Influence of the Acetabular Cup Material on the Shell Deformation and Strain Distribution in the Adjacent Bone—A Finite Element Analysis
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Shell and Liner Deformation
3.2. Strain Distribution
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Herberts, P.; Malchau, H. Long-term registration has improved the quality of hip replacement: A review of the Swedish THR Register comparing 160,000 cases. Acta Orthop. Scand. 2000, 71, 111–121. [Google Scholar] [CrossRef] [Green Version]
- Dickinson, A.S.; Taylor, A.C.; Browne, M. The influence of acetabular cup material on pelvis cortex surface strains, measured using digital image correlation. J. Biomech. 2012, 45, 719–723. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.S.; Callaghan, J.J.; Ahn, P.B.; Brown, T.D. Fracture of the acetabulum during insertion of an oversized hemispherical component. J. Bone Jt. Surg. 1995, 77, 111–117. [Google Scholar] [CrossRef]
- Wright, J.M.; Pellicci, P.M.; Salvati, E.A.; Ghelman, B.; Roberts, M.M.; Koh, J.L. Bone density adjacent to press-fit acetabular components. A prospective analysis with quantitative computed tomography. J. Bone Jt. Surg. 2001, 83, 529–536. [Google Scholar] [CrossRef]
- Meneghini, R.M.; Ford, K.S.; McCollough, C.H.; Hanssen, A.D.; Lewallen, D.G. Bone remodeling around porous metal cementless acetabular components. J. Arthroplast. 2010, 25, 741–747. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Yoon, S.-H.; Kim, J.-S. Changes in the bone mineral density in the acetabulum and proximal femur after cementless total hip replacement: Alumina-on-alumina versus alumina-on-polyethylene articulation. J. Bone Jt. Surg. 2007, 89, 174–179. [Google Scholar] [CrossRef] [Green Version]
- Nakahara, I.; Takao, M.; Bandoh, S.; Bertollo, N.; Walsh, W.R.; Sugano, N. In vivo implant fixation of carbon fiber-reinforced PEEK hip prostheses in an ovine model. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2013, 31, 485–492. [Google Scholar] [CrossRef]
- Pace, N.; Marinelli, M.; Spurio, S. Technical and histologic analysis of a retrieved carbon fiber-reinforced poly-ether-ether-ketone composite alumina-bearing liner 28 months after implantation. J. Arthroplast. 2008, 23, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Scholes, S.C.; Inman, I.A.; Unsworth, A.; Jones, E. Tribological assessment of a flexible carbon-fibre-reinforced poly(ether-ether-ketone) acetabular cup articulating against an alumina femoral head. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2008, 222, 273–283. [Google Scholar] [CrossRef]
- Wang, A.; Lin, R.; Polineni, V.K.; Essner, A.; Stark, C.; Dumbleton, J.H. Carbon fiber reinforced polyether ether ketone composite as a bearing surface for total hip replacement. Tribol. Int. 1998, 31, 661–667. [Google Scholar] [CrossRef]
- Wang, Q.Q.; Wu, J.J.; Unsworth, A.; Briscoe, A.; Jarman-Smith, M.; Lowry, C.; Simpson, D.; Collins, S. Biotribological study of large diameter ceramic-on-CFR-PEEK hip joint including fluid uptake, wear and frictional heating. J. Mater. Sci. Mater. Med. 2012, 23, 1533–1542. [Google Scholar] [CrossRef] [Green Version]
- Vogel, D.; Schulze, C.; Dempwolf, H.; Kluess, D.; Bader, R. Biomechanical behavior of modular acetabular cups made of poly-ether-ether-ketone: A finite element study. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2018, 232, 1030–1038. [Google Scholar] [CrossRef]
- Goebel, P.; Kluess, D.; Wieding, J.; Souffrant, R.; Heyer, H.; Sander, M.; Bader, R. The influence of head diameter and wall thickness on deformations of metallic acetabular press-fit cups and UHMWPE liners: A finite element analysis. J. Orthop. Sci. Off. J. Jpn. Orthop. Assoc. 2013, 18, 264–270. [Google Scholar] [CrossRef]
- Meding, J.B.; Small, S.R.; Jones, M.E.; Berend, M.E.; Ritter, M.A. Acetabular cup design influences deformational response in total hip arthroplasty. Clin. Orthop. Relat. Res. 2013, 471, 403–409. [Google Scholar] [CrossRef] [Green Version]
- Ong, K.L.; Rundell, S.; Liepins, I.; Laurent, R.; Markel, D.; Kurtz, S.M. Biomechanical modeling of acetabular component polyethylene stresses, fracture risk, and wear rate following press-fit implantation. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2009, 27, 1467–1472. [Google Scholar] [CrossRef]
- Schmidig, G.; Patel, A.; Liepins, I.; Thakore, M.; Markel, D.C. The effects of acetabular shell deformation and liner thickness on frictional torque in ultrahigh-molecular-weight polyethylene acetabular bearings. J. Arthroplast. 2010, 25, 644–653. [Google Scholar] [CrossRef]
- Bone, M.C.; Dold, P.; Flohr, M.; Preuss, R.; Joyce, T.J.; Aspden, R.M.; Holland, J.; Deehan, D. The influence of the strength of bone on the deformation of acetabular shells: A laboratory experiment in cadavers. Bone Jt. J. 2015, 97, 473–477. [Google Scholar] [CrossRef] [Green Version]
- Howcroft, D.W.J.; Qureshi, A.; Graham, N.M. Seating of ceramic liners in the uncemented trident acetabular shell: Is there really a problem? Clin. Orthop. Relat. Res. 2009, 467, 2651–2655. [Google Scholar] [CrossRef] [Green Version]
- McAuley, J.P.; Dennis, D.A.; Grostefon, J.; Hamilton, W.G. Factors affecting modular acetabular ceramic liner insertion: A biomechanical analysis. Clin. Orthop. Relat. Res. 2012, 470, 402–409. [Google Scholar] [CrossRef] [Green Version]
- Qiu, C.; Wang, L.; Li, D.; Jin, Z. The influence of metallic shell deformation on the contact mechanics of a ceramic-on-ceramic total hip arthroplasty. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2016, 230, 4–12. [Google Scholar] [CrossRef] [Green Version]
- Sharkey, P.F.; Hozack, W.J.; Callaghan, J.J.; Kim, Y.S.; Berry, D.J.; Hanssen, A.D.; LeWallen, D.G. Acetabular fracture associated with cementless acetabular component insertion: A report of 13 cases. J. Arthroplast. 1999, 14, 426–431. [Google Scholar] [CrossRef]
- Kluess, D.; Martin, H.; Mittelmeier, W.; Schmitz, K.-P.; Bader, R. Influence of femoral head size on impingement, dislocation and stress distribution in total hip replacement. Med. Eng. Phys. 2007, 29, 465–471. [Google Scholar] [CrossRef]
- Kluess, D.; Souffrant, R.; Mittelmeier, W.; Wree, A.; Schmitz, K.-P.; Bader, R. A convenient approach for finite-element-analyses of orthopaedic implants in bone contact: Modeling and experimental validation. Comput. Methods Programs Biomed. 2009, 95, 23–30. [Google Scholar] [CrossRef]
- Schultze, C.; Klüss, D.; Martin, H.; Hingst, V.; Mittelmeier, W.; Schmitz, K.-P.; Bader, R. Finite-Elemente-Analyse einer zementierten, keramischen Femurkomponente unter Berücksichtigung der Einbausituation bei künstlichem Kniegelenkersatz. Biomedizinische Technik. Biomed. Eng. 2007, 52, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Taddei, F.; Pancanti, A.; Viceconti, M. An improved method for the automatic mapping of computed tomography numbers onto finite element models. Med. Eng. Phys. 2004, 26, 61–69. [Google Scholar] [CrossRef]
- Helgason, B.; Perilli, E.; Schileo, E.; Taddei, F.; Brynjólfsson, S.; Viceconti, M. Mathematical relationships between bone density and mechanical properties: A literature review. Clin. Biomech. 2008, 23, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Carter, D.R.; Hayes, W.C. The compressive behavior of bone as a two-phase porous structure. J. Bone Jt. Surg. 1977, 59, 954–962. [Google Scholar] [CrossRef]
- Grifka, J.; Kuster, M. Orthopädie und Unfallchirurgie; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Shirazi-Adl, A.; Dammak, M.; Paiement, G. Experimental determination of friction characteristics at the trabecular bone/porous-coated metal interface in cementless implants. J. Biomed. Mater. Res. 1993, 27, 167–175. [Google Scholar] [CrossRef]
- Kurtz, S.M.; Edidin, A.A.; Bartel, D.L. The role of backside polishing, cup angle, and polyethylene thickness on the contact stresses in metal-backed acetabular components. J. Biomech. 1997, 30, 639–642. [Google Scholar] [CrossRef]
- Bergmann, G.; Bender, A.; Dymke, J.; Duda, G.; Damm, P. Standardized Loads Acting in Hip Implants. PLoS ONE 2016, 11, e0155612. [Google Scholar] [CrossRef]
- Biewener, A.A. Safety factors in bone strength. Calcif. Tissue Int. 1993, 53, S68–S74. [Google Scholar] [CrossRef]
- Frost, H.M. Bone’s mechanostat: A 2003 update. Anat. Rec. Part A Discov. Mol. Cell. Evol. Biol. 2003, 275, 1081–1101. [Google Scholar] [CrossRef]
- Cho, Y.J.; Bae, C.I.; Yoon, W.K.; Chun, Y.S.; Rhyu, K.H. High incidence of early subtrochanteric lateral cortical atrophy after hip arthroplasty using bone-conserving short stem. Int. Orthop. 2018, 42, 303–309. [Google Scholar] [CrossRef]
- Field, R.E.; Rajakulendran, K.; Eswaramoorthy, V.K.; Rushton, N. Three-year prospective clinical and radiological results of a new flexible horseshoe acetabular cup. Hip Int. J. Clin. Exp. Res. Hip Pathol. Ther. 2012, 22, 598–606. [Google Scholar] [CrossRef]
- Manley, M.T.; Ong, K.L.; Kurtz, S.M. The potential for bone loss in acetabular structures following THA. Clin. Orthop. Relat. Res. 2006, 453, 246–253. [Google Scholar] [CrossRef]
- Anderson, A.E.; Peters, C.L.; Tuttle, B.D.; Weiss, J.A. Subject-specific finite element model of the pelvis: Development, validation and sensitivity studies. J. Biomech. Eng. 2005, 127, 364–373. [Google Scholar] [CrossRef] [Green Version]
- Devine, D.M.; Hahn, J.; Richards, R.G.; Gruner, H.; Wieling, R.; Pearce, S.G. Coating of carbon fiber-reinforced polyetheretherketone implants with titanium to improve bone apposition. J. Biomed. Mater. Res. Part B Appl. Biomater. 2013, 101, 591–598. [Google Scholar] [CrossRef]
- Han, C.-M.; Lee, E.-J.; Kim, H.-E.; Koh, Y.-H.; Kim, K.N.; Ha, Y.; Kuh, S.-U. The electron beam deposition of titanium on polyetheretherketone (PEEK) and the resulting enhanced biological properties. Biomaterials 2010, 31, 3465–3470. [Google Scholar] [CrossRef]
- Walsh, W.R.; Bertollo, N.; Christou, C.; Schaffner, D.; Mobbs, R.J. Plasma-sprayed titanium coating to polyetheretherketone improves the bone-implant interface. Spine J. Off. J. N. Am. Spine Soc. 2015, 15, 1041–1049. [Google Scholar] [CrossRef]
- Vogel, D.; Dempwolf, H.; Baumann, A.; Bader, R. Characterization of thick titanium plasma spray coatings on PEEK materials used for medical implants and the influence on the mechanical properties. J. Mech. Behav. Biomed. Mater. 2018, 77, 600–608. [Google Scholar] [CrossRef]
- Everitt, H. Deformation on Impaction of a Large Diameter Carbon Fibre Reinforced Poly-Ether-Ether-Ketone Acetabular Cup and Its Effects on the Tribology of the Bearing. Ph.D. Thesis, University Cardiff, Cardiff, UK, August 2011. [Google Scholar]
- Wu, X.; Liu, X.; Wei, J.; Ma, J.; Deng, F.; Wei, S. Nano-TiO2/PEEK bioactive composite as a bone substitute material: In Vitro and in vivo studies. Int. J. Nanomed. 2012, 7, 1215–1225. [Google Scholar]
- Lin, Z.M.; Meakins, S.; Morlock, M.M.; Parsons, P.; Hardaker, C.; Flett, M.; Isaac, G. Deformation of press-fitted metallic resurfacing cups. Part 1: Experimental simulation. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2006, 220, 299–309. [Google Scholar]
- Park, Y.-S.; Hwang, S.-K.; Choy, W.-S.; Kim, Y.-S.; Moon, Y.-W.; Lim, S.-J. Ceramic failure after total hip arthroplasty with an alumina-on-alumina bearing. J. Bone Jt. Surg. 2006, 88, 780–787. [Google Scholar]
- Ha, Y.-C.; Kim, S.-Y.; Kim, H.J.; Yoo, J.J.; Koo, K.-H. Ceramic liner fracture after cementless alumina-on-alumina total hip arthroplasty. Clin. Orthop. Relat. Res. 2007, 458, 106–110. [Google Scholar] [CrossRef]
- Hasegawa, M.; Sudo, A.; Hirata, H.; Uchida, A. Ceramic acetabular liner fracture in total hip arthroplasty with a ceramic sandwich cup. J. Arthroplast. 2003, 18, 658–661. [Google Scholar] [CrossRef]
- Lopes, R.; Philippeau, J.M.; Passuti, N.; Gouin, F. High rate of ceramic sandwich liner fracture. Clin. Orthop. Relat. Res. 2012, 470, 1705–1710. [Google Scholar] [CrossRef] [Green Version]
- Viste, A.; Chouteau, J.; Desmarchelier, R.; Fessy, M.-H. Fractures of a sandwich ceramic liner at ten year follow-up. Int. Orthop. 2012, 36, 955–960. [Google Scholar] [CrossRef] [Green Version]
- Okita, S.; Hasegawa, M.; Takahashi, Y.; Puppulin, L.; Sudo, A.; Pezzotti, G. Failure analysis of sandwich-type ceramic-on-ceramic hip joints: A spectroscopic investigation into the role of the polyethylene shell component. J. Mech. Behav. Biomed. Mater. 2014, 31, 55–67. [Google Scholar] [CrossRef]
- Wang, T.; Sun, J.-Y.; Zha, G.-C.; Dong, S.-J.; Zhao, X.-J. Mid term results of total hip arthroplasty using polyethylene-ceramic composite (Sandwich) liner. Indian J. Orthop. 2016, 50, 10–15. [Google Scholar]
- Hua, X.; Li, J.; Wang, L.; Jin, Z.; Wilcox, R.; Fisher, J. Contact mechanics of modular metal-on-polyethylene total hip replacement under adverse edge loading conditions. J. Biomech. 2014, 47, 3303–3309. [Google Scholar] [CrossRef] [Green Version]
- Mabuchi, K.; Sakai, R.; Ota, M.; Ujihira, M. Appropriate radial clearance of ceramic-on-ceramic total hip prostheses to realize squeeze-film lubrication. Clin. Biomech. 2004, 19, 362–369. [Google Scholar] [CrossRef]
- Begand, S.; Glien, W.; Oberbach, T. ATZ—A New Material with a High Potential in Joint Replacement. KEM 2005, 284, 983–986. [Google Scholar] [CrossRef]
- Dold, P.; Pandorf, T.; Flohr, M.; Preuss, R.; Bone, M.C.; Joyce, T.J.; Holland, J.; Deehan, D. Acetabular shell deformation as a function of shell stiffness and bone strength. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2016, 230, 259–264. [Google Scholar] [CrossRef] [Green Version]
- Markel, D.; Day, J.; Siskey, R.; Liepins, I.; Kurtz, S.; Ong, K. Deformation of metal-backed acetabular components and the impact of liner thickness in a cadaveric model. Int. Orthop. 2011, 35, 1131–1137. [Google Scholar] [CrossRef] [Green Version]
- Yew, A.; Jin, Z.M.; Donn, A.; Morlock, M.M.; Isaac, G. Deformation of press-fitted metallic resurfacing cups. Part 2: Finite element simulation. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2006, 220, 311–319. [Google Scholar] [CrossRef] [Green Version]
- Thompson, M.S.; Northmore-Ball, M.D.; Tanner, K.E. Effects of acetabular resurfacing component material and fixation on the strain distribution in the pelvis. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2002, 216, 237–245. [Google Scholar] [CrossRef]
- Chatterjee, S.; Kobylinski, S.; Basu, B. Finite Element Analysis to Probe the Influence of Acetabular Shell Design, Liner Material, and Subject Parameters on Biomechanical Response in Periprosthetic Bone. J. Biomech. Eng. 2018, 140, 101014. [Google Scholar] [CrossRef]
- Pitto, R.P.; Bhargava, A.; Pandit, S.; Munro, J.T. Retroacetabular stress-shielding in THA. Clin. Orthop. Relat. Res. 2008, 466, 353–358. [Google Scholar] [CrossRef] [Green Version]
- Widmer, K.-H.; Zurfluh, B.; Morscher, E.W. Load transfer and fixation mode of press-fit acetabular sockets. J. Arthroplast. 2002, 17, 926–935. [Google Scholar] [CrossRef]
- Bergmann, G.; Graichen, F.; Rohlmann, A.; Bender, A.; Heinlein, B.; Duda, G.N.; Heller, M.O.; Morlock, M.M. Realistic loads for testing hip implants. Bio-Med. Mater. Eng. 2010, 20, 65–75. [Google Scholar] [CrossRef]
- Ghosh, R.; Gupta, S. Bone remodelling around cementless composite acetabular components: The effects of implant geometry and implant-bone interfacial conditions. J. Mech. Behav. Biomed. Mater. 2014, 32, 257–269. [Google Scholar] [CrossRef] [PubMed]
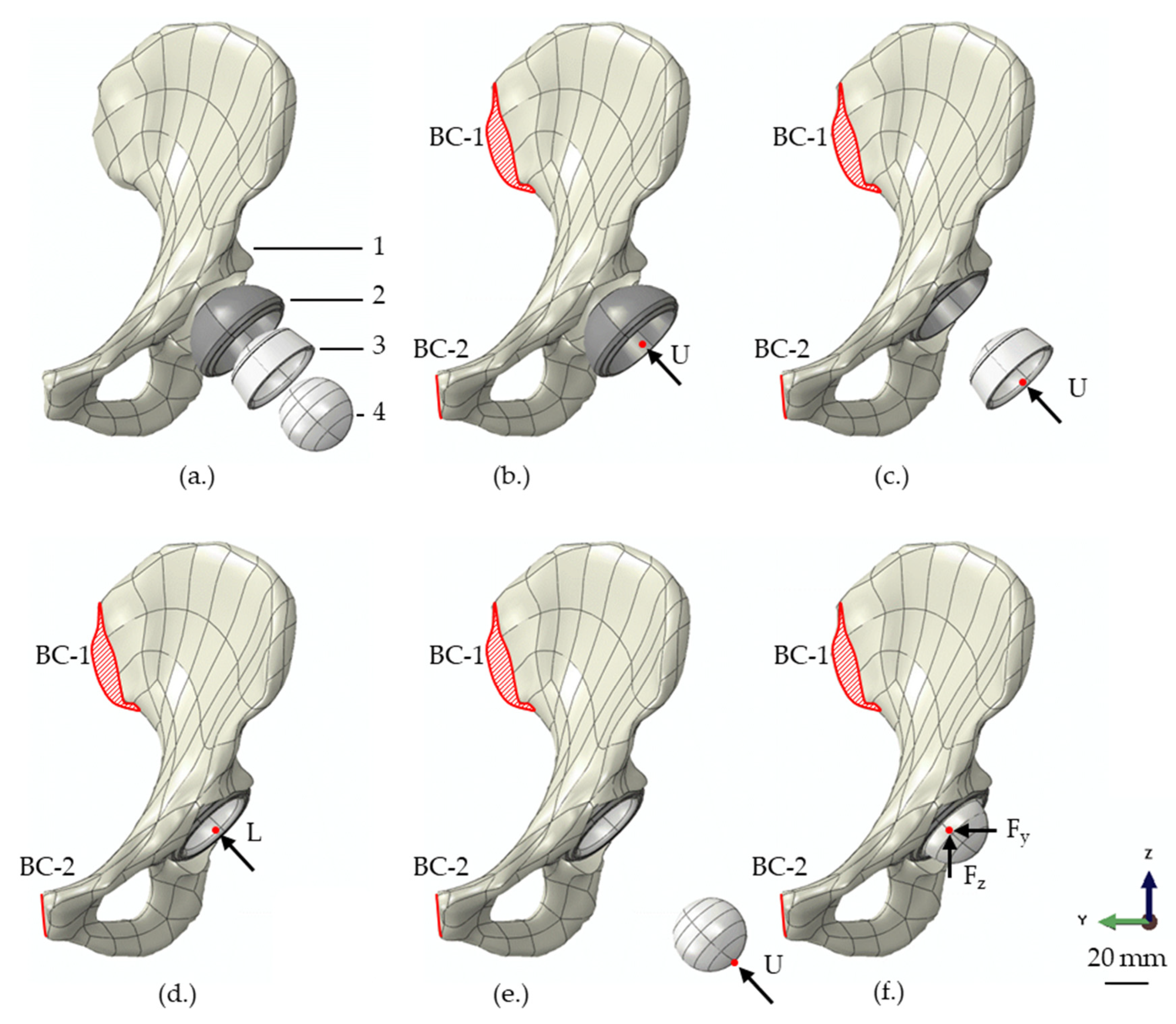
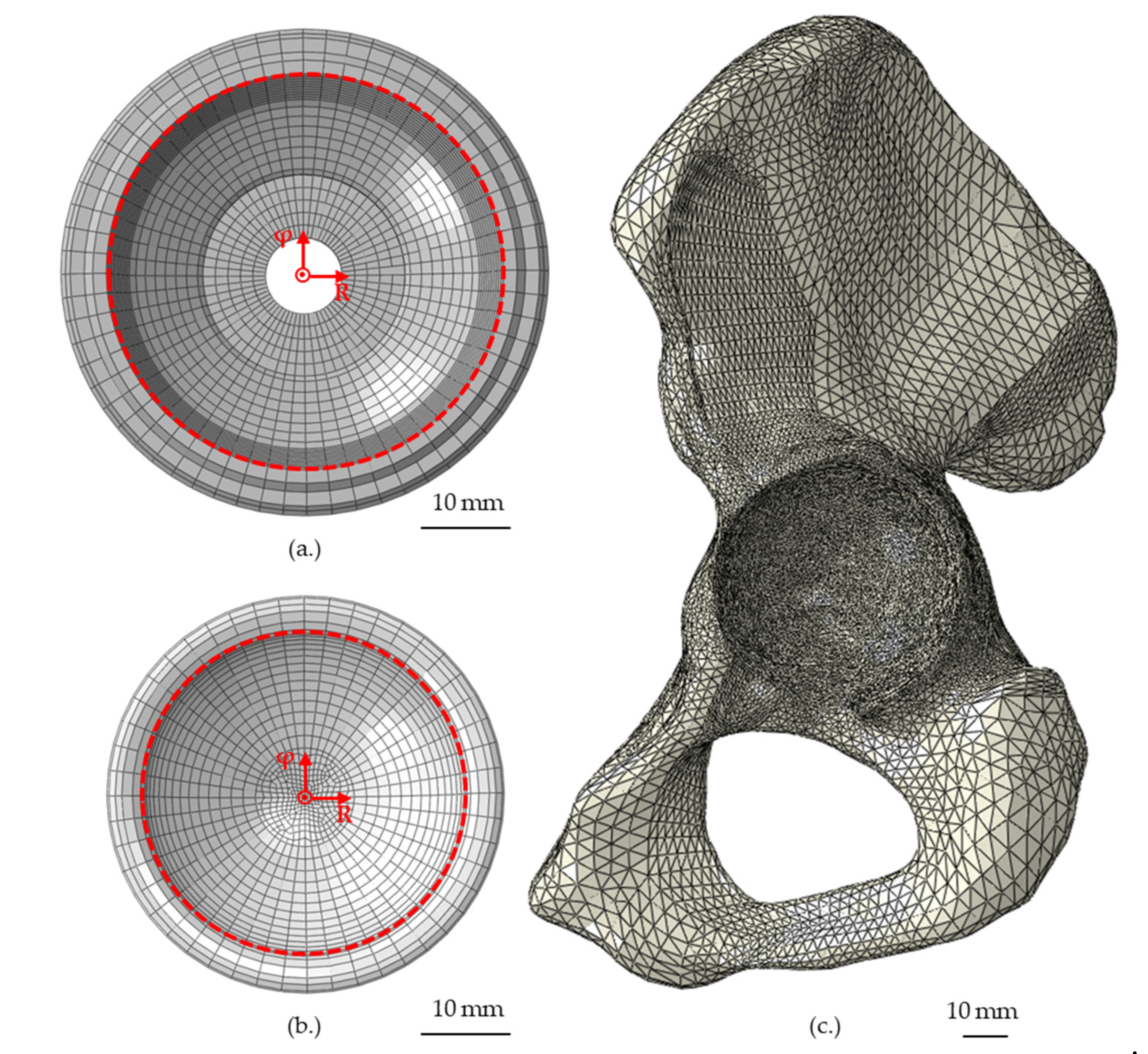

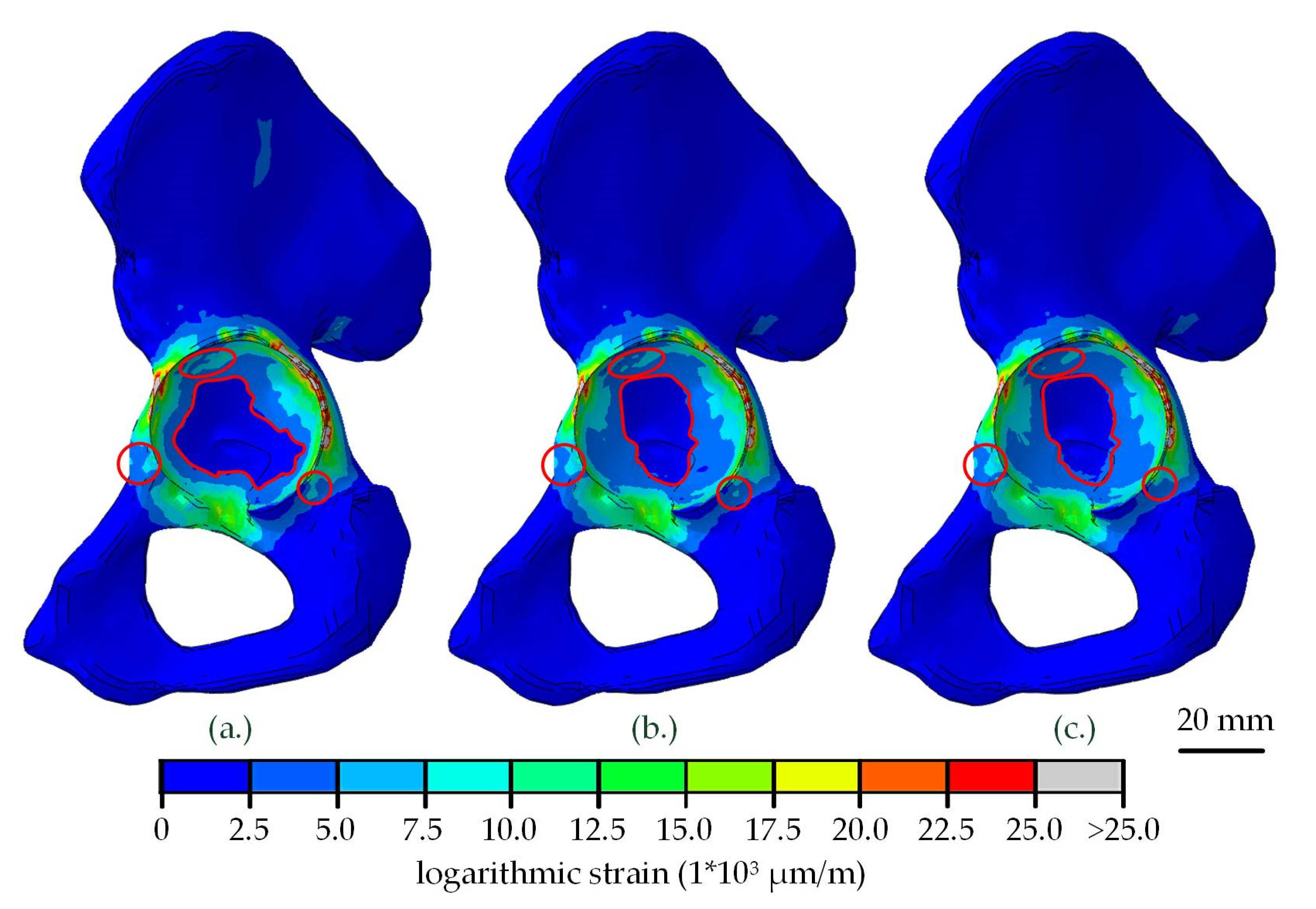
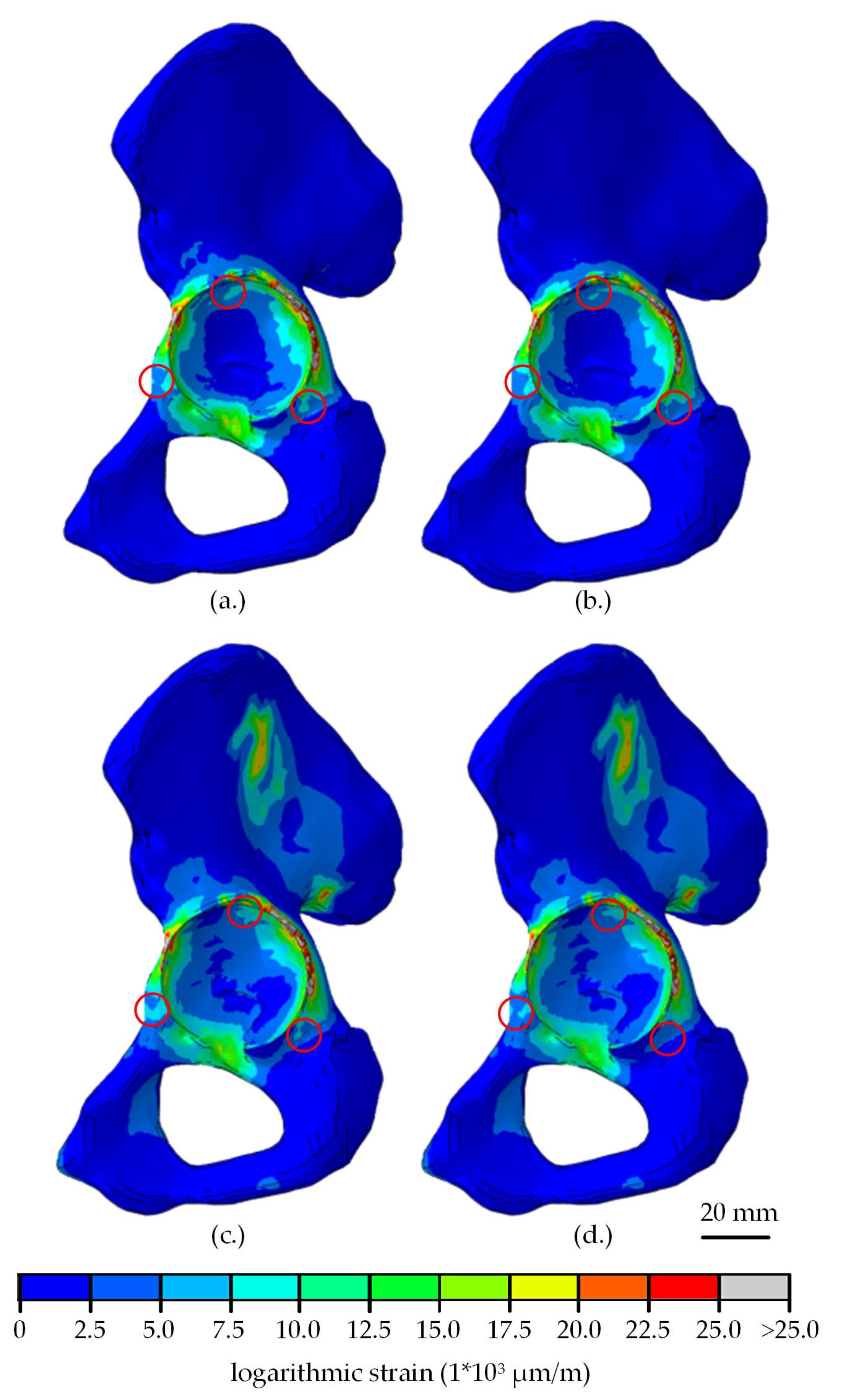
| Part | Material | Young’s Modulus (MPa) | Poisson’s Ratio |
|---|---|---|---|
| Acetabular shell | Titanium | 110,000 | 0.40 |
| Carbon fiber-reinforced poly-ether-ether-ketone (CFR-PEEK-15) | 15,000 | 0.40 | |
| Carbon fiber-reinforced poly-ether-ether-ketone (CFR-PEEK-23) | 23,000 | 0.40 | |
| Liner | Alumina toughened zirconia (ATZ) ceramic | 261,000 | 0.27 |
| Ultra-high-molecular-weight polyethylene (UHMW-PE) [22] | 945 | 0.45 | |
| Ball head | Alumina toughened zirconia (ATZ) ceramic | 261,000 | 0.27 |
| Strain (µm/m) | Bone Response |
|---|---|
| <400 | Atrophy |
| 400–3000 | Bone preserving and building |
| 3000–20,000 | Yielding |
| >20,000 | Fracture |
| Shell Material | Titanium | CFR-PEEK-23 | CFR-PEEK-15 | |||
|---|---|---|---|---|---|---|
| Liner material | Ceramic | UHMWPE | Ceramic | UHMWPE | Ceramic | UHMWPE |
| Maximum deformation (µm) | 2.0 | 149.0 | 1.8 | 184.7 | 4.4 | 180.6 |
| Strain Region (µm/m) | Titanium | CFR-PEEK-23 | CFR-PEEK-15 |
|---|---|---|---|
| <400 (Atrophy) | 17.2% | 18.2% | 18.3% |
| 400 < x < 3000 (Bone preserving and building) | 40.6% | 39.7% | 39.5% |
| 3000 < x < 20,000 (Yielding) | 39.8% | 40.2% | 40.5% |
| >20,000 (Fracture) | 2.4% | 1.8% | 1.7% |
| Strain Regions (µm/m) | Load Scenario | Titanium | CFR-PEEK-23 | CFR-PEEK-15 | |||
|---|---|---|---|---|---|---|---|
| Ceramic | UHMWPE | Ceramic | UHMWPE | Ceramic | UHMWPE | ||
| <400 (Atrophy) | Liner Insertion | 17.3% | 19.3% | 18.9% | 20.2% | 19.3% | 20.6% |
| Loading | 5.3% | 5.3% | 5.6% | 5.5% | 5.4% | 5.6% | |
| 400 < x < 3000 (Bone preserving and building) | Liner Insertion | 41.1% | 39.1% | 41.2% | 39.9% | 41.3% | 40.8% |
| Loading | 47.2% | 47.3% | 48.5% | 48.1% | 48.0% | 48.5% | |
| 3000 < x < 20,000 (Yielding) | Liner Insertion | 39.3% | 39.3% | 38.1% | 38.0% | 37.7% | 36.9% |
| Loading | 45.2% | 45.1% | 44.1% | 44.5% | 44.7% | 44.2% | |
| >20,000 (Fractures) | Liner Insertion | 2.3% | 2.3% | 1.8% | 1.9% | 1.7% | 1.7% |
| Loading | 2.3% | 2.3% | 1.8% | 1.9% | 1.9% | 1.8% | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vogel, D.; Klimek, M.; Saemann, M.; Bader, R. Influence of the Acetabular Cup Material on the Shell Deformation and Strain Distribution in the Adjacent Bone—A Finite Element Analysis. Materials 2020, 13, 1372. https://doi.org/10.3390/ma13061372
Vogel D, Klimek M, Saemann M, Bader R. Influence of the Acetabular Cup Material on the Shell Deformation and Strain Distribution in the Adjacent Bone—A Finite Element Analysis. Materials. 2020; 13(6):1372. https://doi.org/10.3390/ma13061372
Chicago/Turabian StyleVogel, Danny, Matthias Klimek, Michael Saemann, and Rainer Bader. 2020. "Influence of the Acetabular Cup Material on the Shell Deformation and Strain Distribution in the Adjacent Bone—A Finite Element Analysis" Materials 13, no. 6: 1372. https://doi.org/10.3390/ma13061372
APA StyleVogel, D., Klimek, M., Saemann, M., & Bader, R. (2020). Influence of the Acetabular Cup Material on the Shell Deformation and Strain Distribution in the Adjacent Bone—A Finite Element Analysis. Materials, 13(6), 1372. https://doi.org/10.3390/ma13061372





