Geopolymers as Alternative Sustainable Binders for Stabilisation of Clays—A Review
Abstract
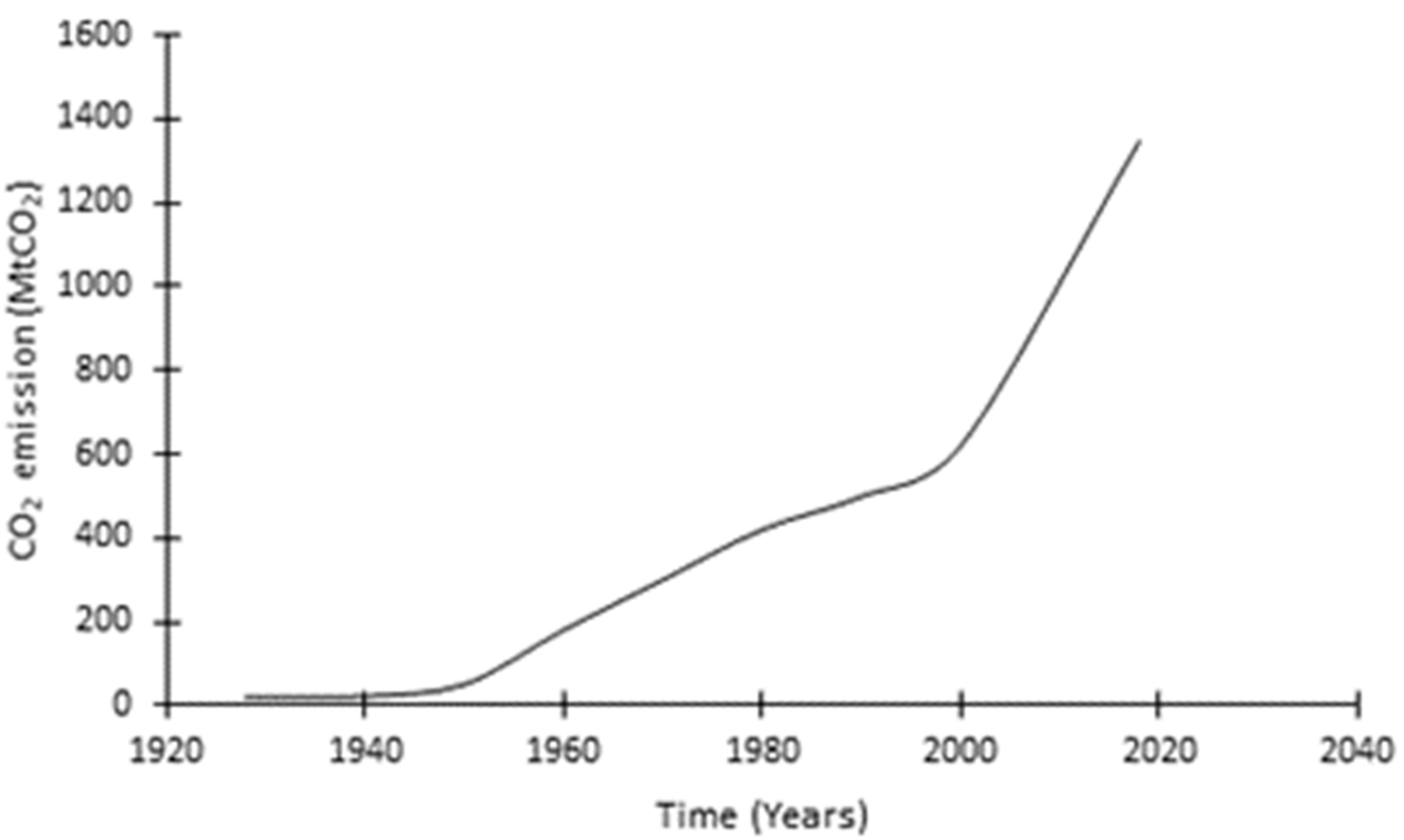
:1. Introduction
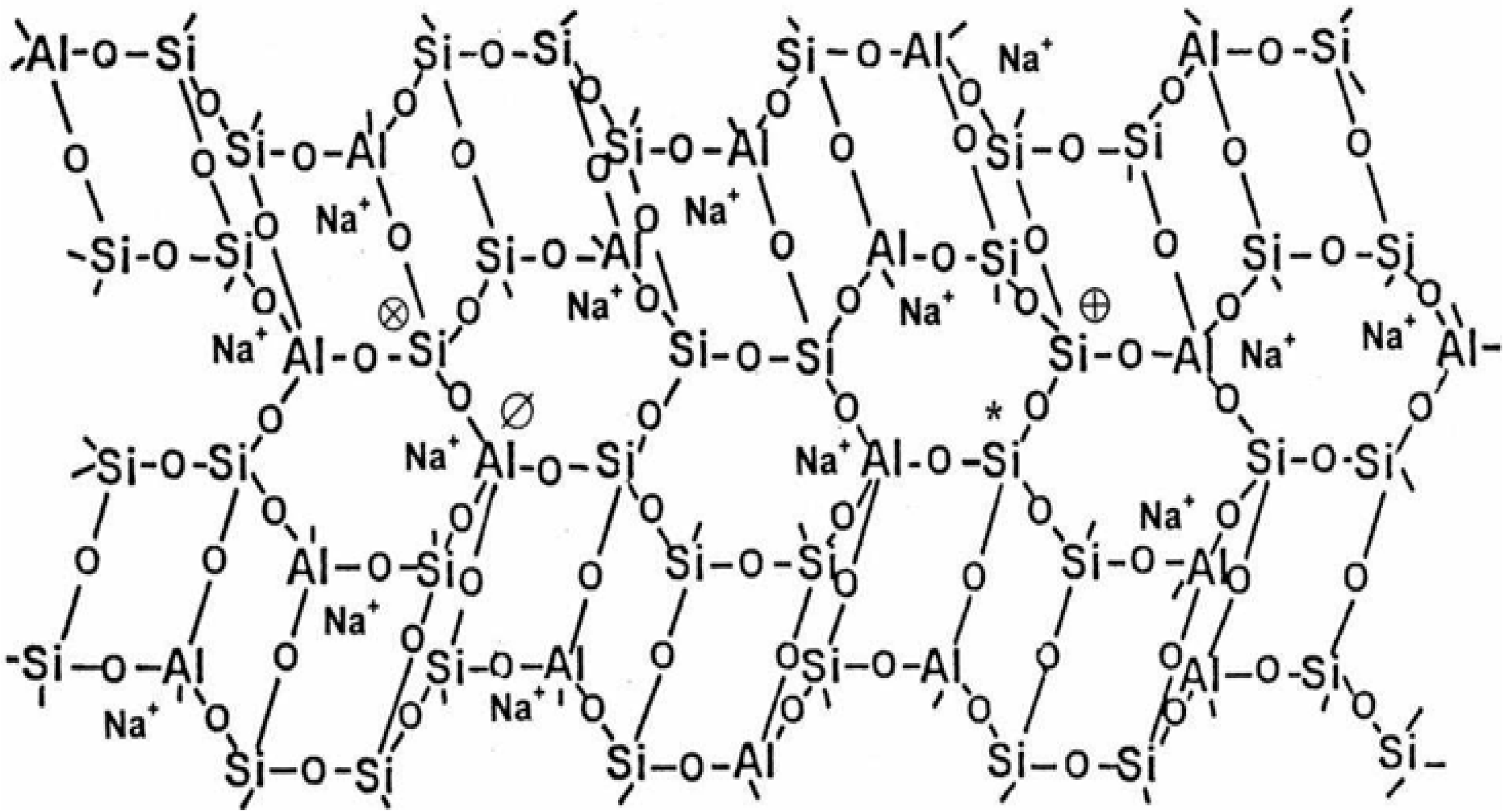
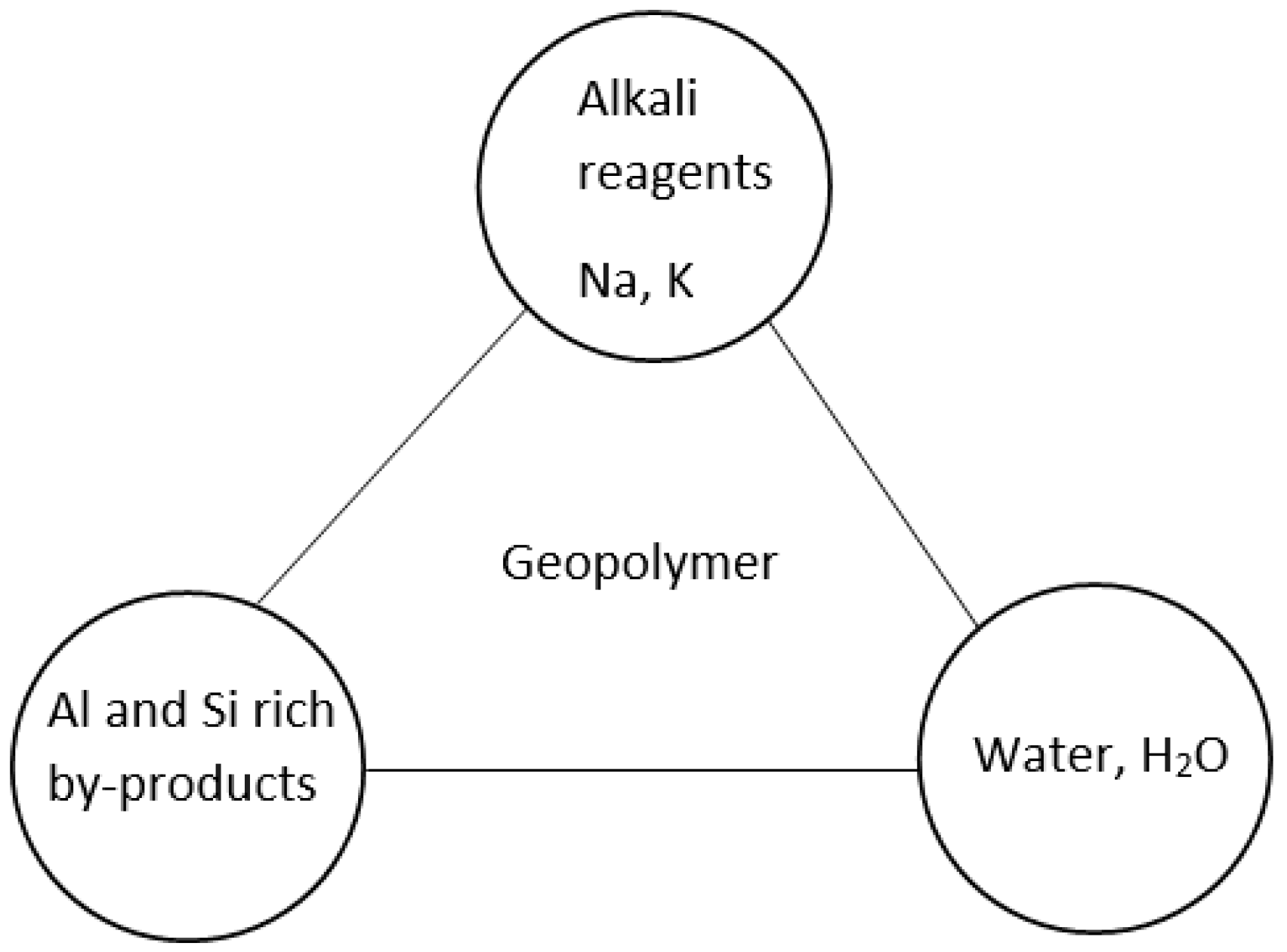
2. Geopolymerisation
2.1. Activator Liquid
2.2. Geopolymer Precursors
2.2.1. Pulverised Fuel Ash-Based Geopolymers
2.2.2. Metakaolin-Based Geopolymers
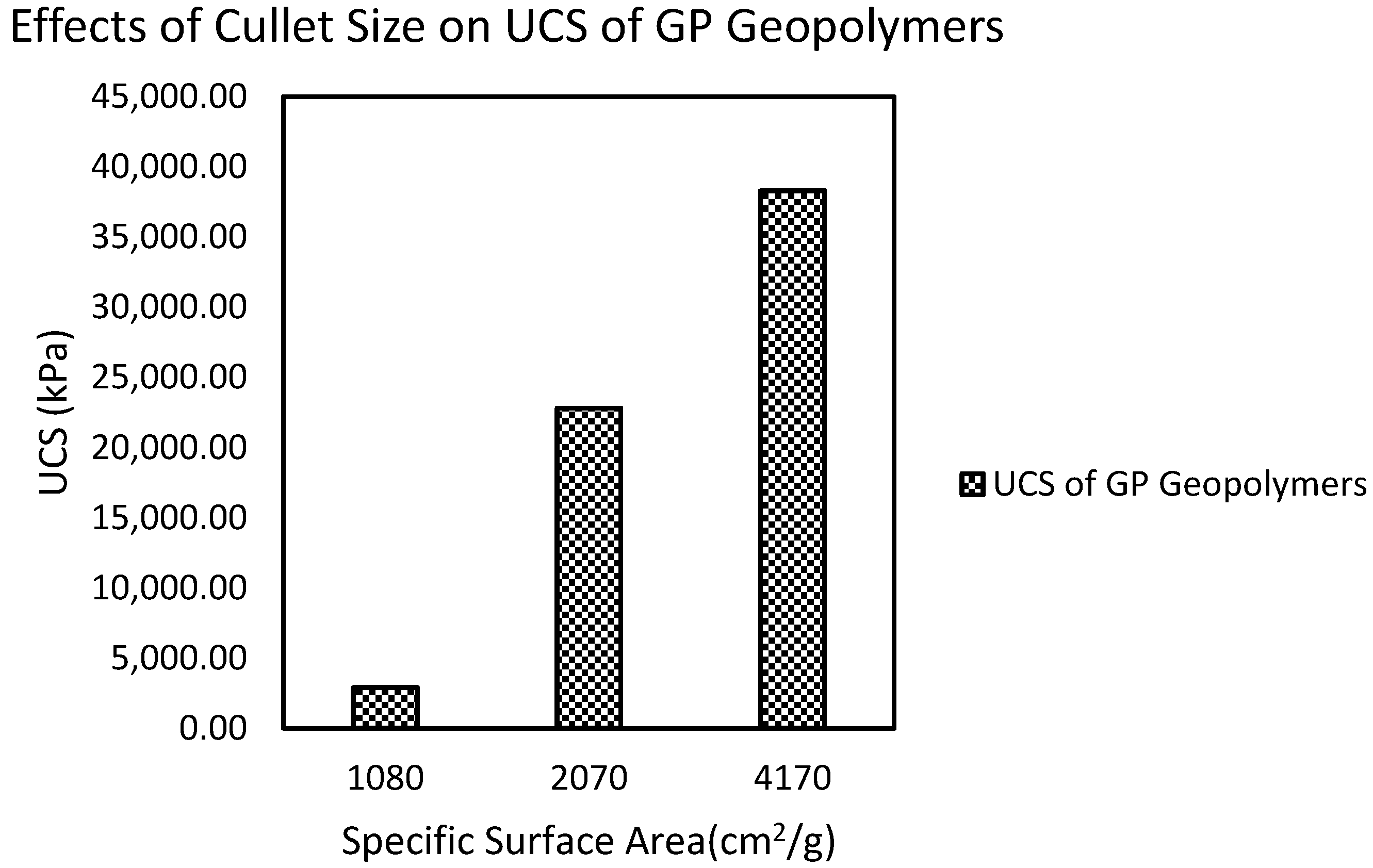
2.2.3. GGBS and Glass Powder-Based Geopolymers
2.2.4. Palm Oil Fuel Ash and Volcanic Ash-Based Geopolymers
3. Discussions
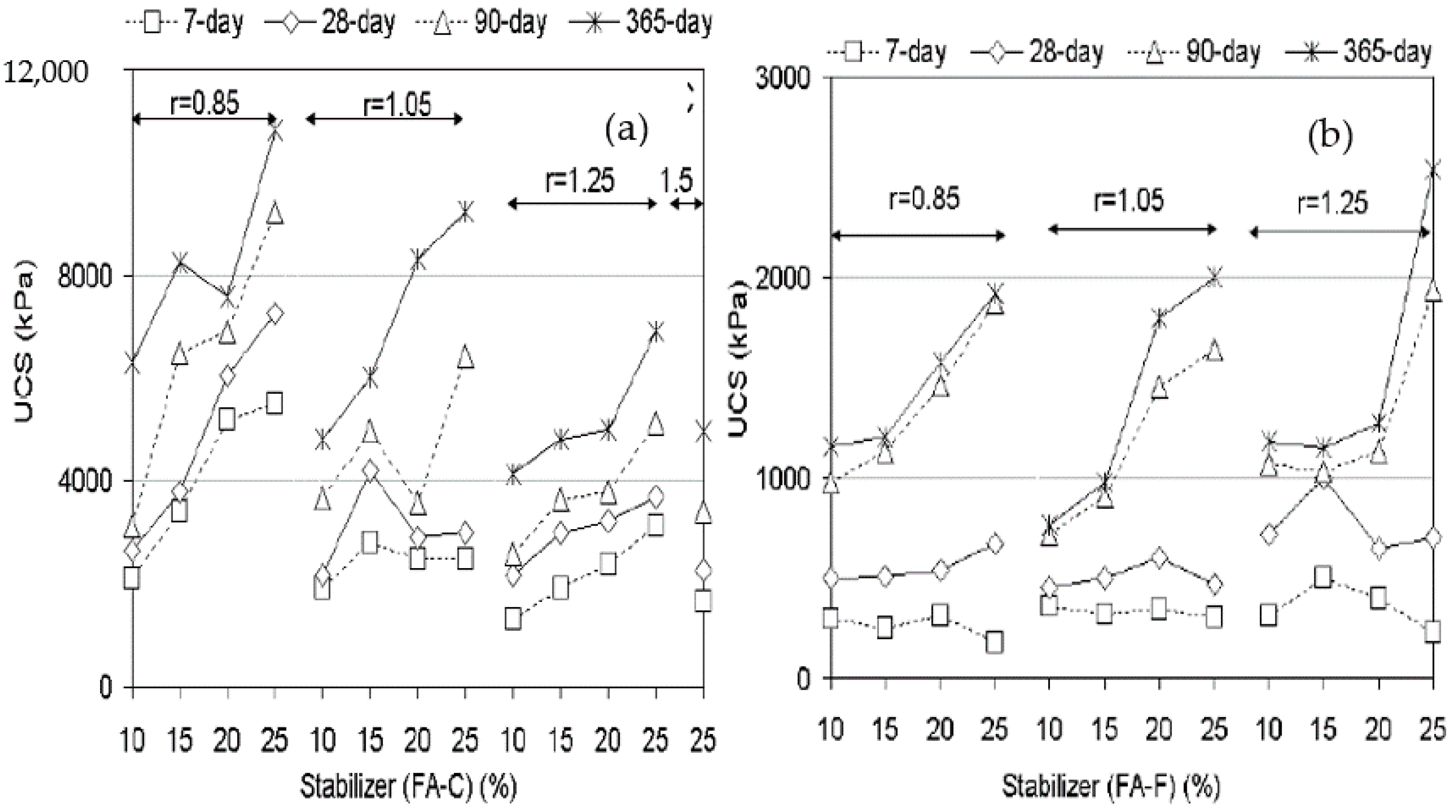
3.1. UCS of Geopolymer Stabilised Clay
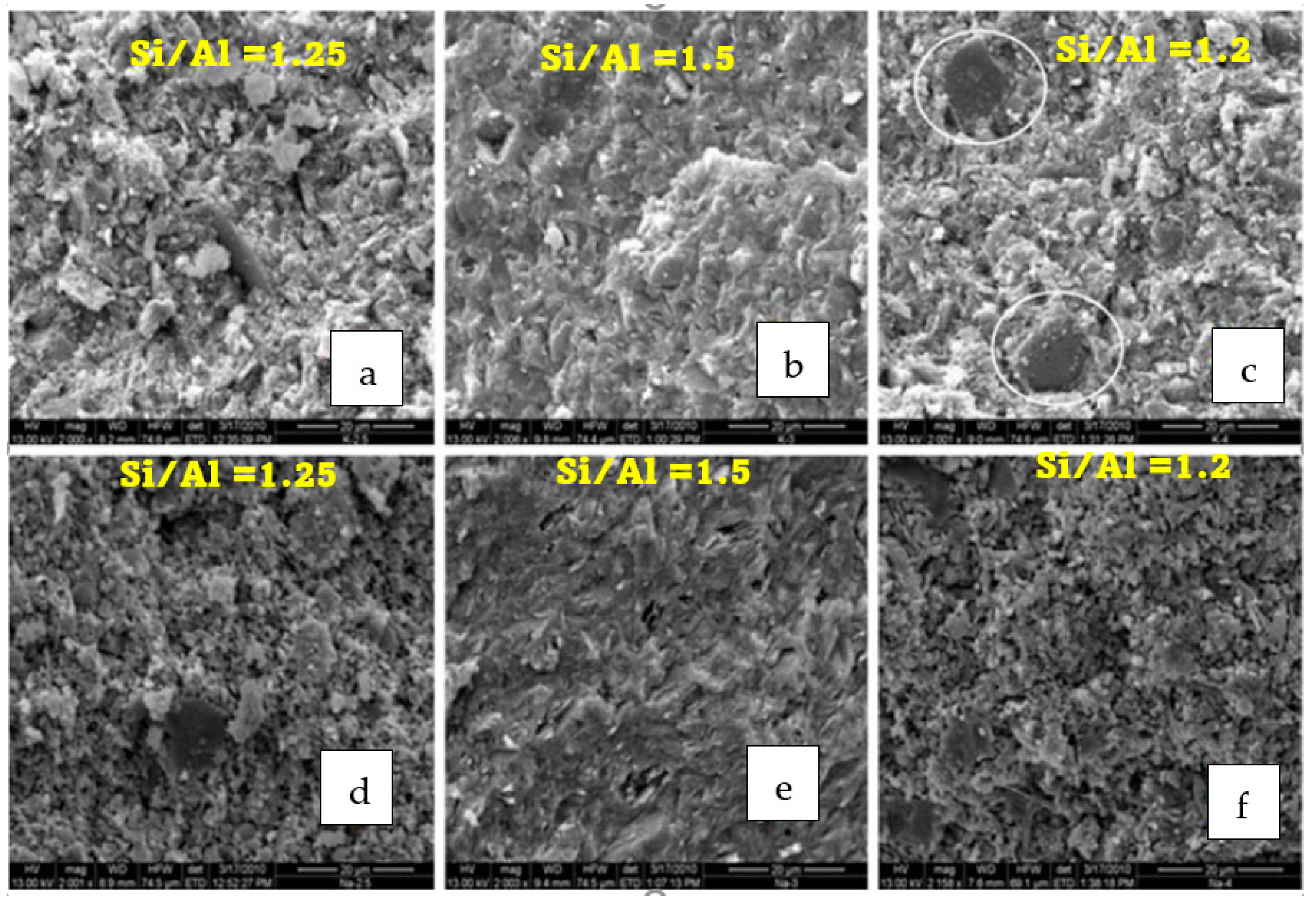
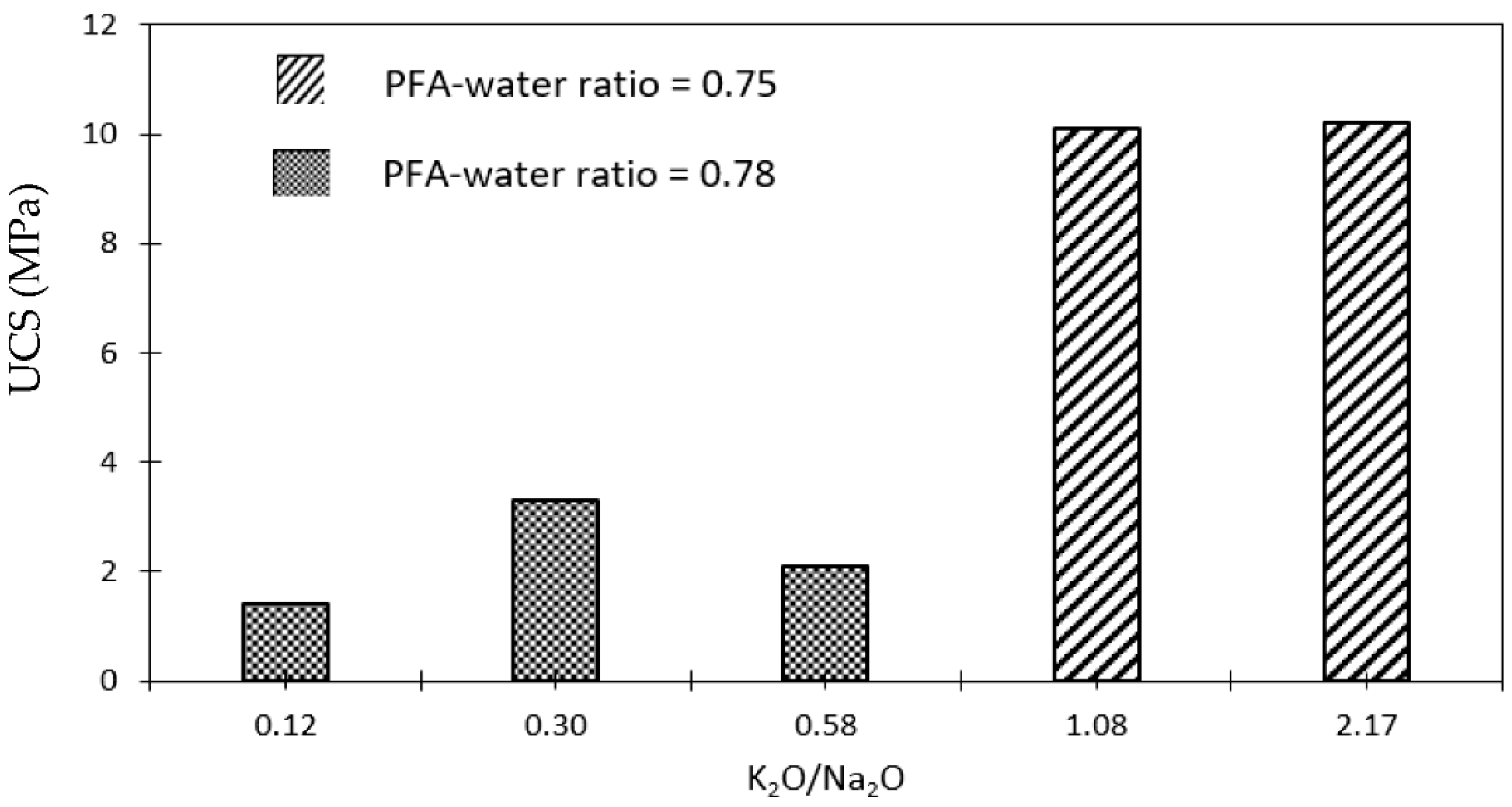
3.2. Effects of Alkali Activator Type on Microstructure and Strength of Geopolymers Binders
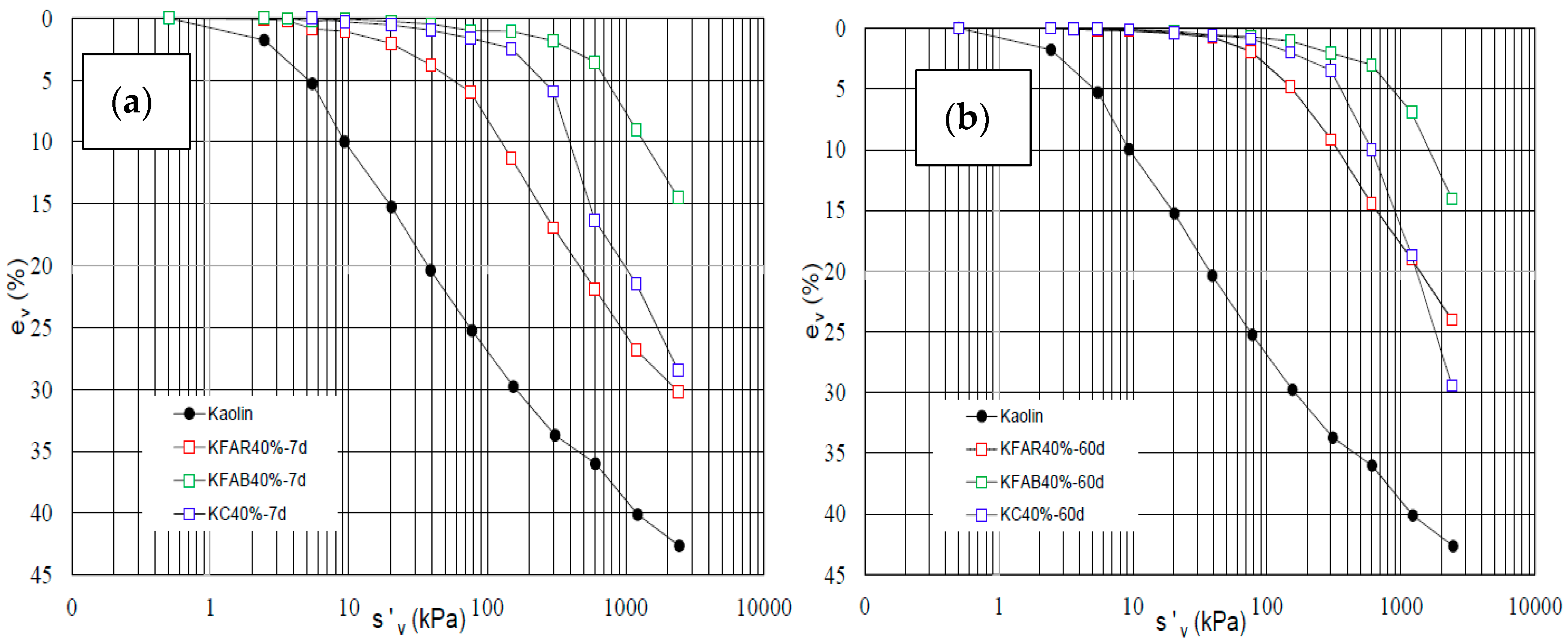
3.3. Durability of Geopolymers Stabilised Clays
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdullah, H.H.; Shahin, M.A.; Walske, M.L. Review of Fly-Ash-Based Geopolymers for Soil Stabilisation with Special Reference to Clay. Geosciences 2020, 10, 249. [Google Scholar] [CrossRef]
- Ayeldeen, M.; Hara, Y.; Kitazume, M.; Negm, A. Unconfined Compressive Strength of Compacted Disturbed Cement-Stabilized Soft Clay. Int. J. Geosynth. Ground Eng. 2016, 2, 28. [Google Scholar] [CrossRef] [Green Version]
- Pongsivasathit, S.; Horpibulsuk, S.; Piyaphipat, S. Assessment of mechanical properties of cement stabilized soils. Case Stud. Constr. Mater. 2019, 11, e00301. [Google Scholar] [CrossRef]
- Horpibulsuk, S.K.; Rachan, R.L.; Chinkulkijniwat, A.; Raksachon, Y.; Suddeepong, A. Analysis of strength development in cement-stabilized silty clay from microstructural considerations. Constr. Build. Mater. 2010, 24, 2011–2021. [Google Scholar] [CrossRef]
- Miyazaki, Y. A Study of Cement Treating Dredged Clay and under-Water Casting Method. Ph.D. Thesis, Kyushu University, Fukuoka, Japan, 2003. [Google Scholar]
- Journals, I.; Muhmed, A.; Wanatowski, D. Effect of Lime Stabilisation on the Strength and Microstructure of Clay. IOSR J. Mech. Civil Eng. (IOSR-JMCE) 2013, 6, 87–94. [Google Scholar]
- Baldovino, J.A.; Moreira, E.B.; Teixeira, W.; Izzo, R.L.; Rose, J.L. Effects of lime addition on geotechnical properties of sedimentary soil in Curitiba, Brazil. J. Rock Mech. Geotech. Eng. 2018, 10, 188–194. [Google Scholar] [CrossRef]
- Ghobadi, M.H.; Abdilor, Y.; Babazadeh, R. Stabilization of clay soils using lime and effect of pH variations on shear strength parameters. Bull. Int. Assoc. Eng. Geol. 2014, 73, 611–619. [Google Scholar] [CrossRef]
- Abbey, S.J.; Ngambi, S.; Coakley, E. Effect of cement and by-product material inclusion on plasticity of deep mixing im-proved soils. Int. J. Civil Eng. Technol. 2016, 7, 265–274. [Google Scholar]
- Abbey, S.J.; Ngambi, S.; Ganjian, E. Development of strength models for prediction of unconfined compressive strength of cement/by-product material improved soils. Geotech. Test J. 2017, 40, 928–935. [Google Scholar] [CrossRef]
- Abbey, S.J.; Eyo, E.U.; Oti, J.; Amakye, S.Y.; Ngambi, S. Mechanical Properties and Microstructure of Fibre-Reinforced Clay Blended with By-Product Cementitious Materials. Geosciences 2020, 10, 241. [Google Scholar] [CrossRef]
- Abbey, S.J.; Ngambi, S.; Olubanwo, A.O.; Tetteh, F.K. Strength and Hydraulic Conductivity of Cement and By–Product Cementitious Materials Improved Soil. Int. J. Appl. Eng. Res. 2018, 13, 8684–8694. [Google Scholar]
- Eyo, E.U.; Ng’Ambi, S.; Abbey, S.J. Performance of clay stabilized by cementitious materials and inclusion of zeo-lite/alkaline metals-based additive. Transp. Geotech. 2020, 23, 100330. [Google Scholar] [CrossRef]
- Jeremiah, J.J.; Abbey, S.J.; Booth, C.A.; Kashyap, A. Results of Application of Artificial Neural Networks in Predicting Geo-Mechanical Properties of Stabilised Clays—A Review. Geotechnics 2021, 1, 8. [Google Scholar] [CrossRef]
- Pourakbar, S.; Huat, B.K. A review of alternatives traditional cementitious binders for engineering improvement of soils. Int. J. Geotech. Eng. 2016, 11, 1–11. [Google Scholar] [CrossRef]
- Horpibulsuk, S.; Phetchuay, C.; Chinkulkijniwat, A.; Cholaphatsorn, A. Strength development in silty clay stabilized with calcium carbide residue and fly ash. Soils Found. 2013, 53, 477–486. [Google Scholar] [CrossRef] [Green Version]
- Wong, B.Y.F.; Wong, K.S.; Phang, I.R.K. A review on geopolymerisation in soil stabilization. IOP Conf. Series: Mater. Sci. Eng. 2019, 495, 012070. [Google Scholar] [CrossRef]
- Andrew, R.M. Global CO2 emissions from cement production, 1928–2018. Earth Syst. Sci. Data 2019, 11, 1675–1710. [Google Scholar] [CrossRef] [Green Version]
- Khale, D.; Chaudhary, R. Mechanism of geopolymerization and factors influencing its development: A review. J. Mater. Sci. 2007, 42, 729–746. [Google Scholar] [CrossRef]
- Davidovits, J. Solid phase synthesis of a mineral block polymer by low temperature polycondensation of alumino-silicate polymers. In Proceedings of the IUPAC International Symposium on Macromolecules, The Royal Institute of Technology, Stockholm, Sweden, 30 August–1 September 1976. Stockholm, Topic III, New Polymers of High Stability. [Google Scholar]
- Nergis, D.D.B.; Abdullah, M.M.A.B.; Vizureanu, P.; Tahir, M.F.M. Geopolymers and Their Uses: Review. IOP Conf. Series: Mater. Sci. Eng. 2018, 374, 012019. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymer Chemistry and Sustainable Development. The Poly(Sialate) Terminology: A Very Useful and Simple Model for the Promotion and Understanding of Green-Chemistry; Geopolymer Institute: Saint-Quentin, France, 2005. [Google Scholar]
- Bayuaji, R.; Yasin, A.K.; Susanto, T.E.; Darmawan, M.S. A review in geopolymer binder with dry mixing method (geopolymer cement). AIP Conf. Proc. 2017, 1887, 020022. [Google Scholar] [CrossRef] [Green Version]
- Mozumder, R.A.; Laskar, A.I. Prediction of unconfined compressive strength of geopolymer-stabilised clayey soils using artificial neural network. Comput. Geotech. 2015, 69, 291–300. [Google Scholar] [CrossRef]
- Lopes, A.V.; Lopes, S.M.R.; Pinto, I. Influence of the Composition of the Activator on Mechanical Characteristics of a Geopolymer. Appl. Sci. 2020, 10, 3349. [Google Scholar] [CrossRef]
- Van Deventer, J.; Provis, J.; Duxson, P.; Lukey, G. Reaction mechanisms in the geopolymeric conversion of inorganic waste to useful products. J. Hazard. Mater. 2007, 139, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Phetchuay, C.; Horpibulsuk, S.; Arulrajah, A.; Suksiripattanapong, C.; Udomchai, A. Strength development in soft marine clay stabilized by fly ash and calcium carbide residue based geopolymer. Appl. Clay Sci. 2016, 127–128, 134–142. [Google Scholar] [CrossRef]
- Damilola, O.M. Syntheses, Characterization and Binding Strength of Geopolymers: A Review. Int. J. Mater. Sci. Appl. 2013, 2, 185. [Google Scholar] [CrossRef] [Green Version]
- Palomoa, A.; Grutzeck, M.W.; Blanco, M.T. Alkali-activated fly ashes: A cement for the future. Cem. Concr. Res. 1999, 29, 1323–1329. [Google Scholar] [CrossRef]
- Rees, C.; Lukey, G.C.; Van Deventer, J.S.J. The role of solid silicates on the formation of geopolymers derived from coal ash. In Proceedings of the International Symposium of Research Student on Material Science and Engineering, Chennai, India, 20–22 December 2004; pp. 1–13. [Google Scholar]
- Jae, E.O.; Paulo, J.M.; Monteiro, S.S.; Jun, S.C.; Simon, M.C. The Evolution of Strength and Crystalline Phases for Alkali-activated Ground Blast Furnace Slag and Fly Ash-based Geopolymers. Cem. Concr. Res. 2010, 40, 189–196. [Google Scholar]
- Altan, E.; Erdoğan, S.T. Alkali activation of a slag at ambient and elevated temperatures. Cem. Concr. Compos. 2012, 34, 131–139. [Google Scholar] [CrossRef]
- Granizo, M.L.; Blanco, M.T. Alkaline activation of metakaolin an isothermal conduction calorimetry study. J. Ther. Anal. 1998, 52, 957–965. [Google Scholar] [CrossRef]
- Davidovits, J. Chemistry of Geopolymeric Systems, Terminology. In Proceedings of the Second International Conference Geopolumère, Saint-Quentin, France, 30 June–2 July 1999; pp. 9–40. [Google Scholar]
- Habert, G.; Roussel, N. Study of two concrete mix-design strategies to reach carbon mitigation objectives. Cem. Concr. Compos. 2009, 31, 397–402. [Google Scholar] [CrossRef]
- Chungsangunsit, T.; Gheewala, S.H.; Patumsawad, S. Emission Assessment of Rice Husk Combustion for Power Production. World Acad. Sci. Eng. Technol. 2009, 29, 1064–1069. [Google Scholar]
- Turner, L.K.; Collins, F.G. Carbon dioxide equivalent (CO2-e) emissions: A comparison between geopolymer and OPC cement concrete. Constr. Build. Mater. 2013, 43, 125–130. [Google Scholar] [CrossRef]
- Azad, N.; Samarakoon, S. Utilization of Industrial By-Products/Waste to Manufacture Geopolymer Cement/Concrete. Sustainability 2021, 13, 873. [Google Scholar] [CrossRef]
- Vitale, E.; Russo, G.; Dell’Agli, G.; Ferone, C.; Bartolomeo, C. Mechanical Behaviour of Soil Improved by Alkali Activated Binders. Environments 2017, 4, 80. [Google Scholar] [CrossRef] [Green Version]
- Ghadir, P.; Ranjbar, N. Clayey soil stabilization using geopolymer and Portland cement. Constr. Build. Mater. 2018, 188, 361–371. [Google Scholar] [CrossRef]
- Canakci, H.; Güllü, H.; Alhashemy, A. Performances of Using Geopolymers Made with Various Stabilizers for Deep Mixing. Materials 2019, 12, 2542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdullah, H.H.; Shahin, M.A.; Walske, M.L.; Karrech, A. Cyclic behaviour of clay stabilised with fly-ash based geopolymer incorporating ground granulated slag. Transp. Geotech. 2021, 26, 100430. [Google Scholar] [CrossRef]
- Phummiphan, I.; Horpibulsuk, S.; Sukmak, P.; Chinkulkijniwat, A.; Arulrajah, A.; Shen, S.-L. Stabilisation of marginal lateritic soil using high calcium fly ash-based geopolymer. Road Mater. Pavement Des. 2016, 17, 877–891. [Google Scholar] [CrossRef]
- Cristelo, N.; Glendinning, S.; Pinto, A.T. Deep soft soil improvement by alkaline activation. Proc. Inst. Civ. Eng.-Ground Improv. 2011, 164, 73–82. [Google Scholar] [CrossRef] [Green Version]
- Sukmak, P.; Horpibulsuk, S.; Shen, S.-L. Strength development in clay–fly ash geopolymer. Constr. Build. Mater. 2013, 40, 566–574. [Google Scholar] [CrossRef]
- Yaghoubi, M.; Arulrajah, A.; Disfani, M.M.; Horpibulsuk, S.; Leong, M. Compressibility and strength development of geopolymer stabilized columns cured under stress. Soils Found. 2020, 60, 1241–1250. [Google Scholar] [CrossRef]
- Phetchuay, C.; Horpibulsuk, S.; Suksiripattanapong, C.; Chinkulkijniwat, A.; Arulrajah, A.; Disfani, M.M. Calcium carbide residue: Alkaline activator for clay–fly ash geopolymer. Constr. Build. Mater. 2014, 69, 285–294. [Google Scholar] [CrossRef]
- Coudert, E.; Paris, M.; Deneele, D.; Russo, G.; Tarantino, A. Use of alkali activated high-calcium fly ash binder for kaolin clay soil stabilisation: Physicochemical evolution. Constr. Build. Mater. 2019, 201, 539–552. [Google Scholar] [CrossRef] [Green Version]
- Abdullah, H.H.; Shahin, M.A.; Walske, M.L. Geo-mechanical behavior of clay soils stabilized at ambient temperature with fly-ash geopolymer-incorporated granulated slag. Soils Found. 2019, 59, 1906–1920. [Google Scholar] [CrossRef]
- Cristelo, N.; Glendinning, S.; Fernandes, L.S.G.; Pinto, A.T. Effect of calcium content on soil stabilisation with alkaline activation. Constr. Build. Mater. 2012, 29, 167–174. [Google Scholar] [CrossRef]
- Araújo, N.; Corrêa-Silva, M.; Miranda, T.; Gomes, A.T.; Castro, F.; Teixeira, T.; Cristelo, N. Unsaturated Response of Clayey Soils Stabilised with Alkaline Cements. Molecules 2020, 25, 2533. [Google Scholar] [CrossRef]
- Alsafi, S.; Farzadnia, N.; Asadi, A.; Huat, B.K. Collapsibility potential of gypseous soil stabilized with fly ash geopolymer; characterization and assessment. Constr. Build. Mater. 2017, 137, 390–409. [Google Scholar] [CrossRef]
- Zhang, M.; Guo, H.; El-Korchi, T.; Zhang, G.; Tao, M. Experimental feasibility study of geopolymer as the next-generation soil stabilizer. Constr. Build. Mater. 2013, 47, 1468–1478. [Google Scholar] [CrossRef]
- Guo, Q.; Wei, M.; Wu, H.; Gu, Y. Strength and micro-mechanism of MK-blended alkaline cement treated high plasticity clay. Constr. Build. Mater. 2019, 236, 117567. [Google Scholar] [CrossRef]
- Samuel, R.; Puppala, A.; Radovic, M. Sustainability Benefits Assessment of Metakaolin-Based Geopolymer Treatment of High Plasticity Clay. Sustainability 2020, 12, 10495. [Google Scholar] [CrossRef]
- Wang, S.; Xue, Q.; Zhu, Y.; Li, G.; Wu, Z.; Zhao, K. Experimental study on material ratio and strength performance of geopolymer-improved soil. Constr. Build. Mater. 2021, 267, 120469. [Google Scholar] [CrossRef]
- Žiūrinkas, D.; Vaiciukyniene, D.; Stelmokaitis, G.; Dorosevas, V. Clayey Soil Strength Improvement by Using Alkali Activated Slag Reinforcing. Minerals 2020, 10, 1076. [Google Scholar] [CrossRef]
- Bilondi, M.P.; Toufigh, M.M.; Toufigh, V. Experimental investigation of using a recycled glass powder-based geopolymer to improve the mechanical behavior of clay soils. Constr. Build. Mater. 2018, 170, 302–313. [Google Scholar] [CrossRef]
- Bilondi, M.P.; Toufigh, M.M.; Toufigh, V. Using calcium carbide residue as an alkaline activator for glass powder–clay geopolymer. Constr. Build. Mater. 2018, 183, 417–428. [Google Scholar] [CrossRef]
- Xiao, R.; Polaczyk, P.; Zhang, M.; Jiang, X.; Zhang, Y.; Huang, B.; Hu, W. Evaluation of Glass Powder-Based Geopolymer Stabilized Road Bases Containing Recycled Waste Glass Aggregate. Natl. Acad. Sci. Transp. Res. Rec. 2020, 2674, 22–32. [Google Scholar] [CrossRef]
- Cyr, M.; Idir, R.; Poinot, T. Properties of inorganic polymer (geopolymer) mortars made of glass cullet. J. Mater. Sci. 2012, 47, 2782–2797. [Google Scholar] [CrossRef]
- Hamada, H.M.; Jokhio, G.A.; Yahaya, F.M.; Humada, A.M.; Gul, Y. The present state of the use of palm oil fuel ash (POFA) in concrete. Constr. Build. Mater. 2018, 175, 26–40. [Google Scholar] [CrossRef]
- Abdeldjouad, L. Effect of Curing Temperature on the Development of Hard Structure of Alkali-Activated Soil. Int. J. GEOMATE 2019, 17, 117–123. [Google Scholar] [CrossRef]
- Phummiphan, P.I.; Horpibulsuk, P.S.; Phoo-Ngernkham, T.; Arulrajah, P.A.; Shen, S.-L. Marginal Lateritic Soil Stabilized with Calcium Carbide Residue and Fly Ash Geopolymers as a Sustainable Pavement Base Material. J. Mater. Civ. Eng. 2017, 29, 04016195. [Google Scholar] [CrossRef]
- European Committee for Standardization (CEN). EN 16907-4:2018: Earthworks 2018 Soil Treatment with Lime And/or Hydraulic Binders; European Committee for Standardization (CEN): Brussels, Belgium, 2018. [Google Scholar]
- Eyo, E.; Ng’Ambi, S.; Abbey, S.J. Incorporation of a nanotechnology-based additive in cementitious products for clay stabilisation. J. Rock Mech. Geotech. Eng. 2020, 12, 1056–1069. [Google Scholar] [CrossRef]
- Abbey, S.J.; Eyo, E.U.; Okeke, C.A.; Ngambi, S. Experimental study on the use of RoadCem blended with by-product cementitious materials for stabilisation of clay soils. Constr. Build. Mater. 2021, 280, 122476. [Google Scholar] [CrossRef]
- Gross, J.; Adaska, W. Guide to Cement-Stabilized Subgrade Soils; Portland Cement Association: Washington, DC, USA; National Concrete Pavement Technology Center, Iowa State University: Ames, IA, USA, 2020. [Google Scholar]
- Van Jaarsveld, J.G.S.; van Deventer, J.S.J. Effect of the Alkali Metal Activator on the Properties of Fly Ash-Based Geopolymers. Ind. Eng. Chem. Res. 1999, 38, 3932–3941. [Google Scholar] [CrossRef]
- Bakri, A.M.M.A.; Kamarudin, H.; Omar, V.; Norazian, M.N.; Ruzaidi, C.M.; Rafiza, A.R. Effects of Alkali Activator Ratio on the Compressive Strength of Fly Ash-Based Geopolymers. Austrialian J. Basic Appl. Sci. 2011, 5, 1916–1922. [Google Scholar]
- Lizcano, M.; Kim, H.S.; Basu, S.; Radovic, M. Mechanical properties of sodium and potassium activated metakaolin-based geopolymers. J. Mater. Sci. 2012, 47, 2607–2616. [Google Scholar] [CrossRef]
- McCormick, A.V.; Bell, T. The Solution Chemistry of Zeolite Precursors. Catal. Rev. Sci. Eng. 1989, 31, 97–127. [Google Scholar] [CrossRef]
- Weldes, H.H.; Lange, K.R. Properties of Soluble Silicates. Ind. Eng. Chem. 1969, 61, 29–44. [Google Scholar] [CrossRef]
- Salimi, M.; Ghorbani, A. Mechanical and compressibility characteristics of a soft clay stabilized by slag-based mixtures and geopolymers. Appl. Clay Sci. 2020, 184, 105390. [Google Scholar] [CrossRef]
- Abbey, S.J.; Eyo, E.U.; Ng’Ambi, S. Swell and microstructural characteristics of high-plasticity clay blended with cement. Bull. Int. Assoc. Eng. Geol. 2019, 79, 2119–2130. [Google Scholar] [CrossRef] [Green Version]
- Leong, H.Y.; Ong, D.E.L.; Sanjayan, J.; Nazari, A. Suitability of Sarawak and Gladstone fly ash to produce geopolymers: A physical, chemical, mechanical, mineralogical and microstructural analysis. Ceram. Int. 2016, 42, 9613–9620. [Google Scholar] [CrossRef] [Green Version]
- Abdullah, H.H.; Shahin, M.A.; Sarker, P. Use of Fly-Ash Geopolymer Incorporating Ground Granulated Slag for Stabilisation of Kaolin Clay Cured at Ambient Temperature. Geotech. Geol. Eng. 2019, 37, 721–740. [Google Scholar] [CrossRef]
- Jiang, N.-J.; Du, Y.-J.; Liu, K. Durability of lightweight alkali-activated ground granulated blast furnace slag (GGBS) stabilized clayey soils subjected to sulfate attack. Appl. Clay Sci. 2018, 161, 70–75. [Google Scholar] [CrossRef]
- Kwasny, T.A.; Soutsos, A.M.N.; McIntosh, J.A.; Cleland, D.J. Sulfate and acid resistance of lithomarge-based geopolymer mortars. Constr. Build. Mater. 2018, 166, 537–553. [Google Scholar] [CrossRef] [Green Version]
- Bakharev, T. Durability of geopolymer materials in sodium and magnesium sulfate solutions. Cem. Concr. Res. 2005, 35, 1233–1246. [Google Scholar] [CrossRef]



| Parameter | Geopolymer | OPC |
|---|---|---|
| Energy consumption (calcination and crushing) | 990 × 106 J/ton * | 3430 × 106 J/ton ** |
| Carbon emission | Low (169 kg CO2/m3) ** | High (306 kg CO2/m3) * |
| Environmental impact | Alternative waste management solution | Release of cement kiln dust (CKD) |
| Major raw material | Industrial and agricultural wastes. | Limestone, shale, rocks etc. |
| Thermal characteristics | Higher resistance to high temperatures | Lower resistance to high temperatures |
| Chemical process | Geopolymerisation | Hydration |
| Calcium requirement | Not necessarily | Required |
| Resultant cementing compound | Sodium or potassium aluminate-silicate hydrates (Na, K, Li, Ca-A-S-H) | Calcium silicate hydrate (C-S-H) and calcium aluminate hydrate (C-A-H) |
| Carbonation effects | Results in lowered pH (10–10.5) which still resists corrosion of steel reinforcement ** | Results in lower Ph (7–8) leading to increased rate of corrosion of steel reinforcement ** |
| Alkali-aggregate reaction (AAR) | No ** | Yes ** |
| Chemical Compound | OPC | FA-F | FA-C | GGBS | BA | RHA | GP | SF | MP | VA |
|---|---|---|---|---|---|---|---|---|---|---|
| CaO | 62.58 | 4.24 | 23.9 | 36.42 | 6.13 | 1.14 | 8.21 | 1.35 | 52.45 | 19.1 |
| Al2O3 | 5.31 | 24.4 | 7.97 | 10.6 | 34.3 | 0.54 | 1.00 | 0.39 | 0.39 | 13.5 |
| Fe2O3 | 4.04 | 7.1 | 5.34 | 1.28 | 15 | 0.16 | 0.52 | 1.21 | 0.78 | 8.5 |
| SO3 | 2.73 | 0.29 | 3.03 | 0.68 | 0.9 | 0.25 | 0.06 | 1.00 | 0.076 | 0.3 |
| MgO | 2.82 | 2.4 | 0.53 | 7.63 | 1.57 | 0.5 | 0.14 | 2.23 | 0.54 | 1.7 |
| SiO2 | 20.25 | 57.2 | 18.27 | 40.4 | 39.4 | 87.2 | 78 | 92.5 | 1.29 | 46.8 |
| K2O | 0.92 | 3.37 | 1.39 | NR | 1.19 | 1.94 | 0.09 | 0.08 | 0.11 | 4.3 |
| Soil Type | Precursor | Precursor (wt.% of Soil) | Alkali Activator | Molarity | Curing Temp. (°C) | Reference | Remarks |
|---|---|---|---|---|---|---|---|
| soft marine clay | PFA (Class F) | 25–45 | NaOH and Na2SiO3 | 3–18 | 25 and 40 | [27] | Improvement UCS |
| Silt | PFA (Class F) and GGBS | 20–30 | NaOH and Na2SiO3 | 8 | [46] | Improved permeability and UCS | |
| Lateritic soil | PFA (Class C) | NR | NaOH and Na2SiO3 | 4.5–5.8 | Ambient | [64] | Improved UCS |
| Lean clay | Mk | 3–15 | NaOH and Na2SiO3 | NR | Ambient | [53] | Increased strength with no increase in MDD |
| soft soil | PFA (Class F) | 20–50 | NaOH and Na2SiO3 | 10–15 | Ambient | [44] | Increased UCS |
| Silty clay | PFA (Class F) | 30 | NaOH and Na2SiO3 | 10 | 65, 75, and 85 | [45] | Increased UCS |
| High plasticity clay | GGBS and Phosphogypsum | 6–30 | NaOH | NR | Ambient | [57] | Increased shear strength |
| Kaolin clay | PFA (Class C and F) | 20 and 40 | Na2OSiO2 | NR | Ambient | [39] | Reduced volume compressibility |
| Silty clay | PFA (Class C) | 0, 5, 10, 15, and 20 | Na2SiO3 and CCR | Na2SiO3/water ratio (0.2, 0.6, 1 and 1.4) CCR of 7% | Ambient | [47] | Reduced PI, increase in UCS |
| Kaolin clay | PFA (Class C) | 100, 50, and 20 | Na2SiO3 | NR | Ambient | [48] | Increased UCS. High calcium content reduced long-term strength |
| Low plasticity clays | GGBS, PFA, MK, MP, GP, RHA, and SF | 10 to 20 | NaOH and Na2SiO3 | 8, 10, 14, 12, and 16 | Initially at ambient and later freeze dried | [41] | Increased UCS |
| Kaolin clay | PFA (Class F) and GGBS | 10 and 20 | NaOH and Na2SiO3 | 14 | Ambient | [42] | Improved MDD, Improved yield strength under cyclic loading, higher durability. |
| high and low plasticity clays | PFA (Class F) and GGBS | 10 and 20 | NaOH and Na2SiO3 | Ambient | [49] | Improved yield strength. | |
| Clay | GP | 3–25% | NaOH | 1–8 | 25–70 | [58] | Improved UCS |
| Clay | POFA | 15–20% | KOH | 10, 12 | Ambient, 50, and 100 | [63] | Improved UCS. Higher UCS with increased temperature. |
| Clay | GP | 3–20% | CCR | 4–13% CCR by mass of dry soil | 25 °C and 70 °C | [59] | Increased UCS. UCS increased with temperatures. |
| Clay | PFA (class C and F) | 10 and 20% | NaOH and Na2SiO3 | 10 | Ambient | [50] | Improved UCS. Higher UCS values for Class F geopolymer treated samples |
| Clay | VA | 0,5, 10, and 15 | NaOH | 4, 8, and 12 | 40 | [40] | |
| High Plasticity Clay | MK and OPC | 30 | NaOH and Na2SiO3 | 1.9 and 3.2 | Ambient | [54] | Increased UCS. Reduced porosity |
| High Plasticity Clay | MK | 4, 10, and 15 | KOH, Silica fume and H2O | - | Ambient, 22 °C | [55] | Increased UCS. Reduced compressibility, swell, and shrinkage |
| Low plasticity clays | PFA (Class F) | 10, 20, and 30 | NaOH and KOH | 8, 10, and 12 | Ambient | [52] | Increased UCS. Reduced collapse potential, and permeability. |
| High plasticity clay | GGBS and PFA (Class F) | GGBS (4–50%) and PFA (4–20%) | NaOH and Na2SiO3 | 4–14.5 | Under water curing | [24] | Increased UCS |
| Clay | MK | 6–12 | CaO and NaHCO3 | 3–11 | 5 | [56] | Improved UCS. Strength reduction after optimum activator content |
| High plasticity clay | Class F PFA | 0.3 | NaOH and Na2SiO3 | 10 | 27–75 | [16] |
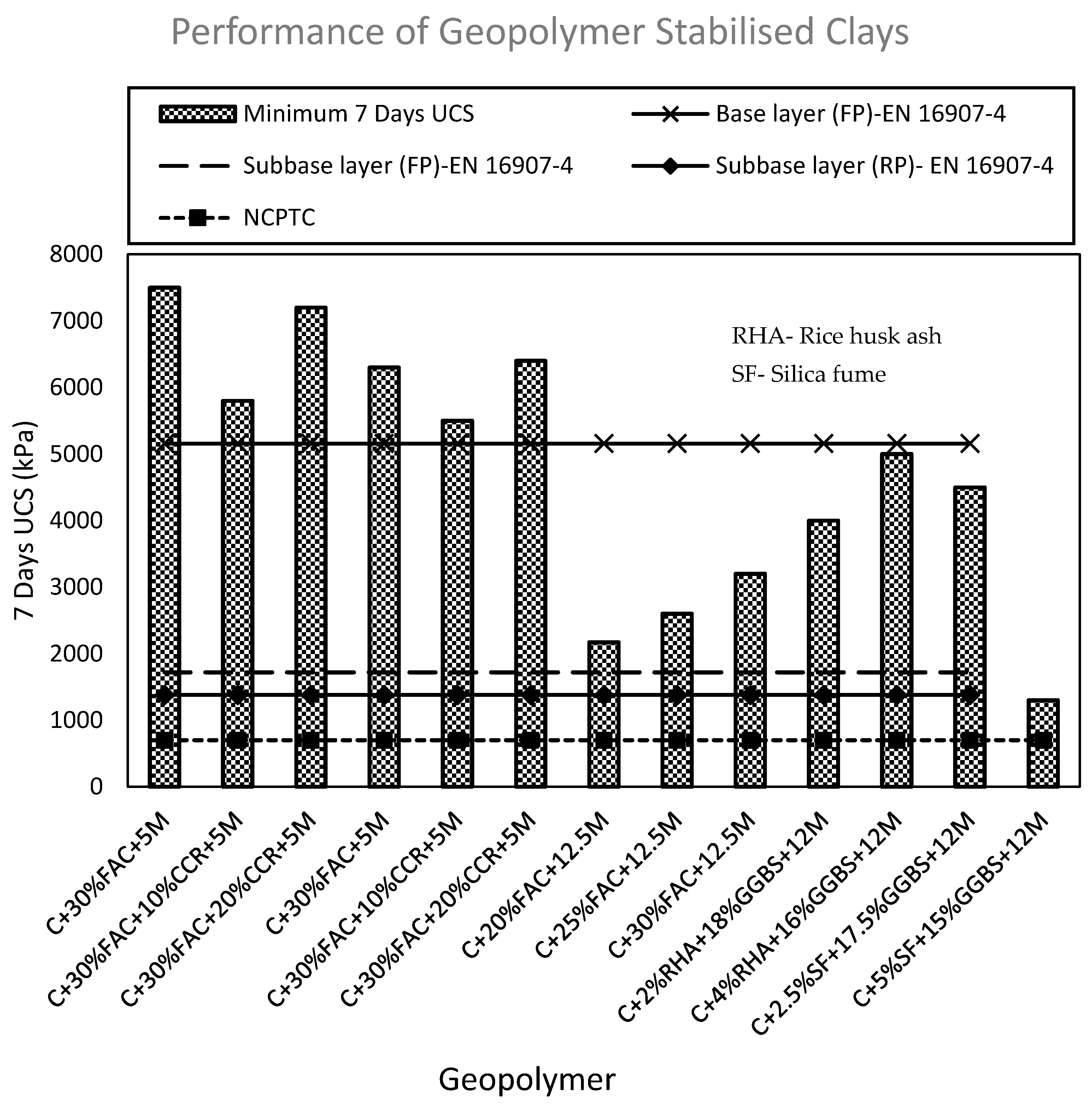
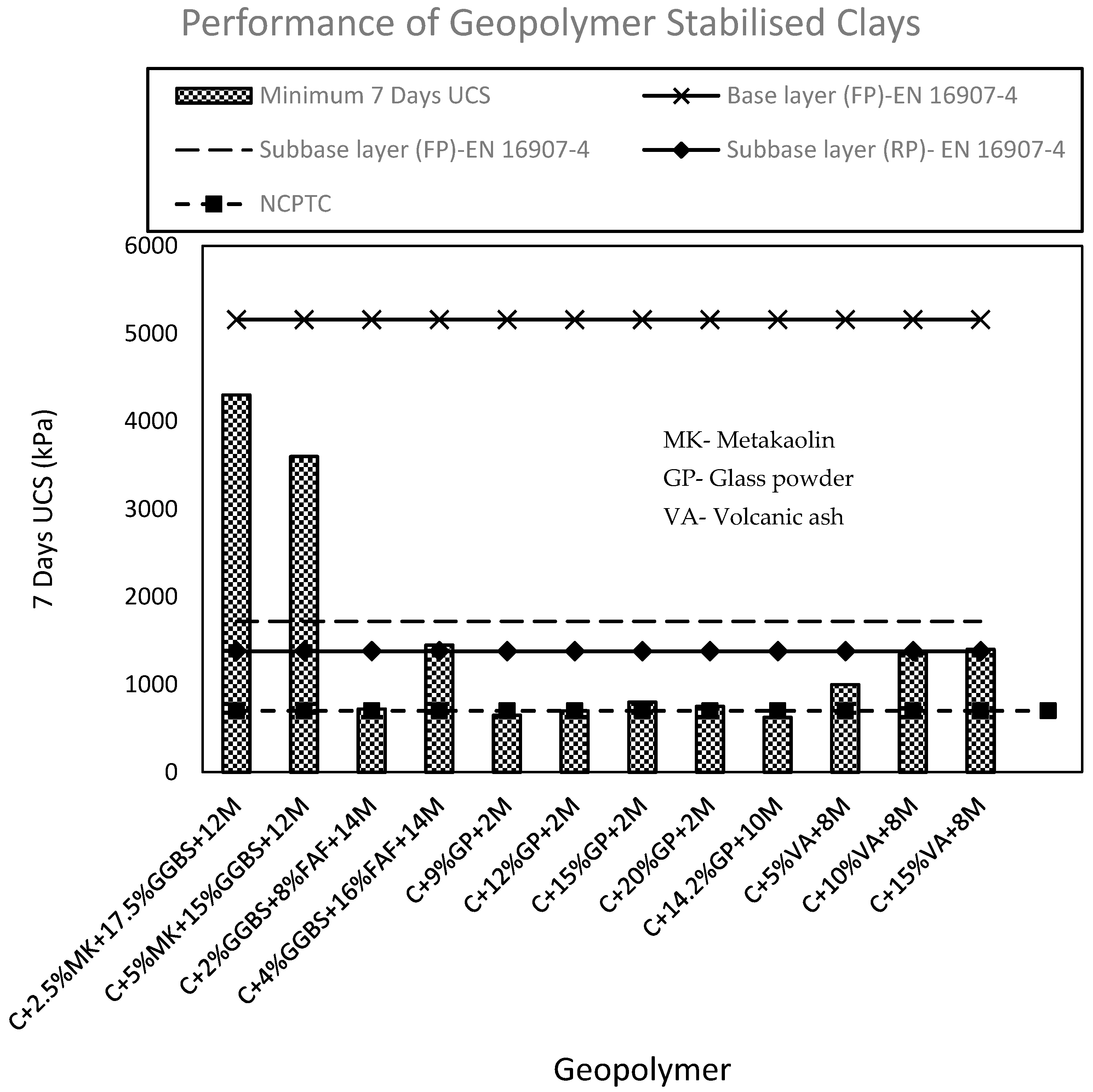
| Application | UCS (kPa) |
|---|---|
| Base layer (FP) | 5160 |
| Subbase layer (FP) | 1720 |
| Subbase layer (RP) | 1380 |
| Lower layers in high embankments (FP) | 500 |
| Lower layers in high embankments (RP) | 1000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeremiah, J.J.; Abbey, S.J.; Booth, C.A.; Kashyap, A. Geopolymers as Alternative Sustainable Binders for Stabilisation of Clays—A Review. Geotechnics 2021, 1, 439-459. https://doi.org/10.3390/geotechnics1020021
Jeremiah JJ, Abbey SJ, Booth CA, Kashyap A. Geopolymers as Alternative Sustainable Binders for Stabilisation of Clays—A Review. Geotechnics. 2021; 1(2):439-459. https://doi.org/10.3390/geotechnics1020021
Chicago/Turabian StyleJeremiah, Jeremiah J., Samuel J. Abbey, Colin A. Booth, and Anil Kashyap. 2021. "Geopolymers as Alternative Sustainable Binders for Stabilisation of Clays—A Review" Geotechnics 1, no. 2: 439-459. https://doi.org/10.3390/geotechnics1020021
APA StyleJeremiah, J. J., Abbey, S. J., Booth, C. A., & Kashyap, A. (2021). Geopolymers as Alternative Sustainable Binders for Stabilisation of Clays—A Review. Geotechnics, 1(2), 439-459. https://doi.org/10.3390/geotechnics1020021








