Morphological Aspects in Ultrasound Visualisation of the Suprascapular Notch Region: A Study Based on a New Four-Step Protocol
Abstract
:1. Introduction
2. Material and Methods
- (a)
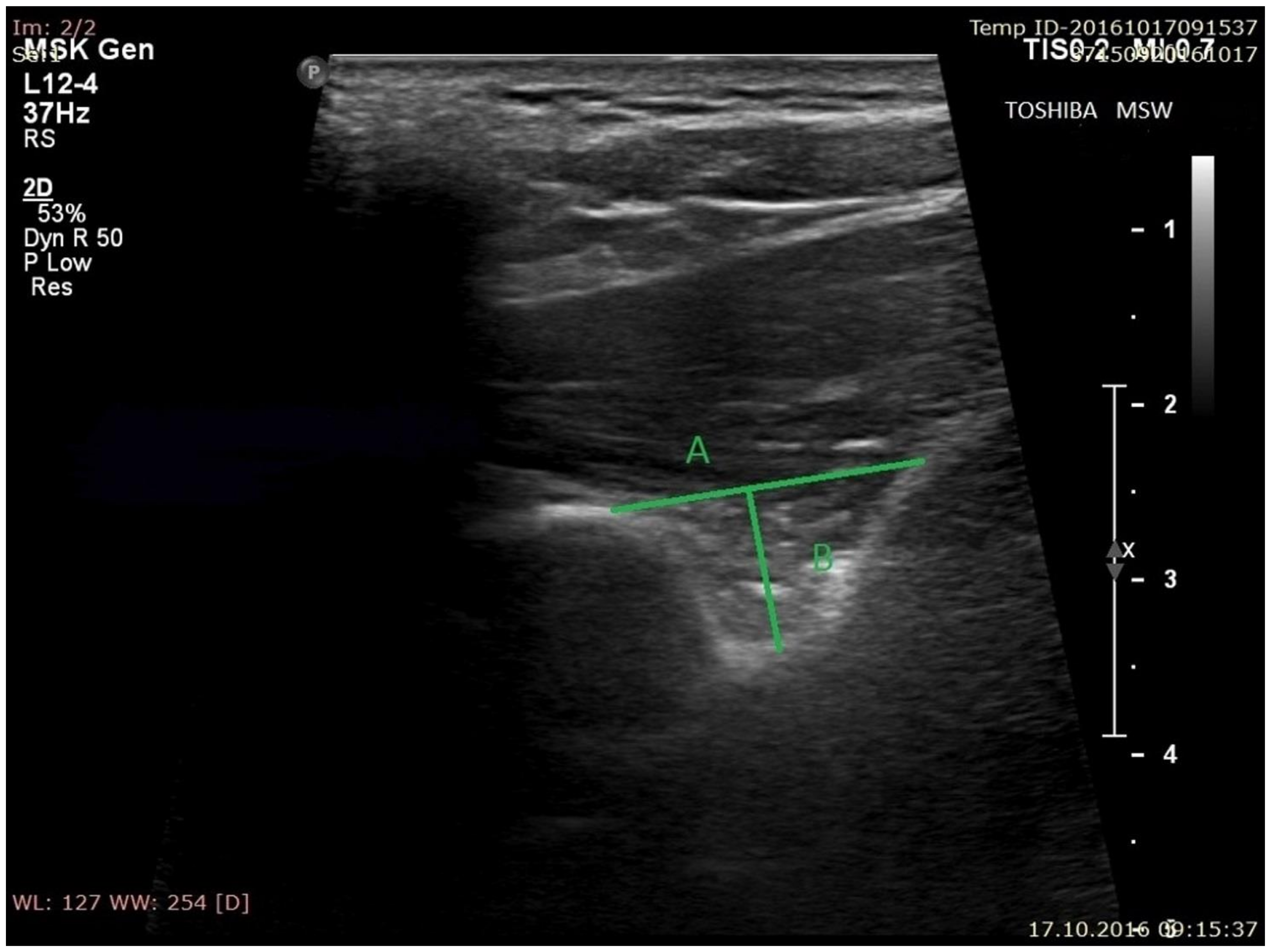
- the superior transverse diameter (STD) of the suprascapular notch: the maximal distance in the horizontal plane between the corners of the suprascapular notch (Figure 2).
- (b)
- the maximal depth (MD) of the suprascapular notch: the distance between the STD and the deepest point of the suprascapular notch measured in a plane perpendicular to the STD (Figure 2).
- (c)
- the diameter of the suprascapular artery (Figure 3A)
- (d)
- the diameter of the suprascapular vein (Figure 3B)
- (e)
- the diameter of the suprascapular nerve (Figure 3C)
- (f)
- the thickness of the soft tissue over the suprascapular artery: the minimal distance between the suprascapular artery and the skin (Figure 4A)
- (g)
- the thickness of the soft tissue over the suprascapular vein: the minimal distance between the suprascapular vein and the skin (Figure 4B)
- (h)
- the thickness of the soft tissue over the suprascapular nerve: the minimal distance between the suprascapular nerve and the skin (Figure 4C).
Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgements
Data Availability
Conflict of interest
References
- Duparc, F.; Coquerel, D.; Ozeel, J.; Noyon, M.; Gerometta, A.; Michot, C. Anatomical basis of the suprascapular nerve entrapment, and clinical relevance of the supraspinatus fascia. Surg. Radiol. Anat. 2010, 32, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Yamakado, K. The suprascapular notch narrows with aging: A preliminary solution of the old conjecture based on a 3D-CT evaluation. Surg. Radiol. Anat. 2016, 38, 693–697. [Google Scholar] [CrossRef] [PubMed]
- Bayramoglu, A.; Demiryurek, D.; Tuccar, E.; Erbil, M.; Aldur, M.M.; Tetik, O.; Doral, M.N. Variations in anatomy at the suprascapular notch possibly causing suprascapular nerve entrapment: An anatomical study. Knee Surg. Sports Trum. Arthrosc. 2003, 11, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Polguj, M.; Jedrzejewski, K.; Podgorski, M.; Majos, A.; Topol, M. A proposal for classification of the superior transverse scapular ligament: Variable morphology and its potential influence on suprascapular nerve entrapment. J. Shoulder Elbow Surg. 2013, 22, 1265–1273. [Google Scholar] [CrossRef] [PubMed]
- Avery, B.W.; Pilon, F.M.; Barclay, J.K. Anterior coracoscapular ligament and suprascapular nerve entrapment. Clin. Anat. 2002, 15, 383–386. [Google Scholar] [CrossRef] [PubMed]
- Polguj, M.; Jędrzejewski, K.; Topol, M. Variable morphology of the anterior coracoscapular ligament—A proposal of classification. Ann. Anat. 2013, 195, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.J.; Gil, Y.C.; Jin, J.D.; Ahn, S.V.; Lee, H.Y. Topographical anatomy of the suprascapular nerve and vessels at the suprascapular notch. Clin. Anat. 2012, 25, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Polguj, M.; Rożniecki, J.; Sibiński, M.; Grzegorzewski, A.; Majos, A.; Topol, M. The variable morphology of suprascapular nerve and vessels at the suprascapular notch—A proposal for classification and its potential clinical implications. Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 1542–1548. [Google Scholar] [CrossRef] [PubMed]
- Gosk, J.; Urban, M.; Rutkowski, R. Entrapment of the suprascapular nerve: Anatomy, etiology, diagnosis, treatment. Ortop. Traumatol. Rehabil. 2007, 9, 68–74. [Google Scholar] [PubMed]
- Moen, T.C.; Babatunde, O.M.; Hsu, S.H.; Ahmad, C.S.; Levine, W.N. Suprascapular neuropathy: What does the literature show? J. Shoulder Elbow Surg. 2012, 21, 835–846. [Google Scholar] [CrossRef] [PubMed]
- Łabętowicz, P.; Synder, M.; Wojciechowski, M.; Orczyk, K.; Jezierski, H.; Topol, M.; Polguj, M. Protective and Predisposing Morphological Factors in Suprascapular Nerve Entrapment Syndrome: A Fundamental Review Based on Recent Observations. BioMed Res. Int. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Polguj, M.; Jędrzejewski, K.; Majos, A.; Topol, M. The trifid superior transverse scapular ligament: A case report and review of the literature. Folia Morph. 2012, 71, 118–120. [Google Scholar]
- Polguj, M.; Jędrzejewski, K.; Majos, A.; Topol, M. Variations in bifid superior transverse scapular ligament as a possible factor of suprascapular entrapment: An anatomic study. Int. Orthop. 2012, 36, 2095–2100. [Google Scholar] [CrossRef] [PubMed]
- Polguj, M.; Sibinski, M.; Grzegorzewski, A.; Waszczykowski, M.; Majos, A.; Topol, M. Morphological and radiological study of ossified superior transverse scapular ligament as potential risk factor of suprascapular nerve entrapment. BioMed Res. Int. 2014. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, B.; Sudoł-Szopińska, I. Normal and sonographic anatomy of selected peripheral nerves. Part II: Peripheral nerves of the upper limb. J. Ultrason. 2012, 12, 131–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinoli, C.; Bianchi, S.; Gandolfo, N.; Valle, M.; Simonetti, S.; Derchi, L.E. US of nerve entrapments in osteofibrous tunnels of the upper and lower limbs. Radiographics 2000, 20, S199–S213. [Google Scholar] [CrossRef] [PubMed]
- Polguj, M.; Synder, M.; Kwapisz, A.; Stefańczyk, K.; Grzelak, P.; Podgórski, M.; Topol, M. Clinical evaluation of the shape of the suprascapular notch—An ultrasonographic and computed tomography comparative study: Application to shoulder pain syndromes. Clin. Anat. 2015, 28, 774–779. [Google Scholar] [CrossRef] [PubMed]
- Karatas, G.K.; Meray, J. Suprascapular nerve block for pain relief in adhesive capsulitis: Comparison of 2 different techniques. Arch. Phys. Med. Rehabil. 2002, 83, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Boykin, R.E.; Friedman, D.J.; Zimmer, Z.R.; Oaklander, A.L.; Higgins, L.D.; Warner, J.J. Suprascapular neuropathy in a shoulder referral practice. J. Shoulder Elbow Surg. 2011, 20, 983–988. [Google Scholar] [CrossRef] [PubMed]
- Casanova, M.G.; Choi, S.; McHardy, P.G. Ultrasound-guided posterior cord and selective suprascapular block for shoulder surgery. Br. J. Anaesth. 2016, 117, 835. [Google Scholar] [CrossRef] [PubMed]
- Jezierski, H.; Podgórski, M.; Stefańczyk, L.; Kachlik, D.; Polguj, M. The influence of suprascapular notch shape on the visualization of structures in the suprascapular notch region—Studies based on a new four-stage ultrasonographic protocol. BioMed Res. Int. 2017. [Google Scholar] [CrossRef] [PubMed]
- Dangoisse, M.J.; Wilson, D.J.; Glynn, C.J. MRI and clinical study of an easy and safe technique of suprascapular nerve blockade. Acta. Anaesthesiol. Belg. 1994, 45, 49–54. [Google Scholar] [PubMed]
- Milowsky, J.; Rovenstine, E.A. Suprascapular nerve block; evaluation in the therapy of shoulder pain. Anesthesiology 1949, 10, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Gorthi, V.; Moon, Y.L.; Kang, J.H. The effectiveness of ultrasonography-guided suprascapular nerve block for perishoulder pain. Orthopedics 2010, 33. [Google Scholar] [CrossRef] [PubMed]
- Rothe, C.; Steen-Hansen, C.; Lund, J.; Jenstrup, M.T.; Lange, K.H. Ultrasound-guided block of the suprascapular nerve—A volunteer study of a new proximal approach. Acta. Anaesthesiol. Scand. 2014, 58, 1228–1232. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, P.J.; Haun, D.W.; Dooley, K.; Kettner, N.W. Sonographic measurement of the normal suprascapular nerve and omohyoid muscle. Man. Ther. 2014, 19, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Laumonerie, P.; Lapegue, F.; Chantalat, E.; Sans, N.; Mansat, P.; Faruch, M. Description and ultrasound targeting of the origin of the suprascapular nerve. Clin. Anat. 2017, 30, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Schneider-Kolsky, M.E.; Pike, J.; Connell, D.A. CT-guided suprascapular nerve blocks: A pilot study. Skeletal. Radiol. 2004, 33, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Marhofer, P.; Greher, M.; Kapral, S. Ultrasound guidance in regional anaesthesia. Br. J. Anaesth. 2005, 94, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Yücesoy, C.; Akkaya, T.; Ozel, O.; Cömert, A.; Tüccar, E.; Bedirli, N.; Unlü, E.; Hekimoğlu, B.; Gümüş, H. Ultrasonographic evaluation and morphometric measurements of the suprascapular notch. Surg. Radiol. Anat. 2009, 31, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Harmon, D.; Hearty, C. Ultrasound-guided suprascapular nerve block technique. Pain Physician 2007, 10, 743–746. [Google Scholar] [PubMed]
- Smoljanovic, T.; Miklic, D.; Grgurevic, L. Diameter of suprascapular nerve in the suprascapular notch. Pain Physician 2008, 11, 263–264. [Google Scholar] [PubMed]
- Moriggl, B. Grundlagen, Moglichkeiten und Grenzen der Sonographie osteobroser Kanale im Schulterbereich, Teil. Ann. Anat. 1997, 179, 375–2392. [Google Scholar] [CrossRef]
- Okur, S.C.; Ozyemisci-Taskiran, O.; Pekindogan, Y.; Mert, M.; Caglar, N.S. Ultrasound-Guided Block of the Suprascapular Nerve in Breast Cancer Survivors with Limited Shoulder Motion—Case Series. Pain Physician 2017, 20, E233–E239. [Google Scholar] [PubMed]





| Type of Suprascapular Notch | All (n = 235) (n(%)) | BMI (n (SD)) | |
|---|---|---|---|
| I | 26 (11.1%) | 24.77 (3.49) | |
| II | 14 (6.0%) | 25.27 (3.67) | |
| III | 151 (64.0%) | 26.43 (4.02) | |
| IV/V | 44 (18.7%) | 27.38 (3.76) | |
| Level p | - | 0.0536 | |
| Suprascapular Vein (n = 235) | Suprascapular Nerve (n = 235) | |
|---|---|---|
| Structure visible (n(%)) | 176 (74.9%) | 113 (48.1%) |
| Structure no visible (n(%)) | 59 (25.1%) | 122 (51.9%) |
| Ultrasonographic Measurements | All (n = 235) | BMI | |
|---|---|---|---|
| R2 | Level p | ||
| Superior transverse diameter (mm) | 14.3 (4.4) | 0.0194 | 0.4881 |
| Maximal depth (mm) | 6.5 (2.2) | 0.0040 | 0.7537 |
| Diameter of suprascapular artery (mm) | 1.8 (0.7) | 0.0009 | 0.8803 |
| Thickness of the soft tissue over the suprascapular artery (mm) | 34.2 (5.7) | 0.3196 | 0.0021 |
| Diameter of suprascapular vein (mm) | 1.4 (0,8) | 0.0779 | 0.1585 |
| Thickness of the soft tissue over the suprascapular vein (mm) | 35.8 (6.0) | 0.2281 | 0.0118 |
| Diameter of suprascapular nerve (mm) | 3.3 (1.0) | 0.0034 | 0.7711 |
| Thickness of the soft tissue over the suprascapular nerve (mm) | 36.0 (5.7) | 0.1052 | 0.0988 |
| Ultrasonographic Measurements | Right Side (n = 116) | Left Side (n = 119) | Level p |
|---|---|---|---|
| Superior transverse diameter (mm) | 14.8 ± 4.8 | 13.8 ± 4.0 | 0.0332 |
| Maximal depth (mm) | 6.3 ± 2.1 | 6.6 ± 2.3 | 0.3586 |
| Diameter of suprascapular nerve (mm) | 3.5 ± 1.1 | 1.3 ± 0.4 | 0.0010 |
| Thickness of the soft tissue over the suprascapular nerve (mm) | 38.0 ± 5.2 | 37.7 ± 6.1 | 1.0000 |
| Diameter of suprascapular artery (mm) | 1.8 ± 0.7 | 1.8 ± 0.7 | 0.5904 |
| Thickness of the soft tissue over the suprascapular artery (mm) | 34.6 ± 5.7 | 33.6 ± 5.7 | 0.0202 |
| Diameter of suprascapular vein (mm) | 1.2 ± 0.7 | 1.5 ± 0.9 | 0.0010 |
| Thickness of the soft tissue over the suprascapular vein (mm) | 36.2 ± 5.9 | 35.2 ± 6.3 | 0.0147 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jezierski, H.; Podgórski, M.; Wysiadecki, G.; Olewnik, Ł.; De Caro, R.; Macchi, V.; Polguj, M. Morphological Aspects in Ultrasound Visualisation of the Suprascapular Notch Region: A Study Based on a New Four-Step Protocol. J. Clin. Med. 2018, 7, 491. https://doi.org/10.3390/jcm7120491
Jezierski H, Podgórski M, Wysiadecki G, Olewnik Ł, De Caro R, Macchi V, Polguj M. Morphological Aspects in Ultrasound Visualisation of the Suprascapular Notch Region: A Study Based on a New Four-Step Protocol. Journal of Clinical Medicine. 2018; 7(12):491. https://doi.org/10.3390/jcm7120491
Chicago/Turabian StyleJezierski, Hubert, Michał Podgórski, Grzegorz Wysiadecki, Łukasz Olewnik, Raffaele De Caro, Veronica Macchi, and Michał Polguj. 2018. "Morphological Aspects in Ultrasound Visualisation of the Suprascapular Notch Region: A Study Based on a New Four-Step Protocol" Journal of Clinical Medicine 7, no. 12: 491. https://doi.org/10.3390/jcm7120491
APA StyleJezierski, H., Podgórski, M., Wysiadecki, G., Olewnik, Ł., De Caro, R., Macchi, V., & Polguj, M. (2018). Morphological Aspects in Ultrasound Visualisation of the Suprascapular Notch Region: A Study Based on a New Four-Step Protocol. Journal of Clinical Medicine, 7(12), 491. https://doi.org/10.3390/jcm7120491








