Study of Carbon Nanotube–Bovine Serum Albumin Interaction Using the Tritium Radiotracer Technique and Supercomputer Simulation †
Abstract
:1. Introduction
2. Materials and Methods
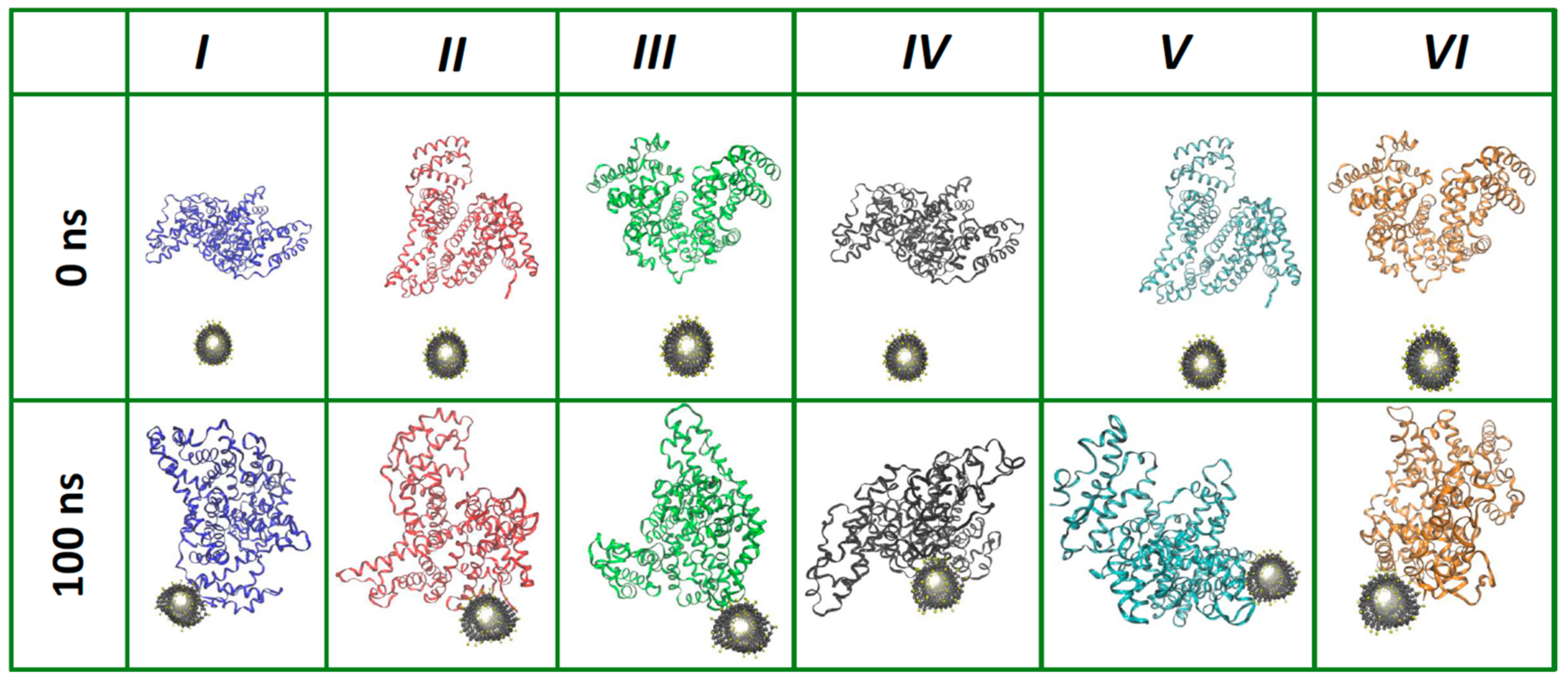
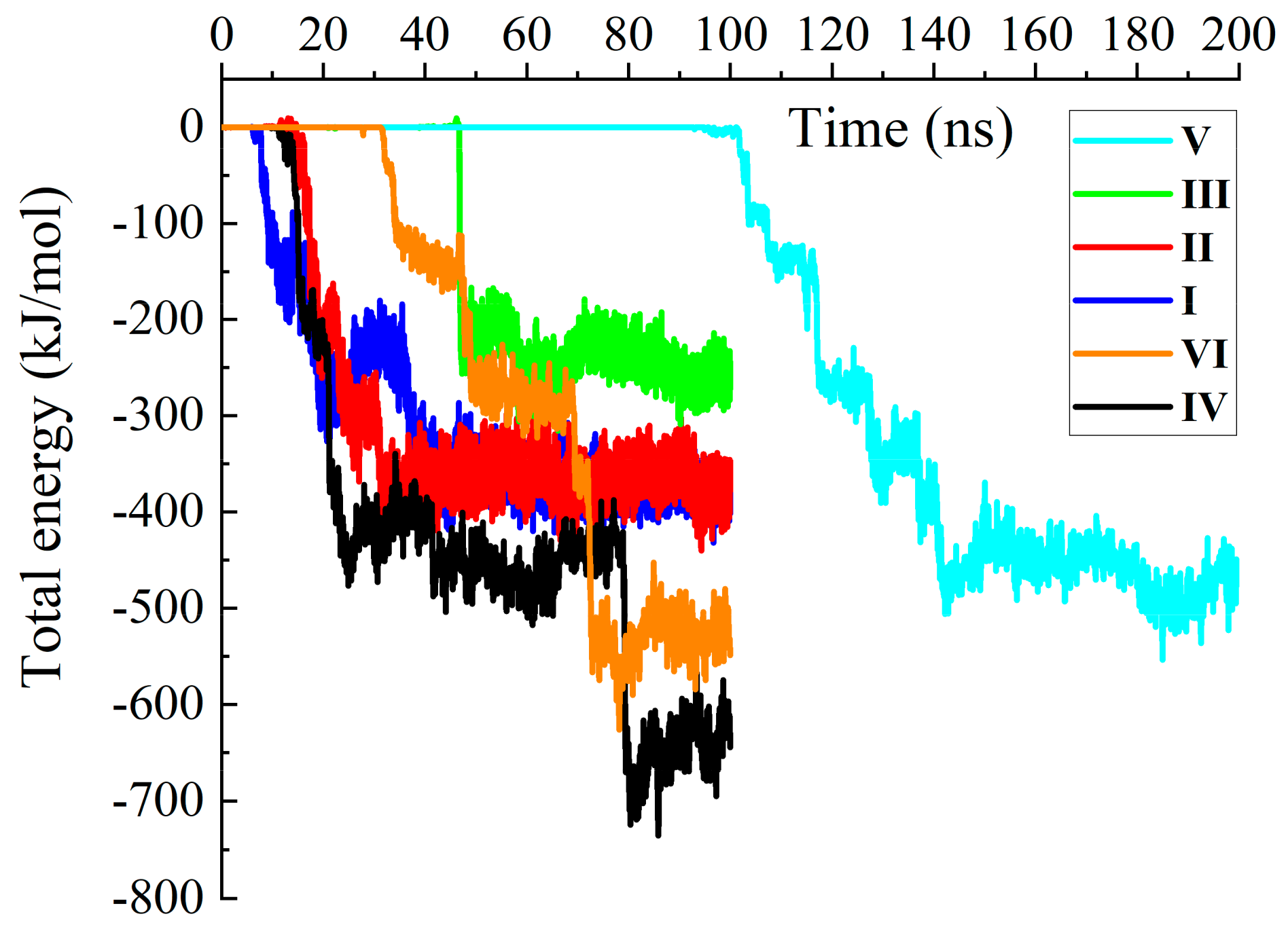
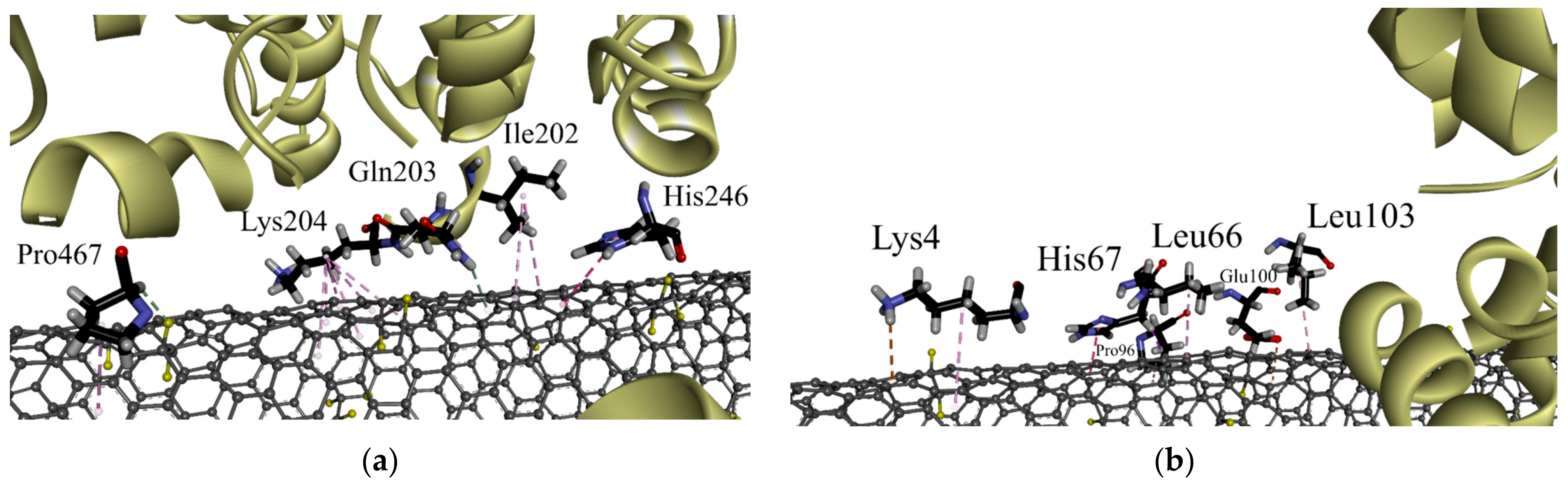
2.1. Preparation of CNT/BSA Model Structure and Supercomputer Simulation
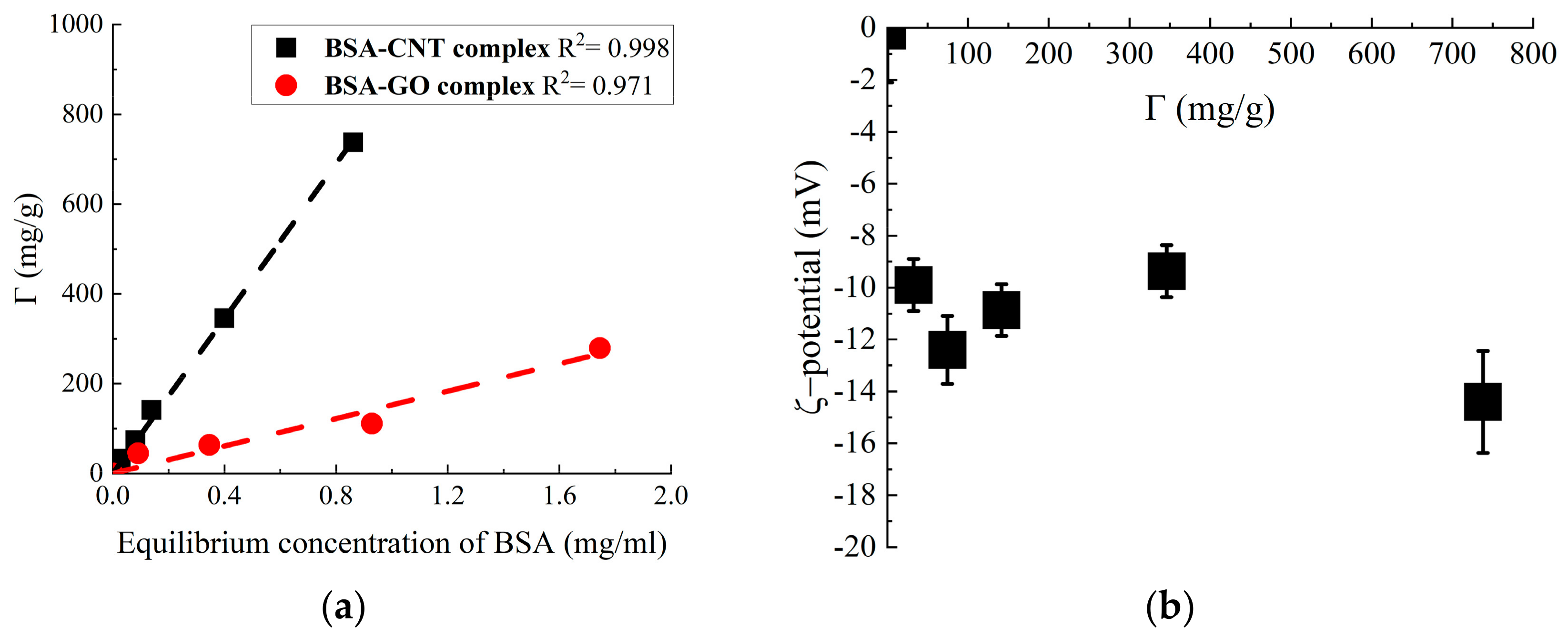
2.2. BSA on SWCNT Adsorption
3. Results and Discussion
- Γ—BSA on SWCNTs adsorption, mg/mg;
- KH—adsorption–desorption equilibrium constant, mL/mg;
- C—BSA concentration in equilibrium solution, mg/mL [22].
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sengupta, J.; Hussain, C.M. CNT and Graphene-Based Transistor Biosensors for Cancer Detection: A Review. Biomolecules 2023, 13, 1024. [Google Scholar] [CrossRef]
- Markov, A.; Wördenweber, R.; Ichkitidze, L.; Gerasimenko, A.; Kurilova, U.; Suetina, I.; Mezentseva, M.; Offenhäusser, A.; Telyshev, D. Biocompatible Swcnt Conductive Composites for Biomedical Applications. Nanomaterials 2020, 10, 2492. [Google Scholar] [CrossRef] [PubMed]
- Saberi, A.; Baltatu, M.S.; Vizureanu, P. The Effectiveness Mechanisms of Carbon Nanotubes (CNTs) as Reinforcements for Magnesium-Based Composites for Biomedical Applications: A Review. Nanomaterials 2024, 14, 756. [Google Scholar] [CrossRef]
- De Simone, G.; Di Masi, A.; Ascenzi, P. Serum Albumin: A Multifaced Enzyme. Int. J. Mol. Sci. 2021, 22, 10086. [Google Scholar] [CrossRef] [PubMed]
- Badun, G.A.; Chernysheva, M.G. Tritium Thermal Activation Method. Features of Application, Modern Achievements, and Further Development Prospects. Radiochemistry 2023, 65, 185–197. [Google Scholar] [CrossRef]
- Önal Acet, B.; Gül, D.; Stauber, R.H.; Odabaşı, M.; Acet, Ö. A Review for Uncovering the “Protein-Nanoparticle Alliance”: Implications of the Protein Corona for Biomedical Applications. Nanomaterials 2024, 14, 823. [Google Scholar] [CrossRef] [PubMed]
- Lu, N.; Sui, Y.; Ding, Y.; Tian, R.; Li, L.; Liu, F. Adsorption of Human Serum Albumin on Functionalized Single-Walled Carbon Nanotubes Reduced Cytotoxicity. Chem. Biol. Interact. 2018, 295, 64–72. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, Y.; Chen, M.; Yan, M.; Zeng, G.; Huang, D. How Do Proteins ‘Response’ to Common Carbon Nanomaterials? Adv. Colloid Interface Sci. 2019, 270, 101–107. [Google Scholar] [CrossRef]
- Park, S.J.; Khang, D. Conformational Changes of Fibrinogen in Dispersed Carbon Nanotubes. Int. J. Nanomedicine 2012, 7, 4325–4333. [Google Scholar] [CrossRef]
- Ma, G.J.; Ferhan, A.R.; Jackman, J.A.; Cho, N.J. Conformational Flexibility of Fatty Acid-Free Bovine Serum Albumin Proteins Enables Superior Antifouling Coatings. Commun. Mater. 2020, 1, 45. [Google Scholar] [CrossRef]
- Chernysheva, M.G.; Badun, G.A. In Vitro Study of Proteins Surface Activity by Tritium Probe. J. Radioanal. Nucl. Chem. 2010, 286, 835–840. [Google Scholar] [CrossRef]
- Hai, Y.; Qu, K.; Liu, Y.; Zhao, C. Binding Mechanism of Single-Walled Carbon Nanotubes (SWCNTs) to Serum Albumin: Spectroscopy and Molecular Modelling Exploration. Environ. Chem. 2018, 15, 278. [Google Scholar] [CrossRef]
- Hampitak, P.; Melendrez, D.; Iliut, M.; Fresquet, M.; Parsons, N.; Spencer, B.; Jowitt, T.A.; Vijayaraghavan, A. Protein Interactions and Conformations on Graphene-Based Materials Mapped Using a Quartz-Crystal Microbalance with Dissipation Monitoring (QCM-D). Carbon 2020, 165, 317–327. [Google Scholar] [CrossRef]
- Sinha, S.; Tam, B.; Wang, S.M. Applications of Molecular Dynamics Simulation in Protein Study. Membranes 2022, 12, 844. [Google Scholar] [CrossRef]
- Balamurugan, K.; Gopalakrishnan, R.; Raman, S.S.; Subramanian, V. Exploring the Changes in the Structure of α-Helical Peptides Adsorbed onto a Single Walled Carbon Nanotube Using Classical Molecular Dynamics Simulation. J. Phys. Chem. B 2010, 114, 14048–14058. [Google Scholar] [CrossRef]
- Vidossich, P.; Magistrato, A. QM/MM Molecular Dynamics Studies of Metal Binding Proteins. Biomolecules 2014, 4, 616–645. [Google Scholar] [CrossRef] [PubMed]
- Voevodin, V.V.; Antonov, A.S.; Nikitenko, D.A.; Shvets, P.A.; Sobolev, S.I.; Sidorov, I.Y.; Stefanov, K.S.; Voevodin, V.V.; Zhumatiy, S.A. Supercomputer Lomonosov-2: Large Scale, Deep Monitoring and Fine Analytics for the User Community. Supercomput. Front. Innov. 2019, 6, 4–11. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A Visualization System for Exploratory Research and Analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Bunyaev, V.A.; Chernysheva, M.G.; Badun, G.A. Chitosan and Serum Albumin as Carbon Nanotube Modifiers. Russ. J. Phys. Chem. A 2024, 98, 1388–1395. [Google Scholar]
- Kusnezov, D.; Bulgac, A.; Bauer, W. Canonical Ensembles from Chaos. Ann. Phys. 1990, 204, 155–185. [Google Scholar] [CrossRef]
- Peters, T. Serum Albumin. Adv. Protein Chem. 1985, 37, 161–245. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.M. VI.—The Adsorption Isotherm at Low Concentrations. Proc. R. Soc. Edinburgh 1920, 39, 48–55. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bunyaev, V.A.; Sinolits, A.V.; Badun, G.A. Study of Carbon Nanotube–Bovine Serum Albumin Interaction Using the Tritium Radiotracer Technique and Supercomputer Simulation. Biol. Life Sci. Forum 2024, 35, 5. https://doi.org/10.3390/blsf2024035005
Bunyaev VA, Sinolits AV, Badun GA. Study of Carbon Nanotube–Bovine Serum Albumin Interaction Using the Tritium Radiotracer Technique and Supercomputer Simulation. Biology and Life Sciences Forum. 2024; 35(1):5. https://doi.org/10.3390/blsf2024035005
Chicago/Turabian StyleBunyaev, Vitalii A., Artem V. Sinolits, and Gennadii A. Badun. 2024. "Study of Carbon Nanotube–Bovine Serum Albumin Interaction Using the Tritium Radiotracer Technique and Supercomputer Simulation" Biology and Life Sciences Forum 35, no. 1: 5. https://doi.org/10.3390/blsf2024035005






