Grape juice concentrate protects reproductive parameters of male rats against cadmium-induced damage: A chronic assay. Br. J. Nutr. 2013, 110, 2020–2029. [Google Scholar] [CrossRef] [Green Version]
- Lamas, C.A.; Gollücke, A.P.; Dolder, H. Grape juice concentrate (G8000®) intake mitigates testicular morphological and ultrastructural damage following cadmium intoxication. Int. J. Exp. Pathol. 2015, 96, 301–310. [Google Scholar] [CrossRef]
- Hasona, N.A. Grape seed extract attenuates dexamethasone-induced testicular and thyroid dysfunction in male albino rats. Andrologia 2018, 50, e13002. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, F.; Liang, M.; Chen, S.; Zhu, Y.; Zou, Z.; Shi, B. Grape seed proanthocyanidin extract attenuates varicocele-induced testicular oxidative injury in rats by activating the Nrf2-antioxidant system. Mol. Med. Rep. 2018, 17, 1799–1806. [Google Scholar] [CrossRef]
- Bayatli, F.; Akkuş, D.; Kilic, E.; Saraymen, R.; Sönmez, M.F. The protective effects of grape seed extract on MDA, AOPP, apoptosis and eNOS expression in testicular torsion: An experimental study. World J. Urol. 2013, 31, 615–622. [Google Scholar] [CrossRef]
- Juan, M.E.; González-Pons, E.; Munuera, T.; Ballester, J.; Rodríguez-Gil, J.E.; Planas, J.M. trans-Resveratrol, a natural antioxidant from grapes, increases sperm output in healthy rats. J. Nutr. 2005, 135, 757–760. [Google Scholar] [CrossRef]
- Musial, C.; Kuban-Jankowska, A.; Gorska-Ponikowska, M. Beneficial Properties of Green Tea Catechins. Int. J. Mol. Sci. 2020, 21, 1744. [Google Scholar] [CrossRef] [Green Version]
- Chacko, S.M.; Thambi, P.T.; Kuttan, R.; Nishigaki, I. Beneficial effects of green tea: A literature review. Chin. Med. 2010, 5, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graham, H.N. Green tea composition, consumption, and polyphenol chemistry. Prev. Med. 1992, 21, 334–350. [Google Scholar] [CrossRef]
- Hasegawa, N.; Niimi, N.; Odani, F. Vitamin C is one of the lipolytic substances in green tea. Phytother. Res. 2002, 16 (Suppl. S1), S91–S92. [Google Scholar] [CrossRef] [PubMed]
- Kavanagh, K.T.; Hafer, L.J.; Kim, D.W.; Mann, K.K.; Sherr, D.H.; Rogers, A.E.; Sonenshein, G.E. Green tea extracts decrease carcinogen-induced mammary tumor burden in rats and rate of breast cancer cell proliferation in culture. J. Cell. Biochem. 2001, 82, 387–398. [Google Scholar] [CrossRef]
- Sartippour, M.R.; Shao, Z.M.; Heber, D.; Beatty, P.; Zhang, L.; Liu, C.; Ellis, L.; Liu, W.; Go, V.L.; Brooks, M.N. Green tea inhibits vascular endothelial growth factor (VEGF) induction in human breast cancer cells. J. Nutr. 2002, 132, 2307–2311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sueoka, N.; Suganuma, M.; Sueoka, E.; Okabe, S.; Matsuyama, S.; Imai, K.; Nakachi, K.; Fujiki, H. A new function of green tea: Prevention of lifestyle-related diseases. Ann. N. Y. Acad. Sci. 2001, 928, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Donà, M.; Dell’Aica, I.; Calabrese, F.; Benelli, R.; Morini, M.; Albini, A.; Garbisa, S. Neutrophil restraint by green tea: Inhibition of inflammation, associated angiogenesis, and pulmonary fibrosis. J. Immunol. 2003, 170, 4335–4341. [Google Scholar] [CrossRef] [Green Version]
- Haqqi, T.M.; Anthony, D.D.; Gupta, S.; Ahmad, N.; Lee, M.S.; Kumar, G.K.; Mukhtar, H. Prevention of collagen-induced arthritis in mice by a polyphenolic fraction from green tea. Proc. Natl. Acad. Sci. USA 1999, 96, 4524–4529. [Google Scholar] [CrossRef] [Green Version]
- Sudano Roccaro, A.; Blanco, A.R.; Giuliano, F.; Rusciano, D.; Enea, V. Epigallocatechin-gallate enhances the activity of tetracycline in staphylococci by inhibiting its efflux from bacterial cells. Antimicrob. Agents Chemother. 2004, 48, 1968–1973. [Google Scholar] [CrossRef] [Green Version]
- Weber, J.M.; Ruzindana-Umunyana, A.; Imbeault, L.; Sircar, S. Inhibition of adenovirus infection and adenain by green tea catechins. Antivir. Res. 2003, 58, 167–173. [Google Scholar] [CrossRef]
- Weinreb, O.; Mandel, S.; Amit, T.; Youdim, M.B. Neurological mechanisms of green tea polyphenols in Alzheimer’s and Parkinson’s diseases. J. Nutr. Biochem. 2004, 15, 506–516. [Google Scholar] [CrossRef]
- Raederstorff, D.G.; Schlachter, M.F.; Elste, V.; Weber, P. Effect of EGCG on lipid absorption and plasma lipid levels in rats. J. Nutr. Biochem. 2003, 14, 326–332. [Google Scholar] [CrossRef]
- Osada, K.; Takahashi, M.; Hoshina, S.; Nakamura, M.; Nakamura, S.; Sugano, M. Tea catechins inhibit cholesterol oxidation accompanying oxidation of low density lipoprotein in vitro. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2001, 128, 153–164. [Google Scholar] [CrossRef]
- Bagherpour, H.; Karimpour Malekshah, A.; Talebpour Amiri, F.; Azadbakht, M. Protective effect of green tea extract on the deltamethrin-induced toxicity in mice testis: An experimental study. Int. J. Reprod. Biomed. 2019, 17, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Soussi, A.; Gaubin, Y.; Beau, B.; Murat, J.C.; Soleilhavoup, J.P.; Croute, F.; El Feki, A. Stress proteins (Hsp72/73, Grp94) expression pattern in rat organs following metavanadate administration. Effect of green tea drinking. Food Chem. Toxicol. 2006, 44, 1031–1037. [Google Scholar] [CrossRef]
- Opuwari, C.; Monsees, T. Green tea consumption increases sperm concentration and viability in male rats and is safe for reproductive, liver and kidney health. Sci. Rep. 2020, 10, 15269. [Google Scholar] [CrossRef] [PubMed]
- Azizi, P.; Soleimani Mehranjani, M. The effect of green tea extract on the sperm parameters and histological changes of testis in rats exposed to para-nonylphenol. Int. J. Reprod. Biomed. 2019, 17, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, R.; Azizi, A.; Abedini, S.; Hemayatkhah Jahromi, V.; Abidi, H.; Jafari Barmak, M. Green tea improves rat sperm quality and reduced cadmium chloride damage effect in spermatogenesis cycle. J. Med. Life 2018, 11, 371–380. [Google Scholar] [CrossRef]
- Abdelrazek, H.M.; Helmy, S.A.; Elsayed, D.H.; Ebaid, H.M.; Mohamed, R.M. Ameliorating effects of green tea extract on cadmium induced reproductive injury in male Wistar rats with respect to androgen receptors and caspase-3. Reprod. Biol. 2016, 16, 300–308. [Google Scholar] [CrossRef]
- Mosbah, R.; Yousef, M.I.; Mantovani, A. Nicotine-induced reproductive toxicity, oxidative damage, histological changes and haematotoxicity in male rats: The protective effects of green tea extract. Exp. Toxicol. Pathol. 2015, 67, 253–259. [Google Scholar] [CrossRef]
- Sato, K.; Sueoka, K.; Tanigaki, R.; Tajima, H.; Nakabayashi, A.; Yoshimura, Y.; Hosoi, Y. Green tea extracts attenuate doxorubicin-induced spermatogenic disorders in conjunction with higher telomerase activity in mice. J. Assist. Reprod Genet. 2010, 27, 501–508. [Google Scholar] [CrossRef] [Green Version]
- Bedrood, Z.; Rameshrad, M.; Hosseinzadeh, H. Toxicological effects of Camellia sinensis (green tea): A review. Phytother. Res. 2018, 32, 1163–1180. [Google Scholar] [CrossRef]
- Saha, S.K.; McHugh, E.; Murray, P.; Walsh, D.J. Microalgae as a source of nutraceuticals. In Phycotoxins; Wiley-Blackwell: Hackensack, NJ, USA, 2015; pp. 255–291. [Google Scholar] [CrossRef]
- Plaza, M.; Herrero, M.; Cifuentes, A.; Ibáñez, E. Innovative natural functional ingredients from microalgae. J. Agric. Food Chem. 2009, 57, 7159–7170. [Google Scholar] [CrossRef]
- Aitken, R.J.; Curry, B.J. Redox regulation of human sperm function: From the physiological control of sperm capacitation to the etiology of infertility and DNA damage in the germ line. Antioxid. Redox Signal. 2011, 14, 367–381. [Google Scholar] [CrossRef]
- Tremellen, K. Oxidative stress and male infertility—A clinical perspective. Hum. Reprod. Update 2008, 14, 243–258. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Re: GRAS Exemption Claim for Chlorella Vulgaris as an Ingredient in Foods. Available online: https://www.isditwelgezond.nl/wp-content/uploads/2017/04/ucm277773.pdf (accessed on 18 March 2019).
- U.S. Food and Drug Administration. Re: GRAS Exemption Claim for Spirulina Platensis as an Ingredient in Foods. Available online: http://sdlbl.in/wp-content/uploads/2014/09/FDA-United-States.pdf (accessed on 18 March 2019).
- Vecina, J.F.; Oliveira, A.G.; Araujo, T.G.; Baggio, S.R.; Torello, C.O.; Saad, M.J.; Queiroz, M.L. Chlorella modulates insulin signaling pathway and prevents high-fat diet-induced insulin resistance in mice. Life Sci. 2014, 95, 45–52. [Google Scholar] [CrossRef]
- Li, L.; Li, W.; Kim, Y.H.; Lee, Y.W. Chlorella vulgaris extract ameliorates carbon tetrachloride-induced acute hepatic injury in mice. Exp. Toxicol. Pathol. 2013, 65, 73–80. [Google Scholar] [CrossRef]
- Saberbaghi, T.; Abbasian, F.; Mohd Yusof, Y.A.; Makpol, S. Modulation of Cell Cycle Profile by Chlorella vulgaris Prevents Replicative Senescence of Human Diploid Fibroblasts. Evid. Based Complement. Altern. Med. 2013, 2013, 780504. [Google Scholar] [CrossRef] [Green Version]
- Osama, E.; Galal, A.A.A.; Abdalla, H.; El-Sheikh, S.M.A. Chlorella vulgaris ameliorates testicular toxicity induced by deltamethrin in male rats via modulating oxidative stress. Andrologia 2019, 51, e13214. [Google Scholar] [CrossRef]
- Eissa, M.M.; Ahmed, M.M. Methanolic extract of Chlorella vulgaris protects against sodium nitrite-induced reproductive toxicity in male rats. Andrologia 2020, 52, e13811. [Google Scholar] [CrossRef]
- Sayed, A.E.-D.H.; El-Sayed, Y.S.; El-Far, A.H. Hepatoprotective efficacy of Spirulina platensis against lead-induced oxidative stress and genotoxicity in catfish; Clarias gariepinus. Ecotoxicol. Environ. Saf. 2017, 143, 344–350. [Google Scholar] [CrossRef]
- Sadek, K.M.; Lebda, M.A.; Nasr, S.M.; Shoukry, M. Spirulina platensis prevents hyperglycemia in rats by modulating gluconeogenesis and apoptosis via modification of oxidative stress and MAPK-pathways. Biomed. Pharmacother. 2017, 92, 1085–1094. [Google Scholar] [CrossRef]
- Farag, M.R.; Abd El-Aziz, R.M.; Ali, H.A.; Ahmed, S.A. Evaluating the ameliorative efficacy of Spirulina platensis on spermatogenesis and steroidogenesis in cadmium-intoxicated rats. Environ. Sci. Pollut. Res. Int. 2016, 23, 2454–2466. [Google Scholar] [CrossRef]
- Abd El-Hakim, Y.M.; Mohamed, W.A.; El-Metwally, A.E. Spirulina platensis attenuates furan reprotoxicity by regulating oxidative stress, inflammation, and apoptosis in testis of rats. Ecotoxicol. Environ. Saf. 2018, 161, 25–33. [Google Scholar] [CrossRef] [PubMed]
- El-Desoky, G.E.; Bashandy, S.A.; Alhazza, I.M.; Al-Othman, Z.A.; Aboul-Soud, M.A.; Yusuf, K. Improvement of mercuric chloride-induced testis injuries and sperm quality deteriorations by Spirulina platensis in rats. PLoS ONE 2013, 8, e59177. [Google Scholar] [CrossRef] [PubMed]
- Barkallah, M.; Slima, A.B.; Elleuch, F.; Fendri, I.; Pichon, C.; Abdelkafi, S.; Baril, P. Protective Role of Spirulina platensis Against Bifenthrin-Induced Reprotoxicity in Adult Male Mice by Reversing Expression of Altered Histological, Biochemical, and Molecular Markers Including MicroRNAs. Biomolecules 2020, 10, 753. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, I.A.; Shalaby, A.A.; Abd Elaziz, R.T.; Bahr, H.I. Chlorella vulgaris or Spirulina platensis mitigate lead acetate-induced testicular oxidative stress and apoptosis with regard to androgen receptor expression in rats. Environ. Sci. Pollut. Res. Int. 2021, 28, 39126–39138. [Google Scholar] [CrossRef] [PubMed]
- Nah, W.H.; Koh, I.K.; Ahn, H.S.; Kim, M.J.; Kang, H.-G.; Jun, J.H.; Gye, M.C. Effect of Spirulina maxima on spermatogenesis and steroidogenesis in streptozotocin-induced type I diabetic male rats. Food Chem. 2012, 134, 173–179. [Google Scholar] [CrossRef]
- Chamorro-Cevallos, G.; Garduño-Siciliano, L.; Martínez-Galero, E.; Mojica-Villegas, A.; Pages, N.; Gutiérrez-Salmeán, G. The protective effect of dietary Arthrospira (Spirulina) maxima against mutagenicity induced by benzo[alpha]pyrene in mice. J. Med. Food 2014, 17, 527–534. [Google Scholar] [CrossRef] [Green Version]
- Chamorro-Cevallos, G.; Garduño-Siciliano, L.; Barrón, B.L.; Madrigal-Bujaidar, E.; Cruz-Vega, D.E.; Pages, N. Chemoprotective effect of Spirulina (Arthrospira) against cyclophosphamide-induced mutagenicity in mice. Food Chem. Toxicol. 2008, 46, 567–574. [Google Scholar] [CrossRef]
- Güner, Ö.; Güner, A.; Yavaşoğlu, A.; Karabay Yavaşoğlu, N.Ü.; Kavlak, O. Ameliorative effect of edible Halopteris scoparia against cadmium-induced reproductive toxicity in male mice: A biochemical and histopathologic study. Andrologia 2020, 52, e13591. [Google Scholar] [CrossRef]
- Kong, Z.L.; Sudirman, S. Fucoxanthin-Rich Brown Algae Extract Improves Male Reproductive Function on Streptozotocin-Nicotinamide-Induced Diabetic Rat Model. Int. J. Mol. Sci. 2019, 20, 4485. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.T.; Sudirman, S.; Hsieh, M.C.; Hu, J.Y.; Kong, Z.L. Oral supplementation of fucoxanthin-rich brown algae extract ameliorates cisplatin-induced testicular damage in hamsters. Biomed. Pharmacother. 2020, 125, 109992. [Google Scholar] [CrossRef]
- Burdock, G.A. Review of the biological properties and toxicity of bee propolis (propolis). Food Chem. Toxicol. 1998, 36, 347–363. [Google Scholar] [CrossRef]
- Zabaiou, N.; Fouache, A.; Trousson, A.; Baron, S.; Zellagui, A.; Lahouel, M.; Lobaccaro, J.A. Biological properties of propolis extracts: Something new from an ancient product. Chem. Phys. Lipids 2017, 207, 214–222. [Google Scholar] [CrossRef]
- Seven, I.; Tatli Seven, P. Bee glue (propolis) improves reproductive organs, sperm quality and histological changes and antioxidant parameters of testis tissues in rats exposed to excess copper. Andrologia 2020, 52, e13540. [Google Scholar] [CrossRef]
- Nna, V.U.; Abu Bakar, A.B.; Ahmad, A.; Eleazu, C.O.; Mohamed, M. Oxidative Stress, NF-κB-Mediated Inflammation and Apoptosis in the Testes of Streptozotocin-Induced Diabetic Rats: Combined Protective Effects of Malaysian Propolis and Metformin. Antioxidants 2019, 8, 465. [Google Scholar] [CrossRef] [Green Version]
- Tohamy, A.A.; Abdella, E.M.; Ahmed, R.R.; Ahmed, Y.K. Assessment of anti-mutagenic, anti-histopathologic and antioxidant capacities of Egyptian bee pollen and propolis extracts. Cytotechnology 2014, 66, 283–297. [Google Scholar] [CrossRef] [Green Version]
- Collodel, G.; Moretti, E.; Del Vecchio, M.T.; Biagi, M.; Cardinali, R.; Mazzi, L.; Brecchia, G.; Maranesi, M.; Manca, D.; Castellini, C. Effect of chocolate and Propolfenol on rabbit spermatogenesis and sperm quality following bacterial lipopolysaccharide treatment. Syst. Biol. Reprod. Med. 2014, 60, 217–226. [Google Scholar] [CrossRef]
- Khalil, M.L. Biological activity of bee propolis in health and disease. Asian Pac. J. Cancer Prev. 2006, 7, 22–31. [Google Scholar]
- Sforcin, J.M. Biological Properties and Therapeutic Applications of Propolis. Phytother. Res. 2016, 30, 894–905. [Google Scholar] [CrossRef]
- Nna, V.U.; Abu Bakar, A.B.; Md Lazin, M.; Mohamed, M. Antioxidant, anti-inflammatory and synergistic anti-hyperglycemic effects of Malaysian propolis and metformin in streptozotocin-induced diabetic rats. Food Chem. Toxicol. 2018, 120, 305–320. [Google Scholar] [CrossRef]
- Orsi, R.O.; Fernandes, A.; Bankova, V.; Sforcin, J.M. The effects of Brazilian and Bulgarian propolis in vitro against Salmonella Typhi and their synergism with antibiotics acting on the ribosome. Nat. Prod. Res. 2012, 26, 430–437. [Google Scholar] [CrossRef]
- Yousef, M.I.; Salama, A.F. Propolis protection from reproductive toxicity caused by aluminium chloride in male rats. Food Chem. Toxicol. 2009, 47, 1168–1175. [Google Scholar] [CrossRef]
- Selamoglu Talas, Z. Propolis reduces oxidative stress in l-NAME-induced hypertension rats. Cell Biochem. Funct. 2014, 32, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Sobocanec, S.; Sverko, V.; Balog, T.; Sarić, A.; Rusak, G.; Likić, S.; Kusić, B.; Katalinić, V.; Radić, S.; Marotti, T. Oxidant/antioxidant properties of Croatian native propolis. J. Agric. Food Chem. 2006, 54, 8018–8026. [Google Scholar] [CrossRef] [PubMed]
- Jasprica, I.; Mornar, A.; Debeljak, Z.; Smolcić-Bubalo, A.; Medić-Sarić, M.; Mayer, L.; Romić, Z.; Bućan, K.; Balog, T.; Sobocanec, S.; et al. In vivo study of propolis supplementation effects on antioxidative status and red blood cells. J. Ethnopharmacol. 2007, 110, 548–554. [Google Scholar] [CrossRef]
- Castaldo, S.; Capasso, F. Propolis, an old remedy used in modern medicine. Fitoterapia 2002, 73 (Suppl. 1), S1–S6. [Google Scholar] [CrossRef]
- Capucho, C.; Sette, R.; de Souza Predes, F.; de Castro Monteiro, J.; Pigoso, A.A.; Barbieri, R.; Dolder, M.A.; Severi-Aguiar, G.D. Green Brazilian propolis effects on sperm count and epididymis morphology and oxidative stress. Food Chem. Toxicol. 2012, 50, 3956–3962. [Google Scholar] [CrossRef]
- Çilenk, K.T.; Öztürk, İ.; Sönmez, M.F. Ameliorative effect of propolis on the cadmium-induced reproductive toxicity in male albino rats. Exp. Mol. Pathol. 2016, 101, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Nna, V.U.; Bakar, A.B.A.; Ahmad, A.; Umar, U.Z.; Suleiman, J.B.; Zakaria, Z.; Othman, Z.A.; Mohamed, M. Malaysian propolis and metformin mitigate subfertility in streptozotocin-induced diabetic male rats by targeting steroidogenesis, testicular lactate transport, spermatogenesis and mating behavior. Andrologia 2020, 8, 731–746. [Google Scholar] [CrossRef]
- Sönmez, M.F.; Çilenk, K.T.; Karabulut, D.; Ünalmış, S.; Deligönül, E.; Öztürk, İ.; Kaymak, E. Protective effects of propolis on methotrexate-induced testis injury in rat. Biomed. Pharmacother. 2016, 79, 44–51. [Google Scholar] [CrossRef]
- Rizk, S.M.; Zaki, H.F.; Mina, M.A. Propolis attenuates doxorubicin-induced testicular toxicity in rats. Food. Chem. Toxicol. 2014, 67, 176–186. [Google Scholar] [CrossRef]
- Abd-Elrazek, A.M.; El-Dash, H.A.; Said, N.I. The role of propolis against paclitaxel-induced oligospermia, sperm abnormality, oxidative stress and DNA damage in testes of male rats. Andrologia 2020, 52, e13394. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martins, R.V.L.; Silva, A.M.S.; Duarte, A.P.; Socorro, S.; Correia, S.; Maia, C.J. Natural Products as Protective Agents for Male Fertility. BioChem 2021, 1, 122-147. https://doi.org/10.3390/biochem1030011
Martins RVL, Silva AMS, Duarte AP, Socorro S, Correia S, Maia CJ. Natural Products as Protective Agents for Male Fertility. BioChem. 2021; 1(3):122-147. https://doi.org/10.3390/biochem1030011
Chicago/Turabian StyleMartins, Roberta V. L., Ana M. S. Silva, Ana Paula Duarte, Sílvia Socorro, Sara Correia, and Cláudio J. Maia. 2021. "Natural Products as Protective Agents for Male Fertility" BioChem 1, no. 3: 122-147. https://doi.org/10.3390/biochem1030011
APA StyleMartins, R. V. L., Silva, A. M. S., Duarte, A. P., Socorro, S., Correia, S., & Maia, C. J. (2021). Natural Products as Protective Agents for Male Fertility. BioChem, 1(3), 122-147. https://doi.org/10.3390/biochem1030011