Anaesthetic Effect of Clove Basil (Ocimum gratissimum) Essential Oil on Macrobrachium rosenbergii Post-Larvae
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Origin and Essential Oil Extraction and Composition
2.2. Experimental Design and Anaesthetic Trial
2.3. Statistical Analyses
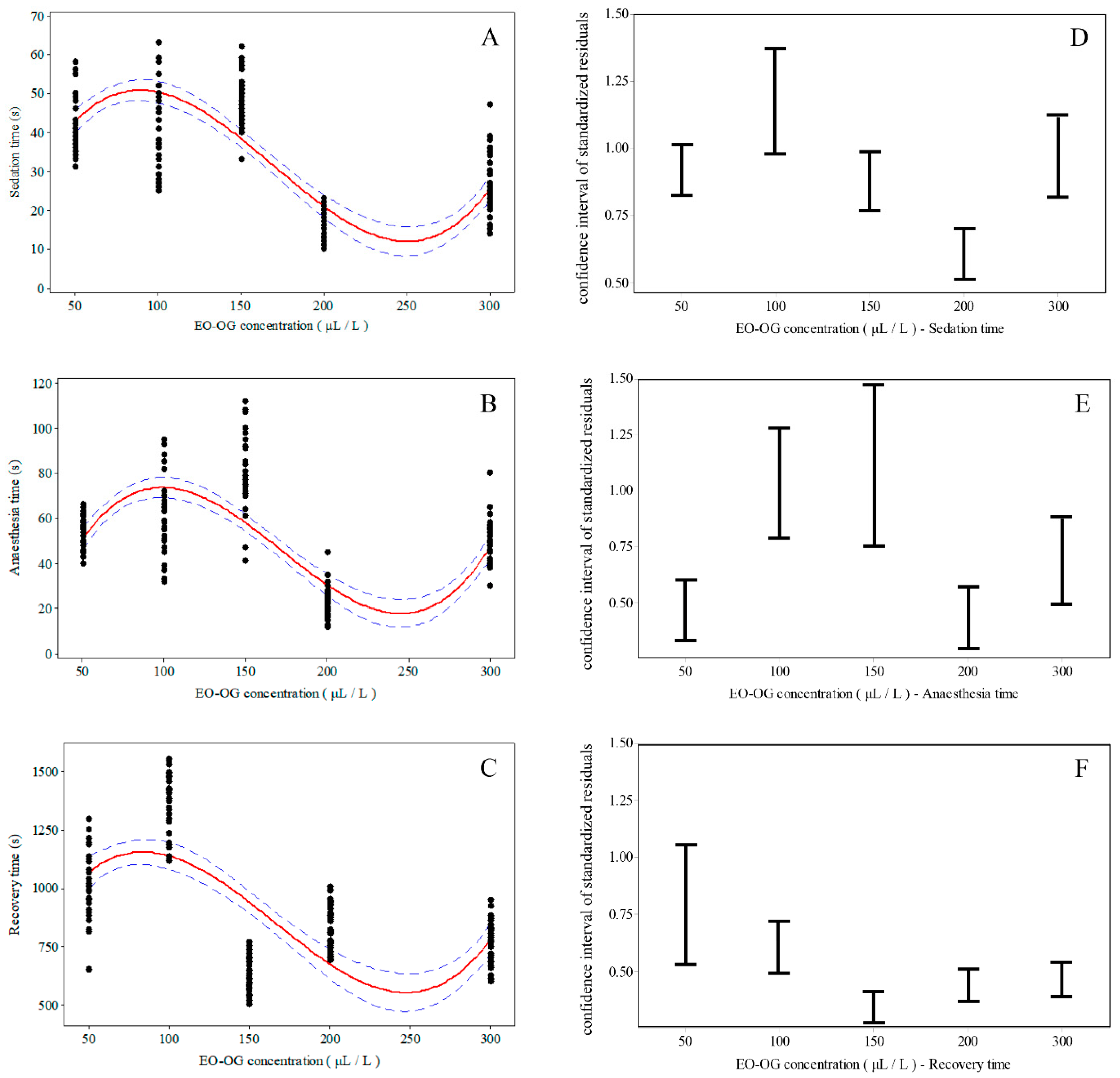
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Cavallo, R.O.; Lavens, P.; Sorgeloos, P. Reproductive Performance of Macrobrachium rosenbergii Females in Captivity. J. World Aquac. Soc. 2001, 32, 60–67. [Google Scholar] [CrossRef]
- Barros, H.P.; Valenti, W.C. Comportamento alimentar do camarão de água doce, Macrobrachium rosenbergii (De Man) (Crustacea, Palaemonidae) durante a fase larval: Análise qualitativa. Rev. Bras. Zool. 1997, 14, 785–793. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2022: Towards Blue Transformation. In The State of World Fisheries and Aquaculture (SOFIA); Food and Agricultural Organization of the United Nations: Rome, Italy, 2022; p. 266. [Google Scholar]
- Machado, I.D.S.; Nunes, C.A.R.; dos Santos, H.B.L.; Lima, J.A.; Meira, T.M.; Da silva Sena, E.; da Silva, W.N. Desempenho do camarão Macrobrachium amazonicum (Heller, 1862) (Crustacea: Decapoda: Palaemonidae), em diferentes densidades. Rev. Bras. Eng. Pesca 2018, 11, 29–37. [Google Scholar] [CrossRef]
- de Souza Valente, C. Anaesthesia of decapod crustaceans. Vet. Anim. Sci. 2022, 16, 100252. [Google Scholar] [CrossRef] [PubMed]
- Zahl, I.H.; Samuelsen, O.; Kiessling, A. Anaesthesia of farmed fish: Implications for welfare. Fish Physiol. Biochem. 2012, 38, 201–218. [Google Scholar] [CrossRef] [PubMed]
- Aydın, B.; Barbas, L.A.L. Sedative and anesthetic properties of essential oils and their active compounds in fish: A review. Aquaculture 2020, 520, 734999. [Google Scholar] [CrossRef]
- Khan, S.; Sahar, A.; Tariq, T.; Sameen, A.; Tariq, F. Chapter 1—Essential oils in plants: Plant physiology, the chemical composition of the oil, and natural variation of the oils (chemotaxonomy and environmental effects, etc.). In Essential Oils; Nayik, G.A., Ansari, M.J., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 1–36. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Pandey, A.K.; Singh, P.; Tripathi, N.N. Chemistry and bioactivities of essential oils of some Ocimum species: An overview. Asian Pac. J. Trop. Biomed. 2014, 4, 682–694. [Google Scholar] [CrossRef]
- Avetisyan, A.; Markosian, A.; Petrosyan, M.; Sahakyan, N.; Babayan, A.; Aloyan, S.; Trchounian, A. Chemical composition and some biological activities of the essential oils from basil Ocimum different cultivars. BMC Complement. Altern. Med. 2017, 17, 60. [Google Scholar] [CrossRef]
- Mbegbu, N.; Nwajinka, C.; Amaefule, D. Thin layer drying models and characteristics of scent leaves (Ocimum gratissimum) and lemon basil leaves (Ocimum africanum). Heliyon 2021, 7, e05945. [Google Scholar] [CrossRef]
- Lorenzi, H.; Matos, F.J.d.A.; Cavalleiro, A.d.S.; Brochini, V.F.; Souza, V.C. Plantas Medicinais no Brasil: Nativas e Exóticas, 3rd ed.; Instituo Plantarum: Nova Odessa, Brazil, 2021; 576p. [Google Scholar]
- Mahapatra, S.K.; Roy, S. Phytopharmacological approach of free radical scavenging and anti-oxidative potential of eugenol and Ocimum gratissimum Linn. Asian Pac. J. Trop. Med. 2014, 7, S391–S397. [Google Scholar] [CrossRef] [PubMed]
- Priyanka, C.; Shivika, S.; Vikas, S. Ocimum gratissimum: A Review on Ethnomedicinal Properties, Phytochemical Constituents, and Pharmacological Profile. In Biotechnological Approaches for Medicinal and Aromatic Plants: Conservation, Genetic Improvement and Utilization; Kumar, N., Ed.; Springer: Singapore, 2018; pp. 251–270. [Google Scholar] [CrossRef]
- Ugbogu, O.C.; Emmanuel, O.; Agi, G.O.; Ibe, C.; Ekweogu, C.N.; Ude, V.C.; Uche, M.E.; Nnanna, R.O.; Ugbogu, E.A. A review on the traditional uses, phytochemistry, and pharmacological activities of clove basil (Ocimum gratissimum L.). Heliyon 2021, 7, e08404. [Google Scholar] [CrossRef] [PubMed]
- Di Stasi, L.C.; Oliveira, G.P.; Carvalhaes, M.A.; Queiroz, M., Jr.; Tien, O.S.; Kakinami, S.H.; Reis, M.S. Medicinal plants popularly used in the Brazilian Tropical Atlantic Forest. Fitoterapia 2002, 73, 69–91. [Google Scholar] [CrossRef] [PubMed]
- de Souza Valente, C.; dos Santos, G.; Becker, A.G.; Heinzmann, B.M.; Caron, B.O.; Baldisserotto, B.; Ballester, E.L.C. Anaesthetic effect of clove basil (Ocimum gratissimum L.) essential oil on the giant river prawn (Macrobrachium rosenbergii, De Man 1879) exposed to different water pHs. Aquac. Int. 2024, 32, 1493–1505. [Google Scholar] [CrossRef]
- Silva, L.d.L.; Parodi, T.V.; Reckziegel, P.; Garcia, V.d.O.; Bürger, M.E.; Baldisserotto, B.; Malmann, C.A.; Pereira, A.M.S.; Heinzmann, B.M. Essential oil of Ocimum gratissimum L.: Anesthetic effects, mechanism of action and tolerance in silver catfish, Rhamdia quelen. Aquaculture 2012, 350–353, 91–97. [Google Scholar] [CrossRef]
- Meneses, J.; do Couto, M.; Sousa, N.; Cunha, F.d.S.; Abe, H.; Ramos, F.; Chagas, E.; Chaves, F.; Martins, M.; Maria, A. Efficacy of Ocimum gratissimum essential oil against the monogenean Cichlidogyrus tilapiae gill parasite of Nile tilapia. Arq. Bras. Med. Veterinária Zootec. 2018, 70, 497–504. [Google Scholar] [CrossRef]
- Bandeira Jr, G.; Pês, T.S.; Saccol, E.M.; Sutili, F.J.; Rossi Jr, W.; Murari, A.L.; Heinzmann, B.M.; Pavanato, M.A.; de Vargas, A.C.; Silva, L.d.L. Potential uses of Ocimum gratissimum and Hesperozygis ringens essential oils in aquaculture. Ind. Crops Prod. 2017, 97, 484–491. [Google Scholar] [CrossRef]
- Coyle, S.D.; Dasgupta, S.; Tidwell, J.H.; Beavers, T.; Bright, L.A.; Yasharian, D.K. Comparative efficacy of anesthetics for the freshwater prawn Macrobrachiurn rosenbergii. J. World Aquac. Soc. 2005, 36, 282–290. [Google Scholar] [CrossRef]
- Yossa, R.; Verdegem, M. Misuse of multiple comparison tests and underuse of contrast procedures in aquaculture publications. Aquaculture 2015, 437, 344–350. [Google Scholar] [CrossRef]
- de Souza Valente, C.; Coates, C.J.; Cagol, L.; Bombardelli, R.A.; Becker, A.G.; Schmidt, D.; Heinzmann, B.M.; Vaz-dos-Santos, A.M.; Baldisserotto, B.; Ballester, E.L.C. Antioxidant status and performance of Macrobrachium rosenbergii juveniles fed diets containing non-nutritive Aloysia triphylla essential oil. Aquac. Int. 2024, 1–14. [Google Scholar] [CrossRef]
- Zuur, A.F.; Ieno, E.N.; Walker, N.J.; Saveliev, A.A.; Smith, G.M. Mixed Effects Models and Extensions in Ecology with R; Springer: Berlin/Heidelberg, Germany, 2009; Volume 574. [Google Scholar]
- Motulsky, H.; Christopoulos, A. Fitting Models to Biological Data Using Linear and Nonlinear Regression: A Practical Guide to Curve Fitting; Oxford University Press: Oxford, UK, 2004. [Google Scholar]
- NIST/SEMATECH. Engineering Statistics: E-Handbook of Statistical Methods; NIST/SEMATECH: Gaithersburg, MD, USA, 2024. [CrossRef]
- Parliament, U. Animal Welfare (Sentience) Bill [HL]. In Bill 255; Parliament, U., Ed.; House of Lords: London, UK, 2022. [Google Scholar]
- Gherardi, F. Behavioural indicators of pain in crustacean decapods. Ann. Dell’istituto Super. Sanità 2009, 45, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Elwood, R.W. Potential pain in fish and decapods: Similar experimental approaches and similar results. Front. Vet. Sci. 2021, 8, 631151. [Google Scholar] [CrossRef] [PubMed]
- Crump, A.; Browning, H.; Schnell, A.; Burn, C.; Birch, J. Sentience in decapod crustaceans: A general framework and review of the evidence. Anim. Sentience 2022, 7, 1. [Google Scholar] [CrossRef]
- Becker, A.J.; Vaz, L.J.; Garcia, L.d.O.; Wasielesky Jr, W.; Heinzmann, B.M.; Baldisserotto, B. Anesthetic potential of different essential oils for two shrimp species, Farfantepenaeus paulensis and Litopenaeus vannamei (Decapoda, Crustacea). Ciência Rural 2021, 51, e20200793. [Google Scholar] [CrossRef]
- Boaventura, T.P.; Souza, C.F.; Ferreira, A.L.; Favero, G.C.; Baldissera, M.D.; Heinzmann, B.M.; Baldisserotto, B.; Luz, R.K. Essential oil of Ocimum gratissimum (Linnaeus, 1753) as anesthetic for Lophiosilurus alexandri: Induction, recovery, hematology, biochemistry and oxidative stress. Aquaculture 2020, 529, 735676. [Google Scholar] [CrossRef]
- Ribeiro, A.S.; Batista, E.d.S.; Dairiki, J.K.; Chaves, F.C.M.; Inoue, L.A.K.A. Anesthetic properties of Ocimum gratissimum essential oil for juvenile matrinxã. Acta Scientiarum. Anim. Sci. 2016, 38, 1–7. [Google Scholar] [CrossRef]
- Benovit, S.C.; Gressler, L.T.; de Lima Silva, L.; de Oliveira Garcia, L.; Okamoto, M.H.; dos Santos Pedron, J.; Sampaio, L.A.; Rodrigues, R.V.; Heinzmann, B.M.; Baldisserotto, B. Anesthesia and transport of Brazilian flounder, Paralichthys orbignyanus, with essential oils of Aloysia gratissima and Ocimum gratissimum. J. World Aquac. Soc. 2012, 43, 896. [Google Scholar] [CrossRef]
- Ferreira, A.L.; Favero, G.C.; Boaventura, T.P.; de Freitas Souza, C.; Ferreira, N.S.; Descovi, S.N.; Baldisserotto, B.; Heinzmann, B.M.; Luz, R.K. Essential oil of Ocimum gratissimum (Linnaeus, 1753): Efficacy for anesthesia and transport of Oreochromis niloticus. Fish Physiol. Biochem. 2021, 47, 135–152. [Google Scholar] [CrossRef]
- da Silva, L.A.; Martins, M.A.; Santo, F.E.; Oliveira, F.C.; Chaves, F.C.M.; Chagas, E.C.; Martins, M.L.; de Campos, C.M. Essential oils of Ocimum gratissimum and Zingiber officinale as anesthetics for the South American catfish Pseudoplatystoma reticulatum. Aquaculture 2020, 528, 735595. [Google Scholar] [CrossRef]
- Abdel-Tawwab, M.; Adeshina, I.; Jenyo-Oni, A.; Ajani, E.K.; Emikpe, B.O. Growth, physiological, antioxidants, and immune response of African catfish, Clarias gariepinus (B.), to dietary clove basil, Ocimum gratissimum, leaf extract and its susceptibility to Listeria monocytogenes infection. Fish Shellfish Immunol. 2018, 78, 346–354. [Google Scholar] [CrossRef]
- Efferth, T.; Koch, E. Complex Interactions between Phytochemicals. The Multi-Target Therapeutic Concept of Phytotherapy. Curr. Drug Targets 2011, 12, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Spoors, F.; James, M.A.; Mendo, T.; McKnight, J.C.; Bønnelycke, E.-M.S.; Khan, N. Investigating clove oil and its derivatives as anaesthetic agents for decapod crustaceans to improve welfare commercially and at slaughter. Front. Anim. Sci. 2023, 4, 1180977. [Google Scholar] [CrossRef]
- Lehotzky, D.; Eske, A.I.; Zupanc, G.K.H. The effect of eugenol anesthesia on the electric organ discharge of the weakly electric fish Apteronotus leptorhynchus. Fish Physiol. Biochem. 2023, 49, 1321–1338. [Google Scholar] [CrossRef] [PubMed]
- Chung, G.; Oh, S.B. Eugenol as Local Anesthetic. In Natural Products: Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes; Ramawat, K.G., Mérillon, J.-M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 4001–4015. [Google Scholar] [CrossRef]
- Kaewmalun, S.; Yata, T.; Kitiyodom, S.; Yostawonkul, J.; Namdee, K.; Kamble, M.T.; Pirarat, N. Clove Oil-Nanostructured Lipid Carriers: A Platform of Herbal Anesthetics in Whiteleg Shrimp (Penaeus vannamei). Foods 2022, 11, 3162. [Google Scholar] [CrossRef]
- Fidyt, K.; Fiedorowicz, A.; Strządała, L.; Szumny, A. β-caryophyllene and β-caryophyllene oxide—Natural compounds of anticancer and analgesic properties. Cancer Med. 2016, 5, 3007–3017. [Google Scholar] [CrossRef]
- Ghelardini, C.; Galeotti, N.; Di Cesare Mannelli, L.; Mazzanti, G.; Bartolini, A. Local anaesthetic activity of β-caryophyllene. Il Farm. 2001, 56, 387–389. [Google Scholar] [CrossRef]
- Moghrovyan, A.; Parseghyan, L.; Sevoyan, G.; Darbinyan, A.; Sahakyan, N.; Gaboyan, M.; Karabekian, Z.; Voskanyan, A. Antinociceptive, anti-inflammatory, and cytotoxic properties of Origanum vulgare essential oil, rich with β-caryophyllene and β-caryophyllene oxide. Korean J Pain 2022, 35, 140–151. [Google Scholar] [CrossRef]
- Boaventura, T.P.; dos Santos, F.A.C.; de Sena Souza, A.; Batista, F.S.; Júlio, G.S.C.; Luz, R.K. Thymol and linalool chemotypes of the essential oil of Thymus vulgaris (thyme) as anesthetic for Colossoma macropomum: Physiology and feed consumption. Aquaculture 2022, 554, 738161. [Google Scholar] [CrossRef]
- Kim, N.S.; Lee, D.S. Headspace solid-phase microextraction for characterization of fragrances of lemon verbena (Aloysia triphylla) by gas chromatography-mass spectrometry. J. Sep. Sci. 2004, 27, 96–100. [Google Scholar] [CrossRef]
- Sousa, O.V.; Silvério, M.S.; Del-Vechio-Vieira, G.; Matheus, F.C.; Yamamoto, C.H.; Alves, M.S. Antinociceptive and anti-inflammatory effects of the essential oil from Eremanthus erythropappus leaves. J. Pharm. Pharmacol. 2008, 60, 771–777. [Google Scholar] [CrossRef]
- de Souza, A.d.S.L.; Peret, A.C.; Hamoy, M.; de Souza, R.A.L.; Torres, M.F.; Barbas, L.A.L. Propofol and essential oil of Nepeta cataria induce anaesthesia and marked myorelaxation in tambaqui Colossoma macropomum: Implications on cardiorespiratory responses. Aquaculture 2019, 500, 160–169. [Google Scholar] [CrossRef]
- Francomano, F.; Caruso, A.; Barbarossa, A.; Fazio, A.; La Torre, C.; Ceramella, J.; Mallamaci, R.; Saturnino, C.; Iacopetta, D.; Sinicropi, M.S. β-Caryophyllene: A Sesquiterpene with Countless Biological Properties. Appl. Sci. 2019, 9, 5420. [Google Scholar] [CrossRef]
- da Silva, E.; Aldegunde, M.; da Silva, D.F.; Lopes, C.; Bertoldi, F.C.; Weber, R.A. Assessment of induction and recovery times of anaesthesia in Astyanax bimaculatus using 2-phenoxyethanol and the essential oils of Melaleuca alternifolia and Ocimum gratissimum. Aquac. Res. 2020, 51, 577–583. [Google Scholar] [CrossRef]
- Rucinque, D.S.; Ferreira, P.F.; Leme, P.R.P.; Lapa-Guimarães, J.; Viegas, E.M.M. Ocimum americanum and Lippia alba essential oils as anaesthetics for Nile tilapia: Induction, recovery of apparent unconsciousness and sensory analysis of fillets. Aquaculture 2021, 531, 735902. [Google Scholar] [CrossRef]
- Yigit, N.O.; Metin, S.; Sabuncu, O.F.; Didinen, B.I.; Didinen, H.; Ozmen, O.; Koskan, O. Efficiency of Ocimum basilicum and Eucalyptus globulus essential oils on anesthesia and histopathology of rainbow trout, Oncorhynchus mykiss. J. World Aquac. Soc. 2022, 53, 1051–1061. [Google Scholar] [CrossRef]
- Can, E.; Kizak, V.; Seyhaneyildiz Can, Ş.; Özçiçek, E. Anesthetic potential of geranium (Pelargonium graveolens) oil for two cichlid species, Sciaenochromis fryeri and Labidochromis caeruleus. Aquaculture 2018, 491, 59–64. [Google Scholar] [CrossRef]
- de Lima Boijink, C.; Queiroz, C.A.; Chagas, E.C.; Chaves, F.C.M.; Inoue, L.A.K.A. Anesthetic and anthelminthic effects of clove basil (Ocimum gratissimum) essential oil for tambaqui (Colossoma macropomum). Aquaculture 2016, 457, 24–28. [Google Scholar] [CrossRef]
- Yigit, N.O.; Metin, S.; Didinen, B.I.; Didinen, H.; Ozmen, O. Effect of lavander (Lavandula angustifolia) and laurel (Laurus nobilis) essential oils as anesthesics in rainbow trout (Oncorhynchus mykiss). Aquaculture 2022, 557, 738328. [Google Scholar] [CrossRef]
- Vilhena, C.S.; do Nascimento, L.A.S.; de Aguiar Andrade, E.H.; da Silva, J.K.d.R.; Hamoy, M.; Torres, M.F.; Barbas, L.A.L. Essential oil of Piper divaricatum induces a general anaesthesia-like state and loss of skeletal muscle tonus in juvenile tambaqui, Colossoma macropomum. Aquaculture 2019, 510, 169–175. [Google Scholar] [CrossRef]
- Ferraz, E.d.O.; Vieira, M.A.R.; Ferreira, M.I.; Fernandes Junior, A.; Marques, M.O.M.; Minatel, I.O.; Albano, M.; Sambo, P.; Lima, G.P.P. Seasonality effects on chemical composition, antibacterial activity and essential oil yield of three species of Nectandra. PLoS ONE 2018, 13, e0204132. [Google Scholar] [CrossRef]
- Myatt, J. Pharmacology of Inhalational Anaesthetics. Update Anaesth. Educ. Anaesth. Worldw. 2008, 24, 102–107. [Google Scholar]
- Khan, K.S.; Hayes, I.; Buggy, D.J. Pharmacology of anaesthetic agents II: Inhalation anaesthetic agents. Contin. Educ. Anaesth. Crit. Care Pain 2013, 14, 106–111. [Google Scholar] [CrossRef]
- Wouden, J.; Miller, K.W. General anesthetic pharmacology. In Principles of Pharmacology: The Pathophysiologic Basis of Drug Therapy, 3rd ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2012; pp. 240–259. [Google Scholar]
- Hudson, A.E.; Herold, K.F.; Hemmings, H.C. Chapter 10—Pharmacology of Inhaled Anesthetics. In Pharmacology and Physiology for Anesthesia; Hemmings, H.C., Egan, T.D., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2013; pp. 159–179. [Google Scholar] [CrossRef]
- Li, Y.; She, Q.; Han, Z.; Sun, N.; Liu, X.; Li, X. Anaesthetic Effects of Eugenol on Grass Shrimp (Palaemonetes sinensis) of Different Sizes at Different Concentrations and Temperatures. Sci. Rep. 2018, 8, 11007. [Google Scholar] [CrossRef] [PubMed]
- Parodi, T.V.; Cunha, M.A.; Heldwein, C.G.; de Souza, D.M.; Martins, Á.C.; Garcia, L.d.O.; Junior, W.W.; Monserrat, J.M.; Schmidt, D.; Caron, B.O. The anesthetic efficacy of eugenol and the essential oils of Lippia alba and Aloysia triphylla in post-larvae and sub-adults of Litopenaeus vannamei (Crustacea, Penaeidae). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2012, 155, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Bodur, T.; Afonso, J.M.; Montero, D.; Navarro, A. Assessment of effective dose of new herbal anesthetics in two marine aquaculture species: Dicentrarchus labrax and Argyrosomus regius. Aquaculture 2018, 482, 78–82. [Google Scholar] [CrossRef]
- Marking, L.L.; Meyer, F.P. Are Better Anesthetics Needed in Fisheries? Fisheries 1985, 10, 2–5. [Google Scholar] [CrossRef]
| Model | a | ±S.E. | b | ±S.E. | c | ±S.E. | d | ±S.E. | F | p-Value | S.E. Regression |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sedation time | 1.862 × 10−5 | 0.0000 | −0.009 | 0.0011 | 1.248 | 0.1657 | 1.7956 | 6.8086 | 73.046 | 2.59 × 10−31 | 10.13 |
| Anaesthesia time | 3.559 × 10−5 | 0.0000 | −0.018 | 0.0018 | 2.591 | 0.2658 | −37.1887 | 10.9733 | 47.013 | 1.74 × 10−22 | 16.32 |
| Recovery time | 2.737 × 10−4 | 0.0000 | −0.136 | 0.0230 | 17.033 | 3.4830 | 522.9480 | 145.1070 | 41.075 | 1.35 × 10−20 | 219.44 |
| EO-OG Concentrations (μL L−1) | Mean (s) | S.E. | Min (s) | Max (s) | |
|---|---|---|---|---|---|
| Sedation | 50 | 44.80 | 1.22 | 31.00 | 58.00 |
| 100 | 41.53 | 1.80 | 25.00 | 63.00 | |
| 150 | 49.24 | 1.18 | 33.00 | 62.00 | |
| 200 | 15.98 | 0.63 | 10.00 | 23.00 | |
| 300 | 25.85 | 1.29 | 14.00 | 47.00 | |
| Anaesthesia | 50 | 54.17 | 1.12 | 40.00 | 66.00 |
| 100 | 60.43 | 2.57 | 32.00 | 95.00 | |
| 150 | 80.10 | 2.97 | 41.00 | 112.00 | |
| 200 | 22.52 | 1.02 | 12.00 | 45.00 | |
| 300 | 48.16 | 1.68 | 30.00 | 80.00 | |
| Recovery | 50 | 1006.60 | 25.20 | 649.00 | 1296.00 |
| 100 | 1367.10 | 19.80 | 1116.00 | 1554.00 | |
| 150 | 630.10 | 11.30 | 502.00 | 770.00 | |
| 200 | 829.60 | 14.40 | 693.00 | 1005.00 | |
| 300 | 765.00 | 15.30 | 600.00 | 948.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Souza Valente, C.; Mendes, A.F.; do Nascimento Ferreira, C.H.; Baldisserotto, B.; Heinzmann, B.M.; Vaz-dos-Santos, A.M.; Ballester, E.L.C. Anaesthetic Effect of Clove Basil (Ocimum gratissimum) Essential Oil on Macrobrachium rosenbergii Post-Larvae. Aquac. J. 2024, 4, 192-202. https://doi.org/10.3390/aquacj4030014
de Souza Valente C, Mendes AF, do Nascimento Ferreira CH, Baldisserotto B, Heinzmann BM, Vaz-dos-Santos AM, Ballester ELC. Anaesthetic Effect of Clove Basil (Ocimum gratissimum) Essential Oil on Macrobrachium rosenbergii Post-Larvae. Aquaculture Journal. 2024; 4(3):192-202. https://doi.org/10.3390/aquacj4030014
Chicago/Turabian Stylede Souza Valente, Cecília, Arielly Fávaro Mendes, Caio Henrique do Nascimento Ferreira, Bernardo Baldisserotto, Berta Maria Heinzmann, André Martins Vaz-dos-Santos, and Eduardo Luis Cupertino Ballester. 2024. "Anaesthetic Effect of Clove Basil (Ocimum gratissimum) Essential Oil on Macrobrachium rosenbergii Post-Larvae" Aquaculture Journal 4, no. 3: 192-202. https://doi.org/10.3390/aquacj4030014
APA Stylede Souza Valente, C., Mendes, A. F., do Nascimento Ferreira, C. H., Baldisserotto, B., Heinzmann, B. M., Vaz-dos-Santos, A. M., & Ballester, E. L. C. (2024). Anaesthetic Effect of Clove Basil (Ocimum gratissimum) Essential Oil on Macrobrachium rosenbergii Post-Larvae. Aquaculture Journal, 4(3), 192-202. https://doi.org/10.3390/aquacj4030014







