Toward Revision of the ‘Best Practice for Diabetic Patients on Hemodialysis 2012’
Abstract
:1. Introduction
2. Which Glycemic Index, GA or HbA1c, Demonstrates Better Performance in Dialysis Patients?
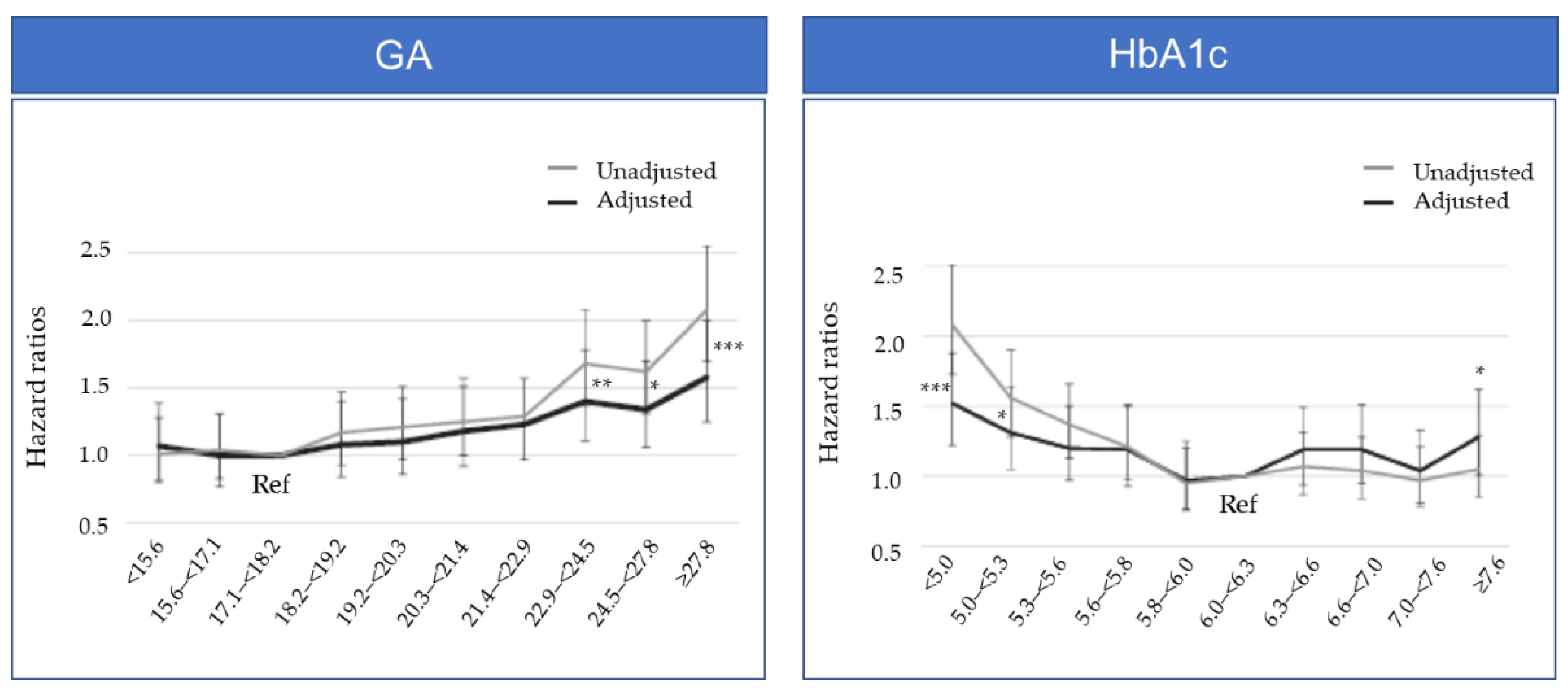
3. Target GA Levels in Hemodialysis Patients from Observational Studies and Meta-analyses
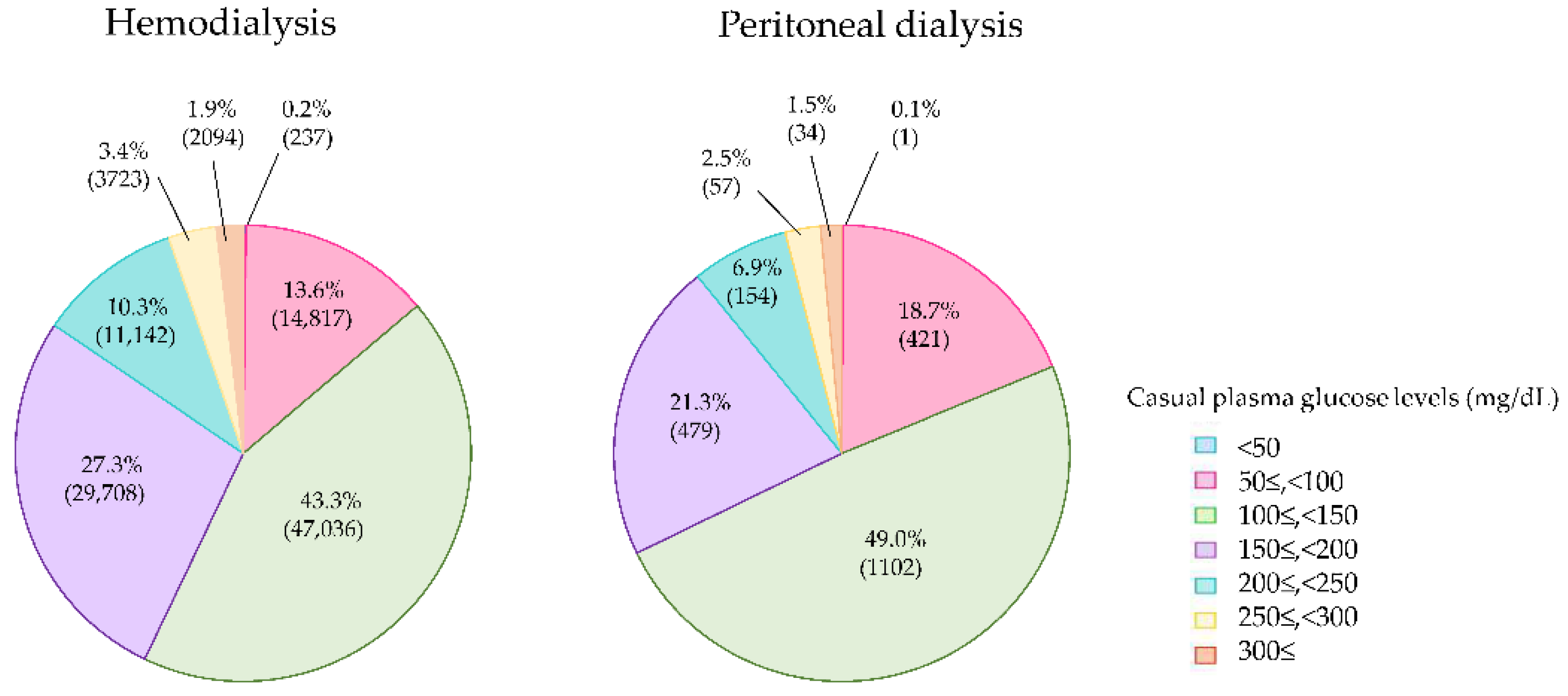
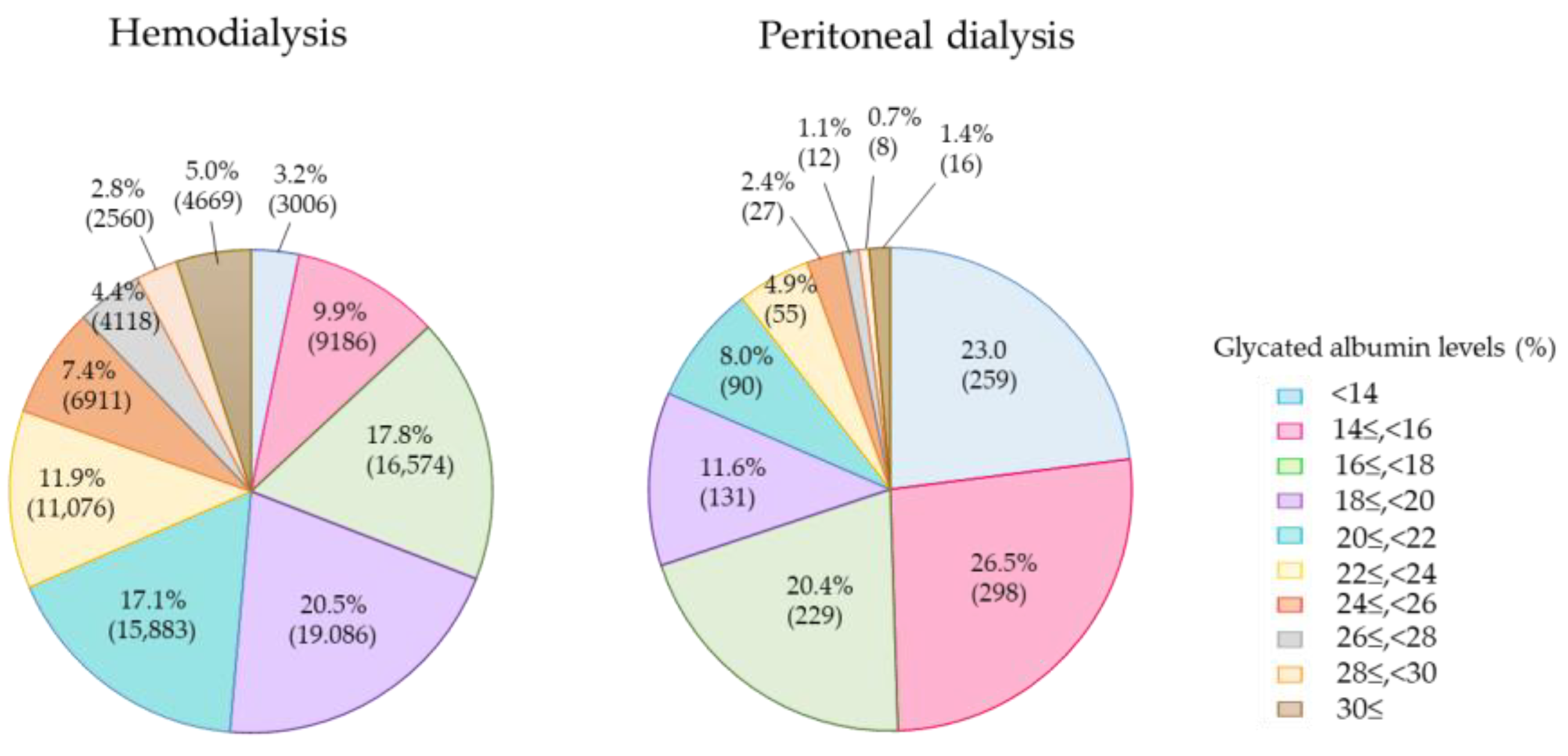
4. Current Glycemic Status in Diabetes Patients on Dialysis
5. Association between GA Levels and Mortality in PD Patients
6. Limitations of GA
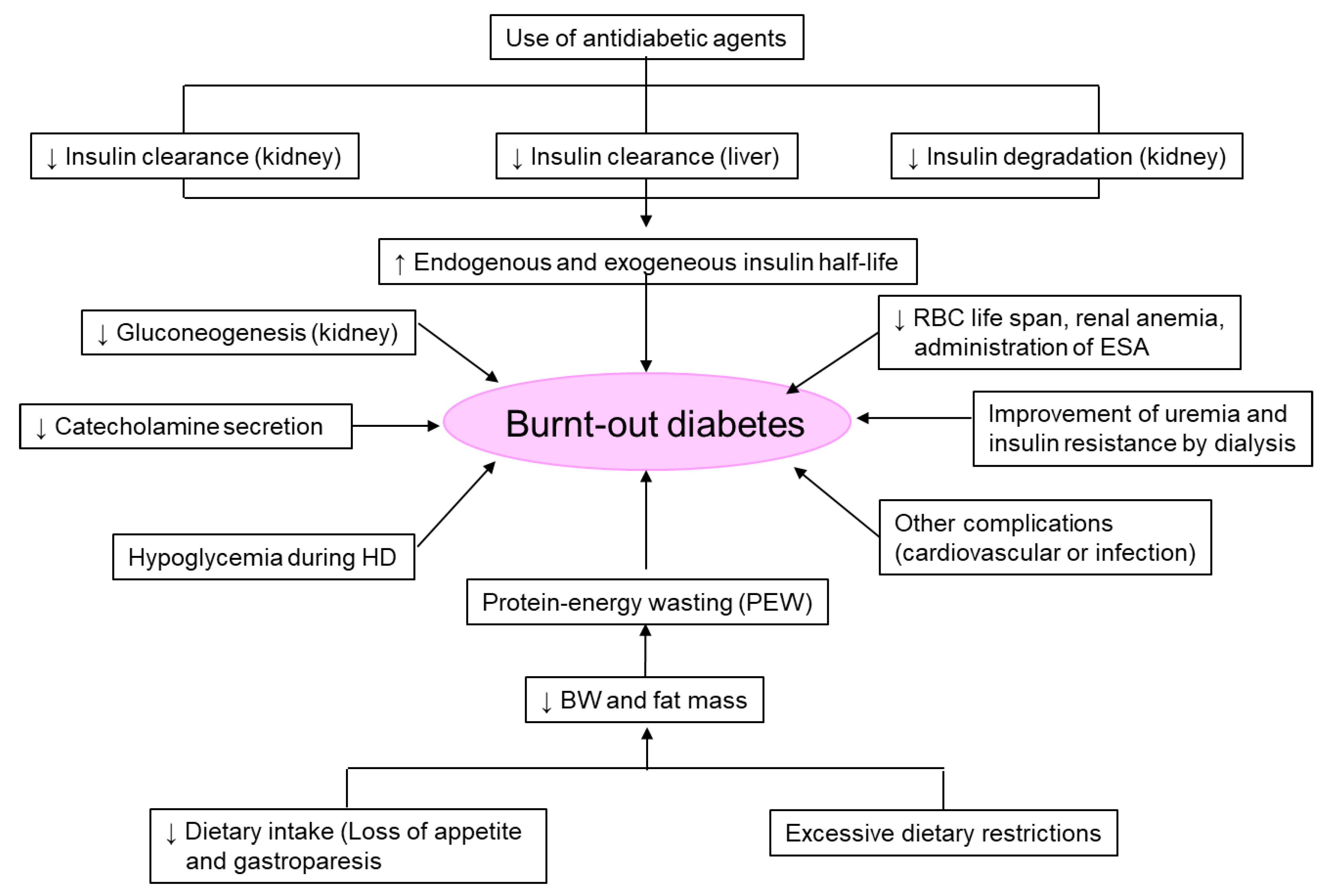
7. ‘Burnt-Out Diabetes’ Phenomenon in Patients on Dialysis
8. Continuous Glucose Monitoring in Patients on Dialysis
9. Peculiarities of Glycemic Control in Dialysis Patients
10. Medications for Glycemic Control in Diabetes Patients on Dialysis
10.1. SUs
10.2. Biguanide
10.3. Fast-Acting Insulin Secretagogues
10.4. α-Glucosidase Inhibitors (α-GIs)
10.5. Thiazolidinedione
10.6. DPP-4 Inhibitors
10.7. SGLT2 Inhibitors
10.8. GLP-1 Receptor Agonists
10.9. Insulin Therapy
11. Dietary Recommendations
12. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Nitta, K.; Goto, S.; Masakane, I.; Hanafusa, N.; Taniguchi, M.; Hasegawa, T.; Nakai, S.; Wada, A.; Hamano, T.; Hoshino, J.; et al. Annual dialysis data report for 2018, JSDT Renal Data Registry: Survey methods, facility data, incidence, prevalence, and mortality. Ren. Replace. Ther. 2020, 6, 41. [Google Scholar] [CrossRef]
- Nakao, T.; Inaba, M.; Abe, M.; Kaizu, K.; Shima, K.; Babazono, T.; Tomo, T.; Hirakata, H.; Akizawa, T.; Japanese Society for Dialysis Therapy. Best practice for diabetic patients on hemodialysis 2012. Ther. Apher. Dial. 2015, 19 (Suppl. S1), 40–66. [Google Scholar] [CrossRef]
- Inaba, M.; Okuno, S.; Kumeda, Y.; Yamada, S.; Imanishi, Y.; Tabata, T.; Okamura, M.; Okada, S.; Yamakawa, T.; Ishimura, E.; et al. Glycated albumin is a better glycemic indicator than glycated hemoglobin values in hemodialysis patients with diabetes: Effect of anemia and erythropoietin injection. J. Am. Soc. Nephrol. 2007, 18, 896–903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abe, M.; Matsumoto, K. Glycated hemoglobin or glycated albumin for assessment of glycemic control in hemodialysis patients with diabetes? Nat. Clin. Pract. Nephrol. 2008, 4, 482–483. [Google Scholar] [CrossRef] [PubMed]
- Peacock, T.P.; Shihabi, Z.K.; Bleyer, A.J.; Dolbare, E.L.; Byers, J.R.; Knovich, M.A.; Calles-Escandon, J.; Russell, G.B.; Freedman, B.I. Comparison of glycated albumin and hemoglobin A(1c) levels in diabetic subjects on hemodialysis. Kidney Int. 2008, 73, 1062–1068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohzuma, T.; Tao, X.; Koga, M. Glycated albumin as biomarker: Evidence and its outcomes. J. Diabetes Complicat. 2021, 35, 108040. [Google Scholar] [CrossRef] [PubMed]
- Okada, T.; Nakao, T.; Matsumoto, H.; Shino, T.; Nagaoka, Y.; Tomaru, R.; Wada, T. Association between markers of glycemic control, cardiovascular complications and survival in type 2 diabetic patients with end-stage renal disease. Intern. Med. 2007, 46, 807–814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukuoka, K.; Nakao, K.; Morimoto, H.; Nakao, A.; Takatori, Y.; Arimoto, K.; Taki, M.; Wada, J.; Makino, H. Glycated albumin levels predict long-term survival in diabetic patients undergoing haemodialysis. Nephrology 2008, 13, 278–283. [Google Scholar] [CrossRef]
- Inaba, M.; Maekawa, K.; Okuno, S.; Imanishi, Y.; Hayashino, Y.; Emoto, M.; Shoji, T.; Ishimura, E.; Yamakawa, T.; Nishizawa, Y. Impact of atherosclerosis on the relationship of glycemic control and mortality in diabetic patients on hemodialysis. Clin. Nephrol. 2012, 78, 273–280. [Google Scholar] [CrossRef]
- Shafi, T.; Sozio, S.M.; Plantinga, L.C.; Jaar, B.G.; Kim, E.T.; Parekh, R.S.; Steffes, M.W.; Powe, N.R.; Coresh, J.; Selvin, E. Serum fructosamine and glycated albumin and risk of mortality and clinical outcomes in hemodialysis patients. Diabetes Care 2013, 36, 1522–1533. [Google Scholar] [CrossRef]
- Freedman, B.I.; Andries, L.; Shihabi, Z.K.; Rocco, M.V.; Byers, J.R.; Cardona, C.Y.; Pickard, M.A.; Henderson, D.L.; Sadler, M.V.; Courchene, L.M.; et al. Glycated albumin and risk of death and hospitalizations in diabetic dialysis patients. Clin. J. Am. Soc. Nephrol. 2011, 6, 1635–1643. [Google Scholar] [CrossRef] [Green Version]
- Murea, M.; Moran, T.; Russell, G.B.; Shihabi, Z.K.; Byers, J.R.; Andries, L.; Bleyer, A.J.; Freedman, B.I. Glycated albumin, not hemoglobin A1c, predicts cardiovascular hospitalization and length of stay in diabetic patients on dialysis. Am. J. Nephrol. 2012, 36, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.L.; Ma, W.Y.; Lin, Y.F.; Shyu, J.F.; Wang, Y.H.; Liu, Y.M.; Wu, C.C.; Lu, K.C. Glycated Albumin Predicts Long-term Survival in Patients Undergoing Hemodialysis. Int. J. Med. Sci. 2016, 13, 395–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoshino, J.; Hamano, T.; Abe, M.; Hasegawa, T.; Wada, A.; Ubara, Y.; Takaichi, K.; Inaba, M.; Nakai, S.; Masakane, I.; et al. Glycated albumin versus hemoglobin A1c and mortality in diabetic hemodialysis patients: A cohort study. Nephrol. Dial. Transplant. 2018, 33, 1150–1158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoshino, J.; Abe, M.; Hamano, T.; Hasegawa, T.; Wada, A.; Ubara, Y.; Takaichi, K.; Nakai, S.; Masakane, I.; Nitta, K. Glycated albumin and hemoglobin A1c levels and cause-specific mortality by patients’ conditions among hemodialysis patients with diabetes: A 3-year nationwide cohort study. BMJ Open Diabetes Res. Care 2020, 8, e001642. [Google Scholar] [CrossRef]
- Hanai, K.; Akamatsu, M.; Fujimori, A.; Higashi, H.; Horie, Y.; Itaya, Y.; Ito, M.; Kanamaru, T.; Kawaguchi, H.; Kikuchi, K.; et al. Usefulness of glycated albumin as a predictor of mortality in chronic hemodialysis patients with diabetes: A multi-center, prospective cohort study. Ren. Replace. Ther. 2020, 6, 17. [Google Scholar] [CrossRef]
- Gan, T.; Liu, X.; Xu, G. Glycated Albumin Versus HbA1c in the Evaluation of Glycemic Control in Patients With Diabetes and CKD. Kidney Int. Rep. 2017, 3, 542–554. [Google Scholar] [CrossRef] [Green Version]
- Copur, S.; Siriopol, D.; Afsar, B.; Comert, M.C.; Uzunkopru, G.; Sag, A.A.; Ortiz, A.; Covic, A.; van Raalte, D.H.; Cherney, D.Z.; et al. Serum glycated albumin predicts all-cause mortality in dialysis patients with diabetes mellitus: Meta-analysis and systematic review of a predictive biomarker. Acta. Diabetol. 2021, 58, 81–91. [Google Scholar] [CrossRef]
- Masakane, I.; Nakai, S.; Ogata, S.; Kimata, N.; Hanafusa, N.; Hamano, T.; Wakai, K.; Wada, A.; Nitta, K. An Overview of Regular Dialysis Treatment in Japan (As of 31 December 2013). Ther. Apher. Dial. 2015, 19, 540–574. [Google Scholar] [CrossRef]
- Nitta, K.; Abe, M.; Masakane, I.; Hanafusa, N.; Taniguchi, M.; Hasegawa, T.; Nakai, S.; Wada, A.; Hamano, T.; Hoshino, J.; et al. Annual dialysis data report 2018, JSDT Renal Data Registry: Dialysis fluid quality, hemodialysis and hemodiafiltration, peritoneal dialysis, and diabetes. Ren. Replace. Ther. 2020, 6, 51. [Google Scholar] [CrossRef]
- Miyabe, M.; Kurajoh, M.; Mori, K.; Okuno, S.; Okada, S.; Emoto, M.; Tsujimoto, Y.; Inaba, M. Superiority of glycated albumin over glycated haemoglobin as indicator of glycaemic control and predictor of all-cause mortality in patients with type 2 diabetes mellitus receiving peritoneal dialysis. Ann. Clin. Biochem. 2019, 56, 684–691. [Google Scholar] [CrossRef] [PubMed]
- Abe, M.; Hamano, T.; Hoshino, J.; Wada, A.; Nakai, S.; Masakane, I. Glycemic control and survival in peritoneal dialysis patients with diabetes: A 2-year nationwide cohort study. Sci. Rep. 2019, 9, 3320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koga, M.; Murai, J.; Saito, H.; Matsumoto, S.; Kasayama, S. Effects of thyroid hormone on serum glycated albumin levels: Study on non-diabetic subjects. Diabetes Res. Clin. Pract. 2009, 84, 163–167. [Google Scholar] [CrossRef]
- Koga, M.; Kasayama, S.; Kanehara, H.; Bando, Y. CLD (chronic liver disease)-HbA1c as a suitable indicator for estimation of mean plasma glucose in patients with chronic liver diseases. Diabetes Res. Clin. Pract. 2008, 81, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Okada, T.; Nakao, T.; Matsumoto, H.; Nagaoka, Y.; Tomaru, R.; Iwasawa, H.; Wada, T. Influence of proteinuria on glycated albumin values in diabetic patients with chronic kidney disease. Intern. Med. 2011, 50, 23–29. [Google Scholar] [CrossRef] [Green Version]
- Freedman, B.I.; Shenoy, R.N.; Planer, J.A.; Clay, K.D.; Shihabi, Z.K.; Burkart, J.M.; Cardona, C.Y.; Andries, L.; Peacock, T.P.; Sabio, H.; et al. Comparison of glycated albumin and hemoglobin A1c concentrations in diabetic subjects on peritoneal and hemodialysis. Perit. Dial. Int. 2010, 30, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Derose, S.F.; Nicholas, S.; Benner, D.; Sharma, K.; Kovesdy, C.P. Burnt-out diabetes: Impact of chronic kidney disease progression on the natural course of diabetes mellitus. J. Ren. Nutr. 2009, 19, 33–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kovesdy, C.P.; Park, J.C.; Kalantar-Zadeh, K. Glycemic control and burnt-out diabetes in ESRD. Semin. Dial. 2010, 23, 148–156. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Lertdumrongluk, P.; Molnar, M.Z.; Kovesdy, C.P.; Kalantar-Zadeh, K. Glycemic control in diabetic dialysis patients and the burnt-out diabetes phenomenon. Curr. Diab. Rep. 2012, 12, 432–439. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Kopple, J.D.; Regidor, D.L.; Jing, J.; Shinaberger, C.S.; Aronovitz, J.; McAllister, C.J.; Whellan, D.; Sharma, K. A1C and survival in maintenance hemodialysis patients. Diabetes Care 2007, 30, 1049–1055. [Google Scholar] [CrossRef]
- Rhee, C.M.; Leung, A.M.; Kovesdy, C.P.; Lynch, K.E.; Brent, G.A.; Kalantar-Zadeh, K. Updates on the management of diabetes in dialysis patients. Semin. Dial. 2014, 27, 135–145. [Google Scholar] [CrossRef] [Green Version]
- Ramirez, S.P.; McCullough, K.P.; Thumma, J.R.; Nelson, R.G.; Morgenstern, H.; Gillespie, B.W.; Inaba, M.; Jacobson, S.H.; Vanholder, R.; Pisoni, R.L.; et al. Hemoglobin A(1c) levels and mortality in the diabetic hemodialysis population: Findings from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Diabetes Care 2012, 35, 2527–2532. [Google Scholar] [CrossRef] [Green Version]
- Abe, M.; Hamano, T.; Hoshino, J.; Wada, A.; Inaba, M.; Nakai, S.; Masakane, I. Is there a “burnt-out diabetes” phenomenon in patients on hemodialysis? Diabetes Res. Clin. Pract. 2017, 130, 211–220. [Google Scholar] [CrossRef]
- Battelino, T.; Danne, T.; Bergenstal, R.M.; Amiel, S.A.; Beck, R.; Biester, T.; Bosi, E.; Buckingham, B.A.; Cefalu, W.T.; Close, K.L.; et al. Clinical Targets for Continuous Glucose Monitoring Data Interpretation: Recommendations From the International Consensus on Time in Range. Diabetes Care 2019, 42, 1593–1603. [Google Scholar] [CrossRef] [Green Version]
- Freckmann, G.; Pleus, S.; Grady, M.; Setford, S.; Levy, B. Measures of Accuracy for Continuous Glucose Monitoring and Blood Glucose Monitoring Devices. J. Diabetes. Sci. Technol. 2019, 13, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Elbalshy, M.; Haszard, J.; Smith, H.; Kuroko, S.; Galland, B.; Oliver, N.; Shah, V.; de Bock, M.I.; Wheeler, B.J. Effect of divergent continuous glucose monitoring technologies on glycaemic control in type 1 diabetes mellitus: A systematic review and meta-analysis of randomised controlled trials. Diabet. Med. 2022, 39, e14854. [Google Scholar] [CrossRef]
- Gordon, I.; Rutherford, C.; Makarounas-Kirchmann, K.; Kirchmann, M. Meta-analysis of average change in laboratory-measured HbA1c among people with type 1 diabetes mellitus using the 14 day flash glucose monitoring system. Diabetes Res. Clin. Pract. 2020, 164, 108158. [Google Scholar] [CrossRef]
- Bianchi, C.; Aragona, M.; Rodia, C.; Baronti, W.; de Gennaro, G.; Bertolotto, A.; Del Prato, S. Freestyle Libre trend arrows for the management of adults with insulin-treated diabetes: A practical approach. J. Diabetes Complicat. 2019, 33, 6–12. [Google Scholar] [CrossRef]
- Gerbaud, E.; Darier, R.; Montaudon, M.; Beauvieux, M.C.; Coffin-Boutreux, C.; Coste, P.; Douard, H.; Ouattara, A.; Catargi, B. Glycemic variability is a powerful independent predictive factor of midterm major adverse cardiac events in patients with diabetes with acute coronary syndrome. Diabetes Care 2019, 42, 674–681. [Google Scholar] [CrossRef] [Green Version]
- Su, G.; Mi, S.H.; Tao, H.; Li, Z.; Yang, H.X.; Zheng, H.; Zhou, Y.; Tian, L. Impact of admission glycemic variability, glucose, and glycosylated hemoglobin on major adverse cardiac events after acute myocardial infarction. Diabetes Care 2013, 36, 1026–1032. [Google Scholar] [CrossRef]
- Aleppo, G.; Ruedy, K.J.; Riddlesworth, T.D.; Kruger, D.F.; Peters, A.L.; Hirsch, I.; Bergenstal, R.M.; Toschi, E.; Ahmann, A.J.; Shah, V.N.; et al. REPLACE-BG: A Randomized Trial Comparing Continuous Glucose Monitoring With and Without Routine Blood Glucose Monitoring in Adults With Well-Controlled Type 1 Diabetes. Diabetes Care 2017, 40, 538–545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martens, T.; Beck, R.W.; Bailey, R.; Ruedy, K.J.; Calhoun, P.; Peters, A.L.; Pop-Busui, R.; Philis-Tsimikas, A.; Bao, S.; Umpierrez, G.; et al. Effect of Continuous Glucose Monitoring on Glycemic Control in Patients With Type 2 Diabetes Treated With Basal Insulin: A Randomized Clinical Trial. JAMA 2021, 325, 2262–2272. [Google Scholar] [CrossRef]
- Karter, A.J.; Parker, M.M.; Moffet, H.H.; Gilliam, L.K.; Dlott, R. Association of Real-time Continuous Glucose Monitoring With Glycemic Control and Acute Metabolic Events Among Patients With Insulin-Treated Diabetes. JAMA 2021, 325, 2273–2284. [Google Scholar] [CrossRef] [PubMed]
- Eversense, X.L. User Guide [Internet]. Available online: https://global.eversensediabetes.com/sites/default/files/2021-11/LBL-1402-31-001_Rev_E_Eversense_User_Guide_mgdL_UK-ENG.pdf (accessed on 8 August 2022).
- Dexcom. Dexcom G6 Continuous Glucose Monitoring System. User Guide [Internet]. Available online: https://s3-us-west-2.amazonaws.com/dexcompdf/G6-CGM-Users-Guide.pdf#page=21 (accessed on 8 August 2022).
- Dexcom. Dexcom G6 Pro Continuous Glucose Monitoring System. User Guide [Internet]. Available online: https://www.dexcom.com/faq/what-dexcom-g6-pro-continuous-glucose-monitoring-cgm-system (accessed on 8 August 2022).
- Abbott. FreeStyle Libre 14 Day System. Available online: https://www.freestyleprovider.abbott/us-en/freestyle-libre-14-day-system.html (accessed on 8 August 2022).
- Abbott. FreeStyle Libre Pro Flash Glucose Monitoring System [Internet]. Available online: https://www.freestyle.abbott/in-en/products/freestyle-libre-pro.html (accessed on 8 August 2022).
- Abbott. FreeStyle Libre 2 System IN-SERVICE GUIDE [Internet]. Available online: https://provider.myfreestyle.com/pdf/In-Service-FreeStyle-Libre-2-HCP-Sales.pdf (accessed on 8 August 2022).
- Medtronic. Guardian Connect System. User Guide [Internet]. Available online: https://www.medtronicdiabetes.com/download-library/guardian-connect (accessed on 8 August 2022).
- Mambelli, E.; Cristino, S.; Mosconi, G.; Göbl, C.; Tura, A. Flash Glucose Monitoring to Assess Glycemic Control and Variability in Hemodialysis Patients: The GIOTTO Study. Front. Med. 2021, 8, 617891. [Google Scholar] [CrossRef]
- Toyoda, M.; Murata, T.; Saito, N.; Kimura, M.; Takahashi, H.; Ishida, N.; Kitamura, M.; Hida, M.; Hayashi, A.; Moriguchi, I.; et al. Assessment of the accuracy of an intermittent-scanning continuous glucose monitoring device in patients with type 2 diabetes mellitus undergoing hemodialysis (AIDT2H) study. Ther. Apher. Dial. 2021, 25, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Narasaki, Y.; Park, E.; You, A.S.; Daza, A.; Peralta, R.A.; Guerrero, Y.; Novoa, A.; Amin, A.N.; Nguyen, D.V.; Price, D.; et al. Continuous glucose monitoring in an end-stage renal disease patient with diabetes receiving hemodialysis. Semin. Dial. 2021, 34, 388–393. [Google Scholar] [CrossRef]
- Képénékian, L.; Smagala, A.; Meyer, L.; Imhoff, O.; Alenabi, F.; Serb, L.; Fleury, D.; Dorey, F.; Krummel, T.; Le Floch, J.P.; et al. Continuous glucose monitoring in hemodialyzedpatients with type 2 diabetes: A multicenter pilot study. Clin. Nephrol. 2014, 82, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Joubert, M.; Fourmy, C.; Henri, P.; Ficheux, M.; Lobbedez, T.; Reznik, Y. Effectiveness of continuous glucose monitoring in dialysis patients with diabetes: The DIALYDIAB pilot study. Diabetes Res. Clin. Pract. 2015, 107, 348–354. [Google Scholar] [CrossRef]
- Bomholt, T.; Kofod, D.; Nørgaard, K.; Rossing, P.; Feldt-Rasmussen, B.; Hornum, M. Can the Use of Continuous Glucose Monitoring Improve Glycemic Control in Patients with Type 1 and 2 Diabetes Receiving Dialysis? Nephron 2022, 13, 1–6. [Google Scholar] [CrossRef]
- Hayashi, A.; Takano, K.; Masaki, T.; Yoshino, S.; Ogawa, A.; Shichiri, M. Distinct biomarker roles for HbA1c and glycated albumin in patients with type 2 diabetes on hemodialysis. J. Diabetes Complicat. 2016, 30, 1494–1499. [Google Scholar] [CrossRef]
- Bomholt, T.; Feldt-Rasmussen, B.; Butt, R.; Borg, R.; Sarwary, M.H.; Elung-Jensen, T.; Almdal, T.; Knop, F.K.; Nørgaard, K.; Ranjan, A.G.; et al. Hemoglobin A1c and Fructosamine Evaluated in Patients with Type 2 Diabetes Receiving Peritoneal Dialysis Using Long-Term Continuous Glucose Monitoring. Nephron 2022, 146, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, D.; Lyu, X.L.; Sun, X.M.; Duan, B.H. Continuous glucose monitoring in diabetes patients with chronic kidney disease on dialysis: A meta-analysis. Minerva Endocrinol. 2022, 47, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group. KDIGO 2020 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease. Kidney Int. 2020, 98, S1–S115. [Google Scholar] [CrossRef] [PubMed]
- Abe, M.; Kalantar-Zadeh, K. Haemodialysis-induced hypoglycaemia and glycaemic disarrays. Nat. Rev. Nephrol. 2015, 11, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Abe, M.; Kaizu, K.; Matsumoto, K. Plasma insulin is removed by hemodialysis: Evaluation of the relation between plasma insulin and glucose by using a dialysate with or without glucose. Ther. Apher. Dial. 2007, 11, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, A.; Kubota, T.; Shibahara, N.; Terasaki, J.; Kagitani, M.; Ueda, H.; Inoue, T.; Katsuoka, Y. The mechanism of hypoglycemia caused by hemodialysis. Clin. Nephrol. 2004, 62, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Abe, M.; Kikuchi, F.; Kaizu, K.; Matsumoto, K. The influence of hemodialysis membranes on the plasma insulin level of diabetic patients on maintenance hemodialysis. Clin. Nephrol. 2008, 69, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Abe, M.; Okada, K.; Matsumoto, K. Plasma insulin and C-peptide concentrations in diabetic patients undergoing hemodialysis: Comparison with five types of high-flux dialyzer membranes. Diabetes Res. Clin. Pract. 2008, 82, e17–e19. [Google Scholar] [CrossRef] [PubMed]
- Abe, M.; Okada, K.; Ikeda, K.; Matsumoto, S.; Soma, M.; Matsumoto, K. Characterization of insulin adsorption behavior of dialyzer membranes used in hemodialysis. Artif. Organs. 2011, 35, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Abe, M.; Kaizu, K.; Matsumoto, K. Evaluation of the hemodialysis-induced changes in plasma glucose and insulin concentrations in diabetic patients: Comparison between the hemodialysis and non-hemodialysis days. Ther. Apher. Dial. 2007, 11, 288–295. [Google Scholar] [CrossRef]
- Hayashi, A.; Shimizu, N.; Suzuki, A.; Matoba, K.; Momozono, A.; Masaki, T.; Ogawa, A.; Moriguchi, I.; Takano, K.; Kobayashi, N.; et al. Hemodialysis-Related Glycemic Disarray Proven by Continuous Glucose Monitoring; Glycemic Markers and Hypoglycemia. Diabetes Care 2021, 44, 1647–1656. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Emoto, M.; Abe, M.; Inaba, M. Visualization of Blood Glucose Fluctuations Using Continuous Glucose Monitoring in Patients Undergoing Hemodialysis. J. Diabetes. Sci. Technol. 2019, 13, 413–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abe, M.; Okada, K.; Soma, M. Antidiabetic agents in patients with chronic kidney disease and end-stage renal disease on dialysis: Metabolism and clinical practice. Curr. Drug Metab. 2011, 12, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Kidney Disease Outcomes Quality Initiative (KDOQI). KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Diabetes and Chronic Kidney Disease. Am. J. Kidney Dis. 2007, 49, S62–S73. [Google Scholar]
- Guideline Development Group. Clinical Practice Guideline on management of patients with diabetes and chronic kidney disease stage 3b or higher (eGFR < 45 mL/min). Nephrol. Dial. Transplant. 2015, 30 (Suppl. S2), ii1–ii142. [Google Scholar]
- Maruyama, N.; Abe, M. Targets and Therapeutics for Glycemic Control in Diabetes Patients on Hemodialysis. Contrib. Nephrol. 2018, 196, 37–43. [Google Scholar]
- Abe, M.; Okada, K. DPP-4 Inhibitors in Diabetic Patients with Chronic Kidney Disease and End-Stage Kidney Disease on Dialysis in Clinical Practice. Contrib. Nephrol. 2015, 185, 98–115. [Google Scholar]
- Park, S.H.; Nam, J.Y.; Han, E.; Lee, Y.H.; Lee, B.W.; Kim, B.S.; Cha, B.S.; Kim, C.S.; Kang, E.S. Efficacy of different dipeptidyl peptidase-4 (DPP-4) inhibitors on metabolic parameters in patients with type 2 diabetes undergoing dialysis. Medicine 2016, 95, e4543. [Google Scholar] [CrossRef]
- Chacra, A.; Gantz, I.; Mendizabal, G.; Durlach, L.; O’Neill, E.A.; Zimmer, Z.; Suryawanshi, S.; Engel, S.S.; Lai, E. A randomised, double-blind, trial of the safety and efficacy of omarigliptin (a once-weekly DPP-4 inhibitor) in subjects with type 2 diabetes and renal impairment. Int. J. Clin. Pract. 2017, 71, e12955. [Google Scholar] [CrossRef] [Green Version]
- Kaku, K.; Ishida, K.; Shimizu, K.; Achira, M.; Umeda, Y. Efficacy and safety of trelagliptin in Japanese patients with type 2 diabetes with severe renal impairment or end-stage renal disease: Results from a randomized, phase 3 study. J. Diabetes Investig. 2020, 11, 373–381. [Google Scholar] [CrossRef] [Green Version]
- Wanner, C.; Inzucchi, S.E.; Lachin, J.M.; Fitchett, D.; von Eynatten, M.; Mattheus, M.; Johansen, O.E.; Woerle, H.J.; Broedl, U.C.; Zinman, B.; et al. Empagliflozin and Progression of Kidney Disease in Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Pocock, S.J.; Carson, P.; Anand, I.; Doehner, W.; Haass, M.; et al. Effect of Empagliflozin on the Clinical Stability of Patients With Heart Failure and a Reduced Ejection Fraction: The EMPEROR-Reduced Trial. Circulation 2021, 143, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Neal, B.; Perkovic, V.; Mahaffey, K.W.; de Zeeuw, D.; Fulcher, G.; Erondu, N.; Shaw, W.; Law, G.; Desai, M.; Matthews, D.R.; et al. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 644–657. [Google Scholar] [CrossRef] [PubMed]
- Perkovic, V.; Jardine, M.J.; Neal, B.; Bompoint, S.; Heerspink, H.J.L.; Charytan, D.M.; Edwards, R.; Agarwal, R.; Bakris, G.; Bull, S.; et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N. Engl. J. Med. 2019, 380, 2295–2306. [Google Scholar] [CrossRef]
- Heerspink, H.J.L.; Stefánsson, B.V.; Correa-Rotter, R.; Chertow, G.M.; Greene, T.; Hou, F.F.; Mann, J.F.E.; McMurray, J.J.V.; Lindberg, M.; Rossing, P.; et al. Dapagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2020, 383, 1436–1446. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef] [Green Version]
- Granhall, C.; Søndergaard, F.L.; Thomsen, M.; Anderson, T.W. Pharmacokinetics, Safety and Tolerability of Oral Semaglutide in Subjects with Renal Impairment. Clin. Pharmacokinet. 2018, 57, 1571–1580. [Google Scholar] [CrossRef] [Green Version]
- Yajima, T.; Yajima, K.; Hayashi, M.; Takahashi, H.; Yasuda, K. Improved glycemic control with once-weekly dulaglutide in addition to insulin therapy in type 2 diabetes mellitus patients on hemodialysis evaluated by continuous glucose monitoring. J. Diabetes Complicat. 2018, 32, 310–315. [Google Scholar] [CrossRef]
- Bomholt, T.; Idorn, T.; Knop, F.K.; Jørgensen, M.B.; Ranjan, A.G.; Resuli, M.; Hansen, P.M.; Borg, R.; Persson, F.; Feldt-Rasmussen, B.; et al. The Glycemic Effect of Liraglutide Evaluated by Continuous Glucose Monitoring in Persons with Type 2 Diabetes Receiving Dialysis. Nephron 2021, 145, 27–34. [Google Scholar] [CrossRef]
- Idorn, T.; Knop, F.K.; Jørgensen, M.B.; Jensen, T.; Resuli, M.; Hansen, P.M.; Christensen, K.B.; Holst, J.J.; Hornum, M.; Feldt-Rasmussen, B. Safety and Efficacy of Liraglutide in Patients With Type 2 Diabetes and End-Stage Renal Disease: An Investigator-Initiated, Placebo-Controlled, Double-Blind, Parallel-Group, Randomized Trial. Diabetes Care 2016, 39, 206–213. [Google Scholar] [CrossRef] [Green Version]
- Fonseca, V.A.; Alvarado-Ruiz, R.; Raccah, D.; Boka, G.; Miossec, P.; Gerich, J.E.; EFC6018 GetGoal-Mono Study Investigators. Efficacy and safety of the once-daily GLP-1 receptor agonist lixisenatide in monotherapy: A randomized, double-blind, placebo-controlled trial in patients with type 2 diabetes (GetGoal-Mono). Diabetes Care 2012, 35, 1225–1231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Snyder, R.W.; Berns, J.S. Use of insulin and oral hypoglycemic medications in patients with diabetes mellitus and advanced kidney disease. Semin. Dial. 2004, 17, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Charpentier, G.; Riveline, J.P.; Varroud-Vial, M. Management of drugs affecting blood glucose in diabetic patients with renal failure. Diabetes. Metab. 2000, 26, 73–85. [Google Scholar] [PubMed]
- Reilly, J.B.; Berns, J.S. Selection and dosing of medications for management of diabetes in patients with advanced kidney disease. Semin. Dial. 2010, 23, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Shoji, T.; Emoto, M.; Mori, K.; Morioka, T.; Fukumoto, S.; Takahashi, T.; Matsumoto, A.; Nishizawa, Y.; Inaba, M. Thrice-weekly insulin injection with nurse’s support for diabetic hemodialysis patients having difficulty with self injection. Osaka City Med. J. 2012, 58, 35–38. [Google Scholar]
- Fouque, D.; Vennegoor, M.; ter Wee, P.; Wanner, C.; Basci, A.; Canaud, B.; Haage, P.; Konner, K.; Kooman, J.; Martin-Malo, A.; et al. European Best Practice Guideline on nutrition. Nephrol. Dial. Transplant. 2007, 22 (Suppl. S2), ii45–ii87. [Google Scholar] [CrossRef] [Green Version]
- National Kidney Foundation. Clinical practice guidelines for nutrition in chronic renal failure. Am. J. Kidney. Dis. 2000, 35, S17–S104. [Google Scholar]
- UK Renal Association. Nutrition in Chronic Kidney Disease Clinical Practice Guidelines. 2009–2010. Available online: https://ukkidney.org/sites/renal.org/files/nutrition-in-ckd-5th-edition-1.pdf (accessed on 8 August 2022).
- Naylor, H.L.; Jackson, H.; Walker, G.H.; Macafee, S.; Magee, K.; Hooper, L.; Stewart, L.; MacLaughlin, H.L.; Renal Nutrition Group of the British Dietetic Association; British Dietetic Association. British Dietetic Association Renal Nutrition Group Evidence Based Dietetic Guidelines Protein Requirements Of Adults On Haemodialysis And Peritoneal Dialysis. J. Hum. Nutr. Diet. 2013, 26, 315–328. [Google Scholar] [CrossRef]
- Leavey, S.F.; McCullough, K.; Hecking, E.; Goodkin, D.; Port, F.K.; Young, E.W. Body mass index and mortality in ‘healthier’ as compared with ‘sicker’ haemodialysis patients: Results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol. Dial. Transplant. 2001, 16, 2386–2394. [Google Scholar] [CrossRef] [Green Version]
- Fissell, R.B.; Bragg-Gresham, J.L.; Gillespie, B.W.; Goodkin, D.A.; Bommer, J.; Saito, A.; Akiba, T.; Port, F.K.; Young, E.W. International variations in vitamin prescription and association with mortality in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am. J. Kidney. Dis. 2004, 44, 293–299. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Block, G.; McAllister, C.J.; Humphreys, M.H.; Kopple, J.D. Appetite and inflammation, nutrition, anemia, and clinical outcome in hemodialysis patients. Am. J. Clin. Nutr. 2004, 80, 299–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pupim, L.B.; Heimburger, O.; Qureshi, A.R. Accelerated lean body mass loss in incident chronic dialysis patients with diabetes mellitus. Kidney Int. 2005, 68, 2638–2674. [Google Scholar] [CrossRef] [Green Version]
- Cano, N.J.; Roth, H.; Aparicio, M.; Azar, R.; Canaud, B.; Chauveau, P.; Combe, C.; Fouque, D.; Laville, M.; Leverve, X.M.; et al. Malnutrition in haemodialysis diabetic patients:evaluation and prognostic influence. Kidney Int. 2002, 62, 593–601. [Google Scholar] [CrossRef]
- Fouque, D.; Kalantar-Zadeh, K.; Kopple, J.; Cano, N.; Chauveau, P.; Cuppari, L.; Franch, H.; Guarnieri, G.; Ikizler, T.A.; Kaysen, G.; et al. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int. 2008, 73, 391–398. [Google Scholar] [CrossRef] [Green Version]
- Pupim, L.B.; Flakoll, P.J.; Majchrzak, K.M.; Aftab Guy, D.L.; Stenvinkel, P.; Ikizler, T.A. Increased muscle protein breakdown in chronic haemodialysis patient with type 2 diabetes mellitus. Kidney Int. 2005, 68, 1857–1865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noori, N.; Kopple, J.D. Effect of diabetes mellitus on protein-energy wasting and protein wasting in end-stage renal disease. Semin. Dial. 2010, 23, 178–184. [Google Scholar] [CrossRef]
- Kopple, J.D. Pathophysiology of protein-energy wasting in chronic renal failure. J. Nutr. 1999, 129, 2475–2515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuo, S.; Nakamoto, M.; Nishihara, G.; Yasunaga, C.; Yanagida, T.; Matsuo, K.; Sakemi, T. Impaired taste acuity in patients with diabetes on maintenance haemodialysis. Nephron Clin. Pract. 2003, 94, 46–50. [Google Scholar] [CrossRef]
- Masakane, I.; Taniguchi, M.; Nakai, S.; Tsuchida, K.; Goto, S.; Wada, A.; Ogata, S.; Hasegawa, T.; Hamano, T.; Hanafusa, N.; et al. Annual Dialysis Data Report 2015, JSDT Renal Data Registry. Ren. Replace. Ther. 2018, 4, 19. [Google Scholar] [CrossRef]

| Eversense XL | Dexcom G6 | Dexcom G6 Pro | FreeStyle Libre | FreeStyle Libre Pro | Freestyle Libre 2 | Medtronic Guardian Connect | |
|---|---|---|---|---|---|---|---|
| Approved for CKD (not on dialysis) | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Approved for hemodialysis | Yes | No | No | Yes | Yes | No | Yes |
| Approved for peritoneal dialysis | Yes | No | No | Yes | Yes | No | Yes |
| Classification | Drug | Regular Dose (mg/Day) | Optimal Dose for Dialysis Patients (mg/Day) |
|---|---|---|---|
| Sulfonylureas | Tolbutamide | 250–2000 | Contraindication |
| Acetohexamide | 250–1000 | Contraindication | |
| Chlorpropamide | 100–500 | Contraindication | |
| Glyclopyramide | 250–500 | Contraindication | |
| Glibenclamide | 1.25–10 | Contraindication | |
| Glipizide *1 | 2.5–20 | Regular dose | |
| Gliclazide | 40–160 | Regular dose *2 | |
| Glimepiride | 0.5–6 | Contraindication | |
| Gliquidone *1 | 15–60 | Regular dose | |
| Fast-acting insulin secretagogues | Nateglinide | 270–360 | Contraindication |
| Mitiglinide | 15–30 | Careful administration | |
| Repaglinide | 0.75–3 | Careful administration | |
| Biguanides | Metformin | 500–2250 | Contraindication |
| Buformin | 50–150 | Contraindication | |
| Thiazolidinediones | Pioglitazone | 15–45 | Regular dose *2 |
| α-Glucosidase inhibitors | Acarbose | 150–300 | Regular dose *3 |
| Voglibose | 0.6–0.9 | Regular dose | |
| Miglitol | 150–225 | Careful administration | |
| DPP-4 inhibitors | Sitagliptin | 50–100 | 12.5–25 |
| Vildagliptin | 50–100 | Careful administration | |
| Alogliptin | 25 | 6.25 | |
| Linagliptin | 5 | Regular dose | |
| Teneligliptin *4 | 20–40 | Regular dose | |
| Anagliptin *4 | 200–400 | 100 | |
| Saxagliptin | 5 | 2.5 | |
| Trelagliptin *4 | 100 mg once a week | 25 mg once a week | |
| Omarigliptin *4 | 25 mg once a week | 12.5 mg once a week | |
| GLP-1 receptor agonist | Liraglutide | 0.3–0.9 | Careful administration |
| Exenatide | 10–20 µg | Contraindication | |
| Exenatide | 2 mg once a week | Contraindication | |
| Lixisenatide | 10–20 µg | Careful administration | |
| Dulaglutide | 0.75 mg once a week | Regular dose | |
| Semaglutide | 0.25–1.0 mg once a week | Regular dose | |
| Semaglutide (oral) | 3–14 mg | Regular dose | |
| SGLT2 inhibitor | Ipragliflozin *4 | 50–100 | Avoid; not effective |
| Dapagliflozin | 5–10 | Avoid; not effective | |
| Luseogliflozin *4 | 2.5–5 | Avoid; not effective | |
| Tofogliflozin *4 | 20 | Avoid; not effective | |
| Canagliflozin | 100 | Avoid; not effective | |
| Empagliflozin | 10–25 | Avoid; not effective |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abe, M.; Matsuoka, T.; Kawamoto, S.; Miyasato, K.; Kobayashi, H. Toward Revision of the ‘Best Practice for Diabetic Patients on Hemodialysis 2012’. Kidney Dial. 2022, 2, 495-511. https://doi.org/10.3390/kidneydial2040045
Abe M, Matsuoka T, Kawamoto S, Miyasato K, Kobayashi H. Toward Revision of the ‘Best Practice for Diabetic Patients on Hemodialysis 2012’. Kidney and Dialysis. 2022; 2(4):495-511. https://doi.org/10.3390/kidneydial2040045
Chicago/Turabian StyleAbe, Masanori, Tomomi Matsuoka, Shunsuke Kawamoto, Kota Miyasato, and Hiroki Kobayashi. 2022. "Toward Revision of the ‘Best Practice for Diabetic Patients on Hemodialysis 2012’" Kidney and Dialysis 2, no. 4: 495-511. https://doi.org/10.3390/kidneydial2040045







