Rapid Serological Test for COVID-19, One-Step-COVID-2019: Accuracy and Implications for Pandemic Control
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Serological Tests
2.2.1. One-Step-COVID-2019-Test
2.2.2. ELISA Test
2.3. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mina, M.J.; Parker, R.; Larremore, D.B. Rethinking COVID-19 Test Sensitivity—A Strategy for Containment. N. Engl. J. Med. 2020, 383, e120. [Google Scholar] [CrossRef]
- Long, Q.-X.; Tang, X.-J.; Shi, Q.-L.; Li, Q.; Deng, H.-J.; Yuan, J.; Hu, J.-L.; Xu, W.; Zhang, Y.; Lv, F.-J.; et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat. Med. 2020, 26, 1200–1204. [Google Scholar] [CrossRef] [PubMed]
- Gong, F.; Wei, H.X.; Li, Q.; Liu, L.; Li, B. Evaluation and Comparison of Serological Methods for COVID-19 Diagnosis. Front. Mol. Biosci. 2021, 8, 682405. [Google Scholar] [CrossRef] [PubMed]
- Lisboa Bastos, M.; Tavaziva, G.; Abidi, S.K.; Campbell, J.R.; Haraoui, L.-P.; Johnston, J.C.; Lan, Z.; Law, S.; MacLean, E.; Trajman, A.; et al. Diagnostic accuracy of serological tests for COVID-19: Systematic review and meta-analysis. BMJ 2020, 370, m2516. [Google Scholar] [CrossRef]
- Whitman, J.D.; Hiatt, J.; Mowery, C.T.; Shy, B.R.; Yu, R.; Yamamoto, T.N.; Rathore, U.; Goldgof, G.M.; Whitty, C.; Woo, J.M.; et al. Evaluation of SARS-CoV-2 serology assays reveals a range of test performance. Nat. Biotechnol. 2020, 38, 1174–1183. [Google Scholar] [CrossRef] [PubMed]
- Meireles, A.L.; Lourenção, L.G.; de Menezes-Júnior, L.A.A.; Coletro, H.N.; Sousa Justiniano, I.C.; de Moura, S.S.; Diniz, A.P.; da Silva Sabião, T.; Sampaio Rocha, A.M.; Batista, A.P.; et al. COVID-Inconfidentes—SARS-CoV-2 seroprevalence in two Brazilian urban areas during the pandemic first wave: Study protocol and initial results. Poblac. Salud Mesoam. 2023, 21, 1–22. [Google Scholar] [CrossRef]
- WHO. Population-Based Age-Stratified Seroepidemiological Investigation Protocol for COVID-19 Virus Infection; WHO: Genewa, Switzerland, 2020.
- Raggio Luiz, R.; Magnanini, M.M.F. A Lógica Da Determinação Do Tamanho Da Amostra Em Investigações. Cad. Saude Colet. 2000, 8, 9–28. [Google Scholar]
- Cordeiro, R. Efeito do desenho em amostragem de conglomerado para estimar a distribuição de ocupações entre trabalhadores. Rev. Saude Publica 2001, 35, 10–15. [Google Scholar] [CrossRef]
- Meireles, A.L.; Xavier, C.C.; Proietti, F.A.; Caiaffa, W.T. Influence of individual and socio-environmental factors on self-rated health in adolescents. Rev. Bras. Epidemiol. 2015, 18, 538–551. [Google Scholar] [CrossRef] [PubMed]
- do Silva, P.L.N.; Pessoa, D.G.C.; Lila, M.F. Análise estatística de dados da PNAD: Incorporando a estrutura do plano amostral. Cien Saude Colet. 2002, 7, 659–670. [Google Scholar] [CrossRef]
- Instituto Brasileiro de Geografia e Estatística (IBGE). Pesquisa de Orçamentos Familiares 2017–2018: Primeiros Resultados; IBGE: Rio de Janeiro, RJ, Brazil, 2019; ISBN 9788524045059. Available online: https://biblioteca.ibge.gov.br/index.php/biblioteca-catalogo?view=detalhes&id=2101670 (accessed on 14 August 2024).
- Hallal, P.C.; Hartwig, F.P.; Horta, B.L.; Silveira, M.F.; Struchiner, C.J.; Vidaletti, L.P.; Neumann, N.A.; Pellanda, L.C.; Dellagostin, O.A.; Burattini, M.N.; et al. SARS-CoV-2 antibody prevalence in Brazil: Results from two successive nationwide serological household surveys. Lancet Glob. Health 2020, 8, e1390-8. [Google Scholar] [CrossRef] [PubMed]
- Silveira, M.F.; Barros, A.J.D.; Horta, B.L.; Pellanda, L.C.; Victora, G.D.; Dellagostin, O.A.; Struchiner, C.J.; Burattini, M.N.; Valim, A.R.M.; Berlezi, E.M.; et al. Population-based surveys of antibodies against SARS-CoV-2 in Southern Brazil. Nat. Med. 2020, 26, 1196–1199. [Google Scholar] [CrossRef] [PubMed]
- Bagno, F.F.; Sérgio, S.A.; Figueiredo, M.M.; Godoi, L.C.; Andrade, L.A.; Salazar, N.C.; Soares, C.P.; Aguiar, A.; Almeida, F.J.; da Silva, E.D.; et al. Development and validation of an enzyme-linked immunoassay kit for diagnosis and surveillance of COVID-19. J. Clin. Virol. Plus 2022, 2, 100101. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Y.P.; Lin, R.T.P.; Renia, L.; Ng, L.F.P. Serological Approaches for COVID-19: Epidemiologic Perspective on Surveillance and Control. Front. Immunol. 2020, 11, 879. [Google Scholar] [CrossRef] [PubMed]
- Anvisa. Aprovados Primeiros Testes Rápidos Para COVID-19. Agência Nacional de Vigilância Sanitária. 2020. Available online: https://www.gov.br/anvisa/pt-br/assuntos/noticias-anvisa/2020/aprovados-primeiros-testes-rapidos-para-covid-19 (accessed on 1 August 2024).
- Umemneku Chikere, C.M.; Wilson, K.; Graziadio, S.; Vale, L.; Allen, A.J. Diagnostic test evaluation methodology: A systematic review of methods employed to evaluate diagnostic tests in the absence of gold standard—An update. PLoS ONE 2019, 14, e0223832. [Google Scholar] [CrossRef] [PubMed]
- Sui, Z.; Dai, X.; Lu, Q.; Zhang, Y.; Huang, M.; Li, S.; Peng, T.; Xie, J.; Zhang, Y.; Wu, C.; et al. Viral dynamics and antibody responses in people with asymptomatic SARS-CoV-2 infection. Signal Transduct. Target. Ther. 2021, 6, 181. [Google Scholar] [CrossRef] [PubMed]
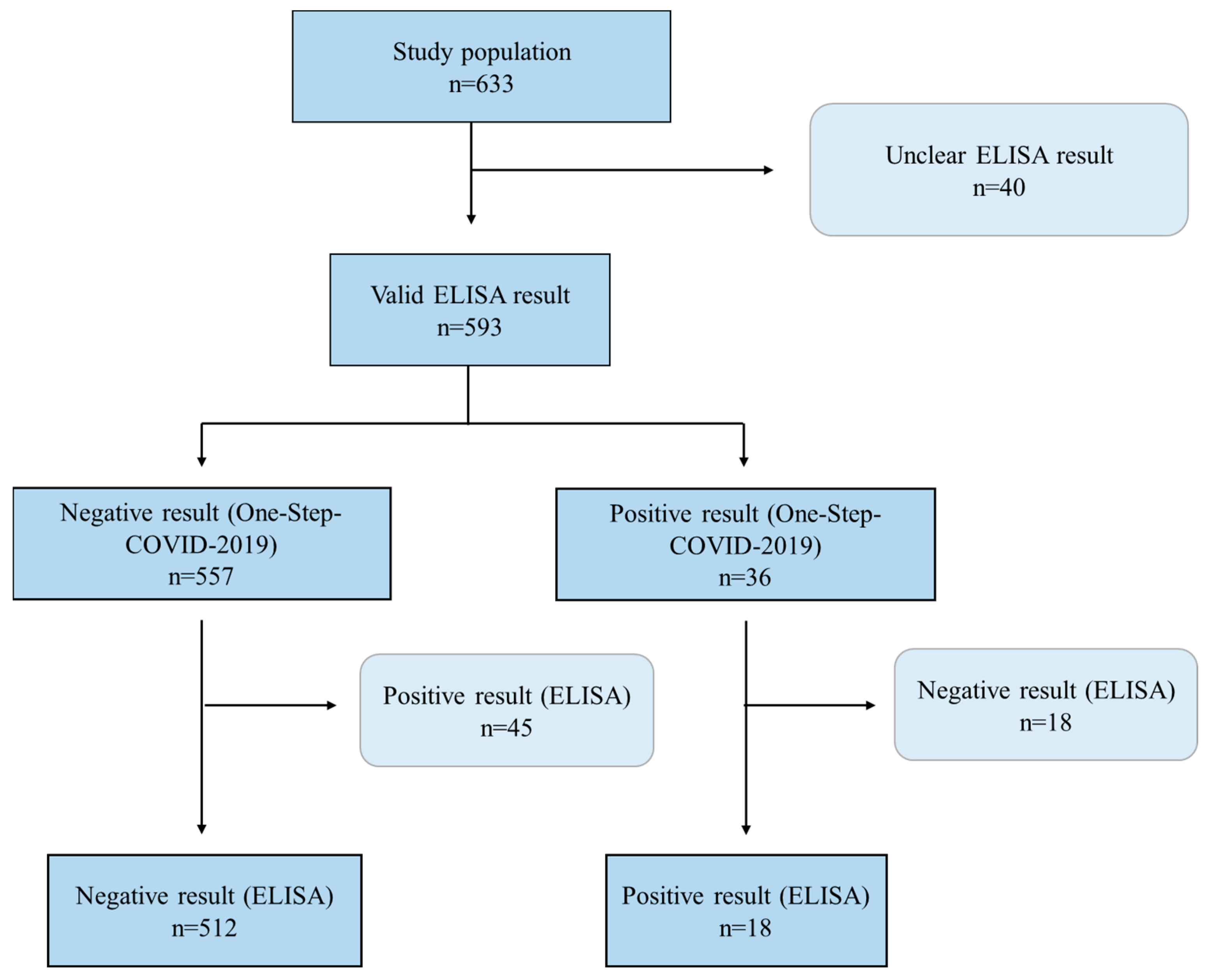
| N | TP | FP | TN | FN | Sens. (%) | Spec. (%) | PPV (%) | NPV (%) | AUC | Kappa | Youden | p-Value a | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | 593 | 18 | 18 | 512 | 45 | 28.6 | 96.6 | 50.0 | 91.9 | 0.626 | 0.310 | 0.252 | <0.001 |
| Symptoms of COVID-19 | |||||||||||||
| Non-symptomatic | 398 | 10 | 12 | 349 | 27 | 27.0 | 96.7 | 45.5 | 92.8 | 0.619 | 0.290 | 0.237 | 0.016 |
| Symptomatic | 192 | 8 | 18 | 160 | 6 | 30.8 | 96.4 | 57.1 | 89.9 | 0.636 | 0.337 | 0.272 | 0.014 |
| ELISA | One-Step-COVID-2019 | ||||||
|---|---|---|---|---|---|---|---|
| Characteristics | Total % (CI95%) | Non-Reactive % (CI95%) | Reactive % (CI95%) | p * | Non-Reactive % (CI95%) | Reactive % (CI95%) | p * |
| Total | 88.9 (85.4–91.6) | 11.1 (8.4–14.6) | 93.9 (91.2–95.8) | 6.1 (4.2–8.8) | |||
| Sex | |||||||
| Male | 49.8 (42.7–56.8) | 94.1 (87.9–97.2) | 5.9 (2.8–12.0) | 0.023 | 92.0 (88.9–94.2) | 8.0 (5.8–11.1) | 0.099 |
| Female | 50.2 (43.2–57.2) | 83.8 (76.6–89.0) | 16.2 (10.9–23.4) | 95.8 (91.1–98.1) | 4.2 (1.9–8.9) | ||
| Age | |||||||
| 18–34 years | 30.8 (25.3–36.8) | 87.3 (75.0–94.1) | 12.7 (5.9–25.0) | 0.514 | 93.6 (88.7–96.5) | 6.4 (3.5–11.3) | 0.514 |
| 35–59 years | 49.2 (43.5–54.9) | 91.3 (85.3–94.9) | 8.7 (5.0–14.7) | 95.3 (89.0–98.0) | 4.7 (2.0–11.0) | ||
| ≥60 years | 20.0 (15.6–25.3) | 85.6 (76.5–91.6) | 14.4 (8.4–23.5) | 90.8 (79.5–96.2) | 9.2 (3.8–20.5) | ||
| Skin color 1 | |||||||
| White | 29.9 (22.7–38.1) | 90.8 (80.9–95.8) | 9.2 (4.2–19.1) | 0.560 | 96.0 (91.8–98.0) | 4.0 (2.0–8.2) | 0.267 |
| BBYI | 70.1 (61.9–77.3) | 88.1 (83.7–91.4) | 11.9 (8.6–16.3) | 93.0 (88.6–95.7) | 7.0 (4.2–11.4) | ||
| Marital status 2 | |||||||
| Married | 52.2 (45.4–58.9) | 88.9 (82.0–93.4) | 11.1 (6.6–17.9) | 0.990 | 93.2 (86.9–96.6) | 6.8 (3.4–13.1) | 0.648 |
| Not married | 47.8 (41.1–54.5) | 88.7 (79.6–94.2) | 11.1 (5.8–20.3) | 94.6 (91.1–96.7) | 5.4 (3.3–8.9) | ||
| Education level | |||||||
| 0–8 years | 24.4 (19.5–30.0) | 80.1 (68.6–88.2) | 19.9 (11.8–31.4) | 0.048 | 87.8 (76.4–94.1) | 12.2 (5.9–23.6) | 0.016 |
| ≥9 years | 75.6 (69.9–80.5) | 91.5 (86.2–94.9) | 8.5 (5.1–13.8) | 95.8 (93.5–97.3) | 4.2 (2.7–6.5) | ||
| Family income 3 | |||||||
| ≤2 MW | 39.3 (32.3–46.8) | 85.5 (75.2–91.9) | 14.5 (8.0–24.8) | 0.232 | 94.7 (90.0–97.3) | 5.3 (2.7–10.3) | 0.520 |
| >2 to ≤4 MW | 32.3 (27.0–37.9) | 88.4 (79.4–93.8) | 11.6 (6.2–20.5) | 91.7 (83.0–96.1) | 8.3 (3.9–17.0) | ||
| >4 MW | 28.4 (20.0–38.6) | 94.3 (89.0–97.1) | 5.7 (2.9–11.0) | 95.2 (89.6–97.8) | 4.8 (2.1–10.4) | ||
| Working 4 | |||||||
| Not workers | 45.1 (41.2–49.1) | 86.4 (78.0–91.9) | 13.6 (8.0–21.9) | 0.165 | 91.7 (86.4–95.0) | 8.3 (5.0–13.6) | 0.001 |
| Active workers | 54.9 (50.9–58.8) | 92.2 (86.6–95.6) | 7.8 (4.4–13.4) | 96.9 (94.6–98.3) | 3.1 (1.7–5.4) | ||
| Work from home 5 | |||||||
| No | 71.9 (65.7–77.3) | 86.6 (82.2–90.1) | 13.4 (9.8–17.8) | 0.033 | 92.8 (89.8–94.9) | 7.2 (5.1–10.2) | 0.045 |
| Yes | 28.1 (22.6–34.3) | 94.6 (88.5–97.6) | 5.4 (2.4–11.5) | 96.6 (92.2–98.6) | 3.4 (1.4–7.8) | ||
| Symptoms of COVID-19 6 | |||||||
| No | 69.7 (64.9–74.2) | 84.0 (77.7–88.8) | 16.0 (11.2–22.3) | 0.196 | 94.6 (91.2–96.7) | 5.4 (3.3–8.8) | 0.404 |
| Yes | 30.3 (25.8–35.1) | 78.6 (71.3–84.5) | 21.4 (15.5–28.7) | 92.2 (84.9–96.1) | 7.8 (3.9–15.1) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menezes-Júnior, L.A.A.d.; Batista, A.P.; Lourenção, L.G.; Rocha, A.M.S.; Lage, N.N.; Barbosa, K.F.; Machado-Coelho, G.L.L.; Meireles, A.L. Rapid Serological Test for COVID-19, One-Step-COVID-2019: Accuracy and Implications for Pandemic Control. COVID 2024, 4, 1315-1323. https://doi.org/10.3390/covid4080093
Menezes-Júnior LAAd, Batista AP, Lourenção LG, Rocha AMS, Lage NN, Barbosa KF, Machado-Coelho GLL, Meireles AL. Rapid Serological Test for COVID-19, One-Step-COVID-2019: Accuracy and Implications for Pandemic Control. COVID. 2024; 4(8):1315-1323. https://doi.org/10.3390/covid4080093
Chicago/Turabian StyleMenezes-Júnior, Luiz Antônio Alves de, Aline Priscila Batista, Luciano Garcia Lourenção, Ana Maria Sampaio Rocha, Nara Nunes Lage, Keila Furbino Barbosa, George Luiz Lins Machado-Coelho, and Adriana Lúcia Meireles. 2024. "Rapid Serological Test for COVID-19, One-Step-COVID-2019: Accuracy and Implications for Pandemic Control" COVID 4, no. 8: 1315-1323. https://doi.org/10.3390/covid4080093







