Preparation and Characterization of an Antibrowning Nanosized Ag-CeO2 Composite with Synergistic Antibacterial Ability
Abstract
:1. Introduction
2. Experiment
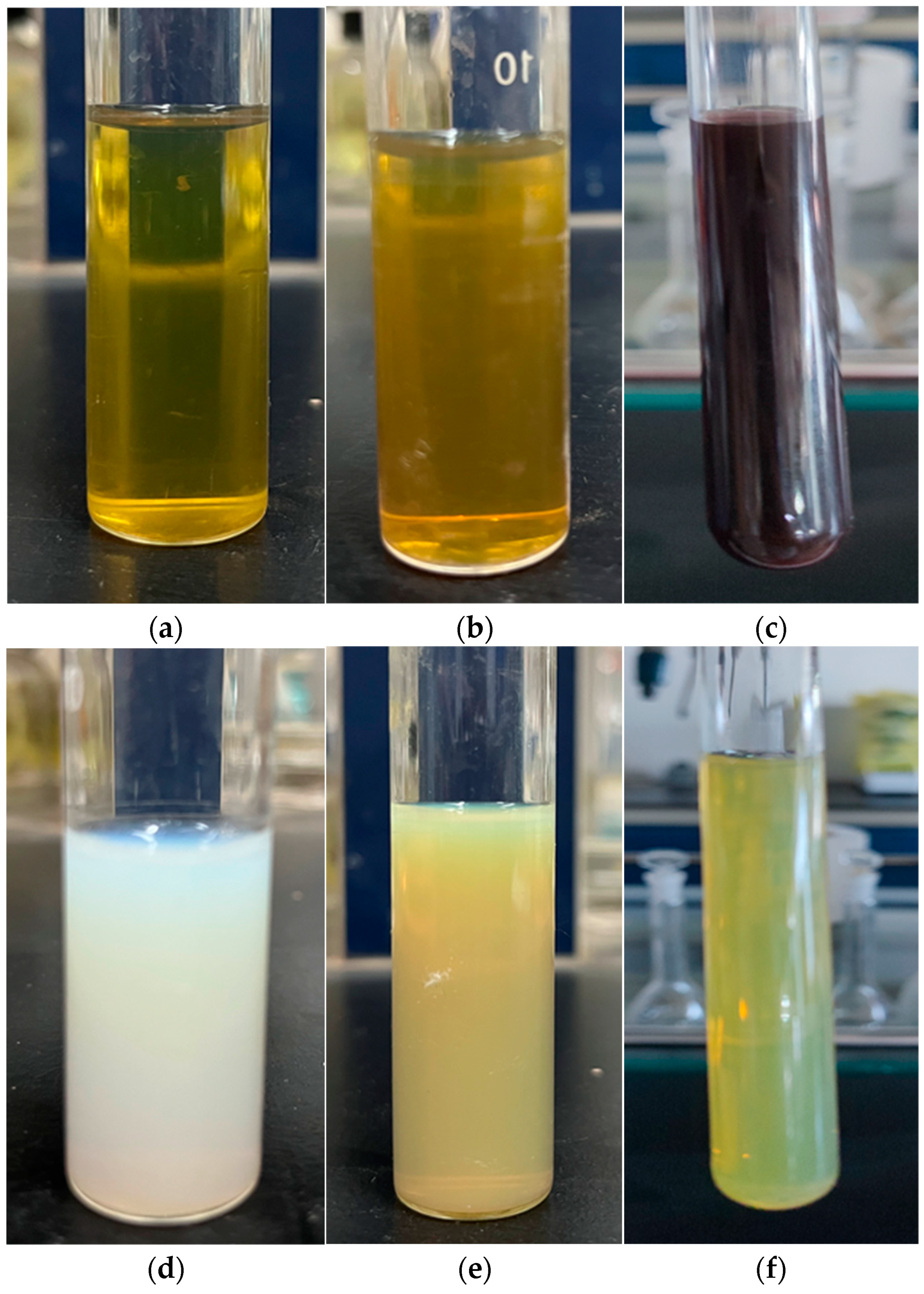
2.1. Synthesis of the Nanosized Ag, CeO2 and Ag-CeO2 Composite
2.2. Characterization
2.3. Antibacterial Test
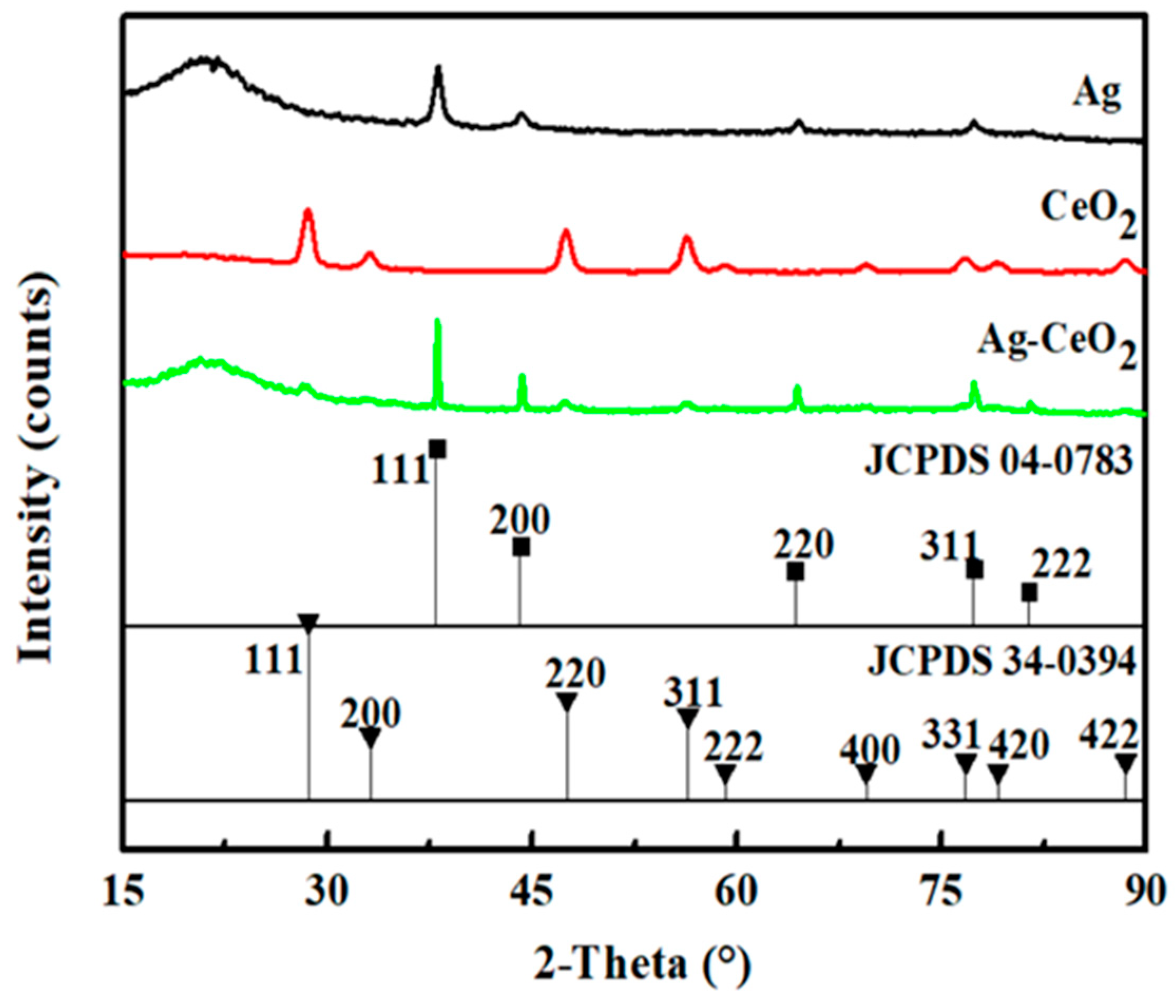
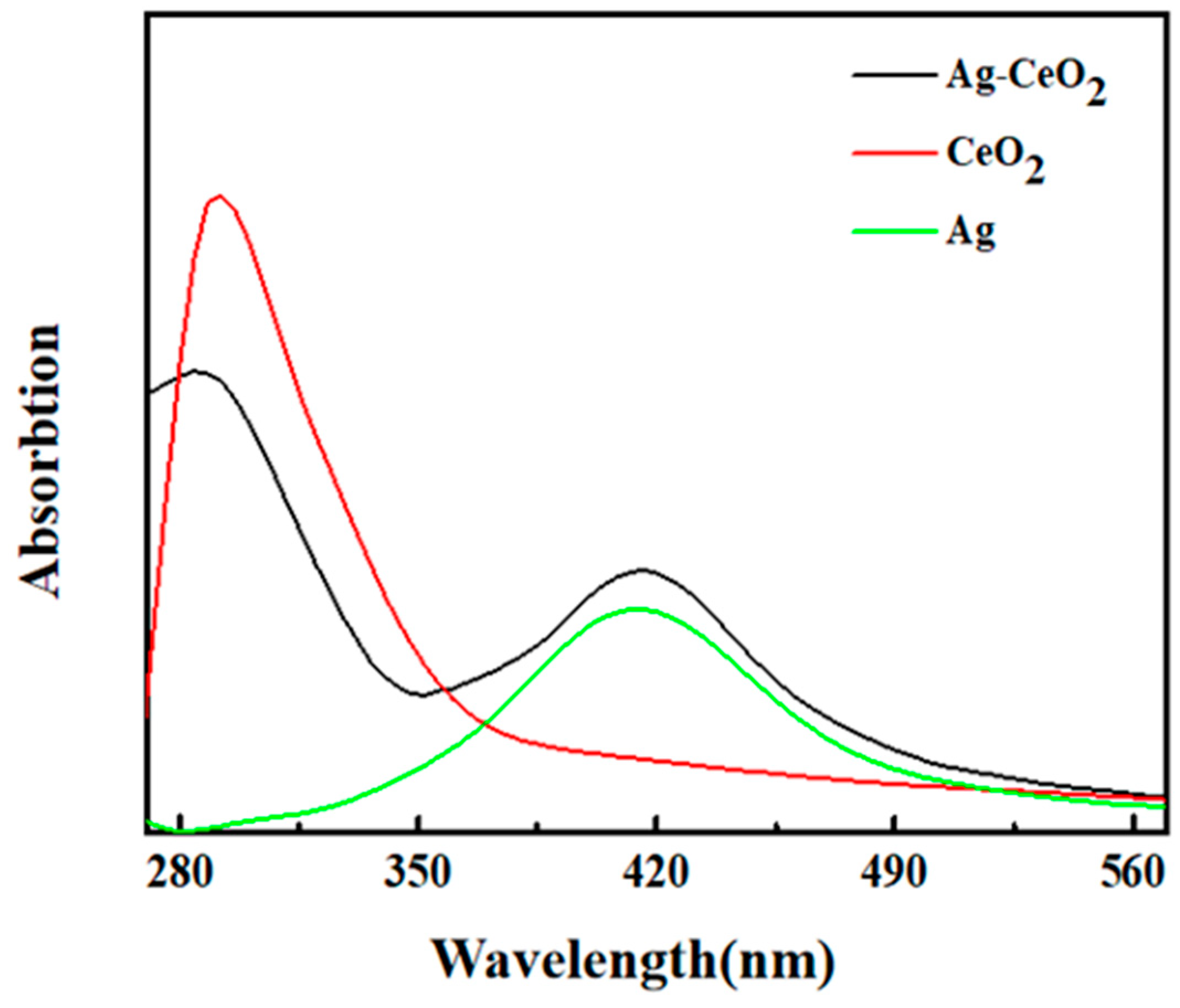
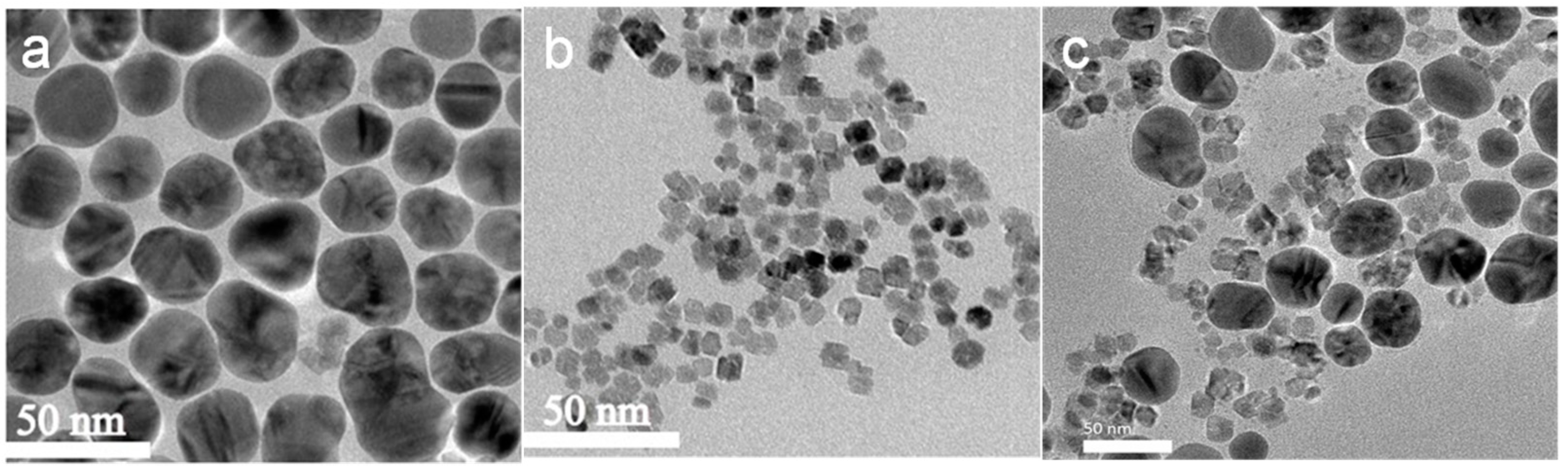
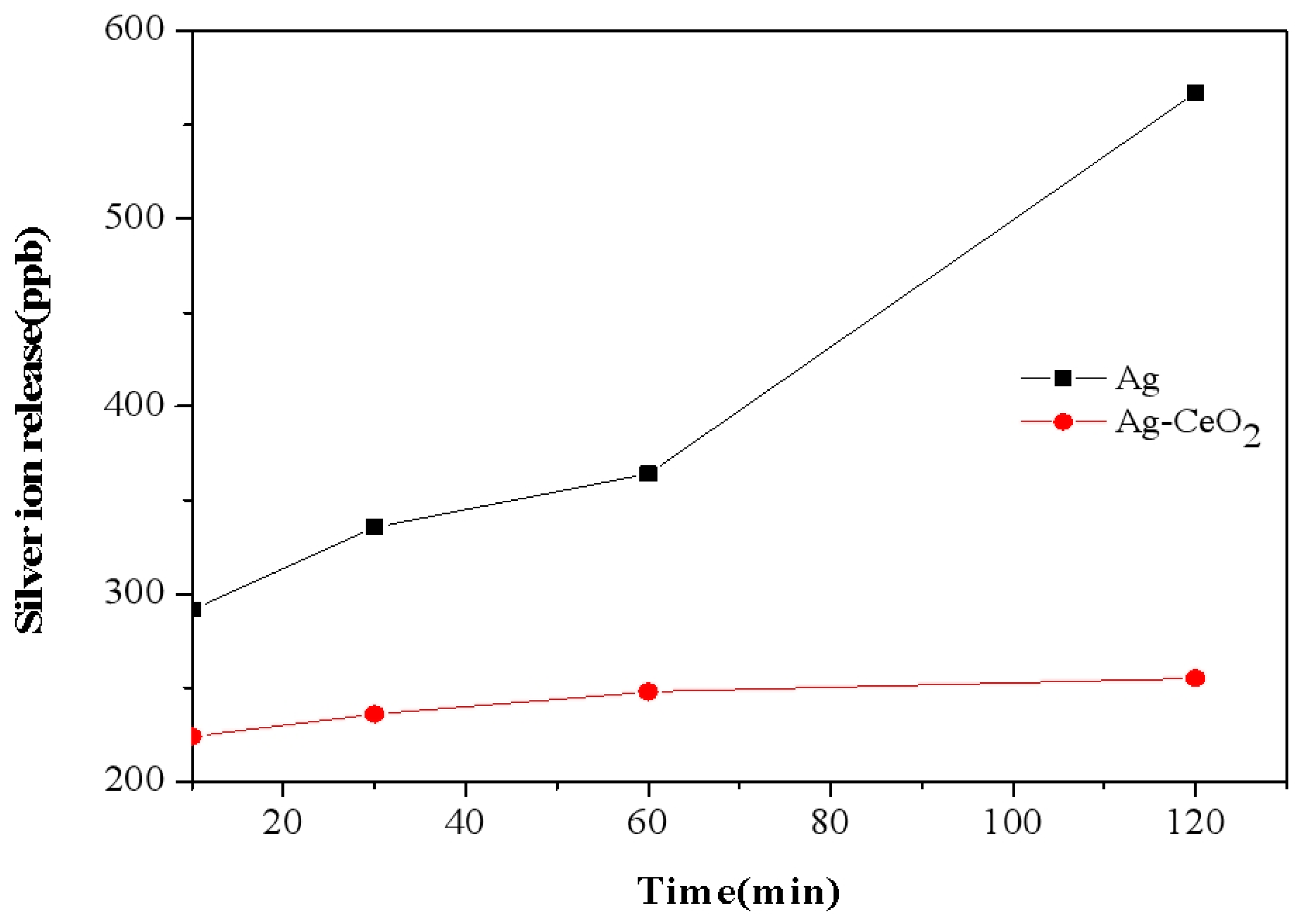
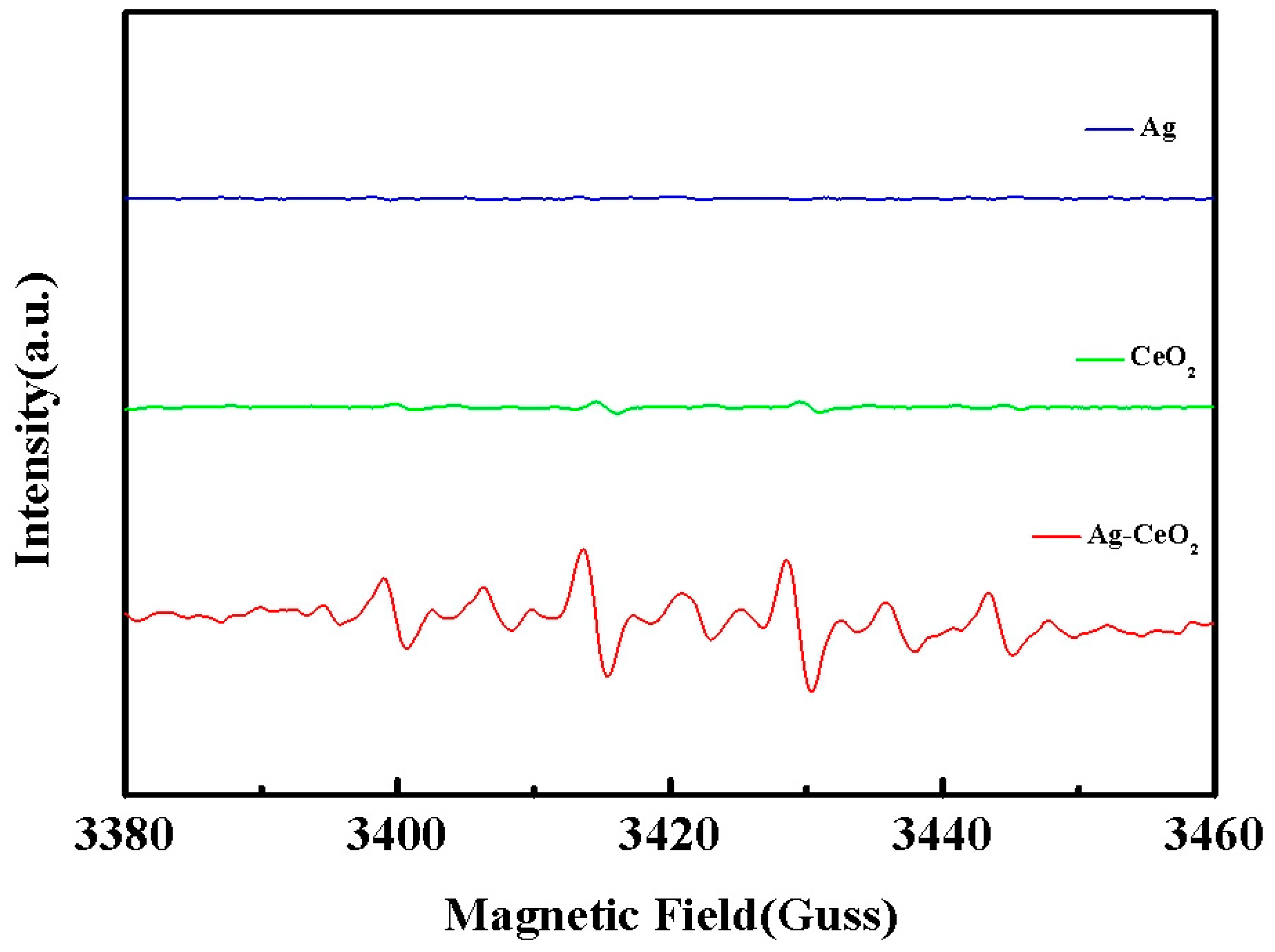
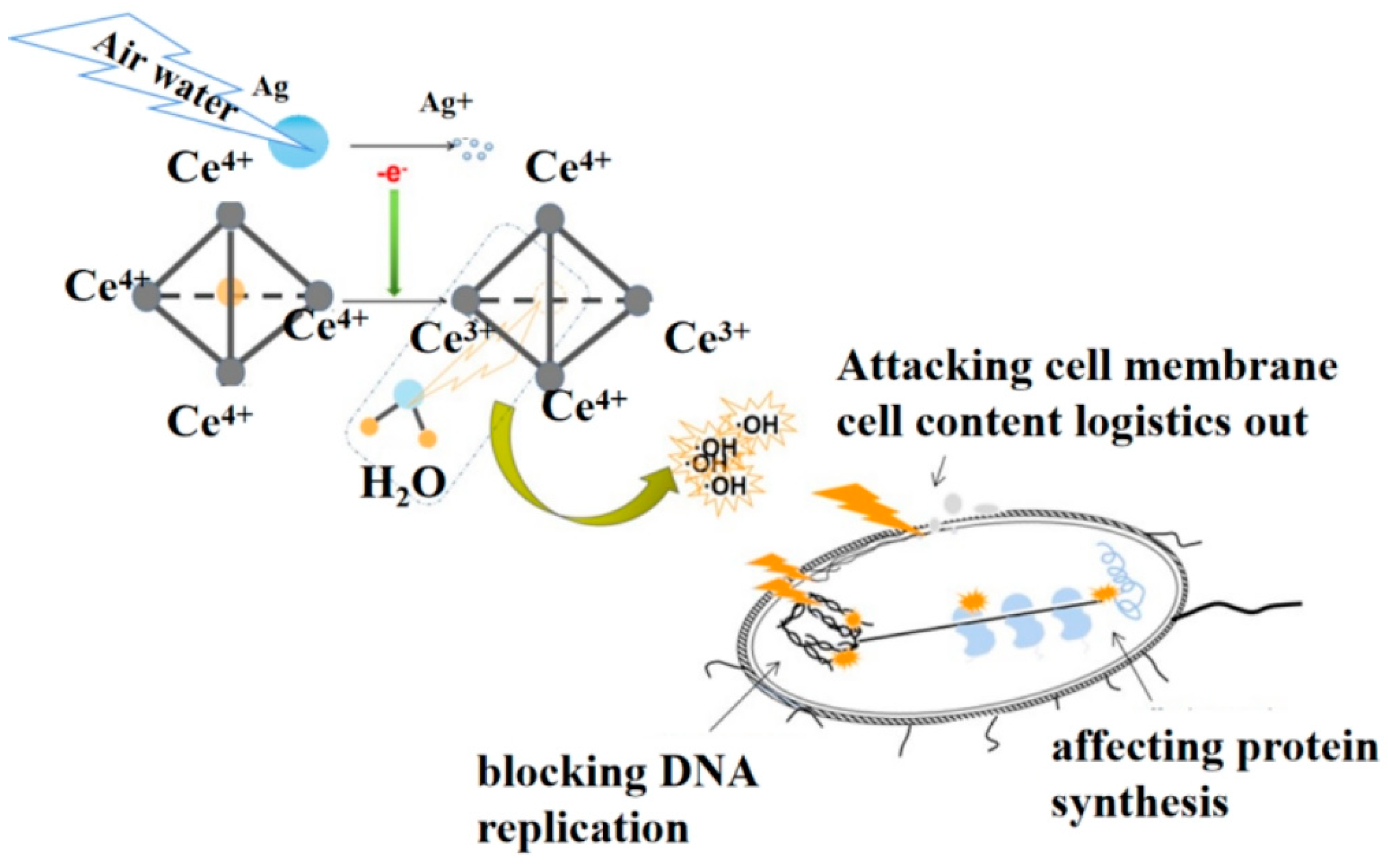
3. Result and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hannah, L. Antibiotic resistance and the biology of history. Body Soc. 2016, 22, 19–52. [Google Scholar]
- Antony, A.C.; Silvester, R.; Divya, P.S.; Aneesa, P.A.; Francis, B.; Ajith Joseph, C.; Hussain, M.S.; Umesh, B.T.; George, J.; Abdulla, M.H. Faecal contamination and prevalence of pathogenic E. coli in shellfish growing areas along south-west coast of India. Reg. Stud. Mar. Sci. 2021, 21, 101774. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, L.; Liu, X.H.; Chen, L.; Yang, Y.Z.; Yu, S.P. Antibacterial carbon-based nanomaterials. Adv. Mater. 2020, 34, 53–57. [Google Scholar]
- Jain, K.K. Nanomedicine: Application of nanobiotechnology in medical practice. Med. Princ. Pract. 2008, 17, 89–101. [Google Scholar] [CrossRef]
- Patil, S.; Desai, N.; Mahadik, K.; Paradkar, A. Can green synthesized propolis loaded silver nanoparticulate gel enhance wound healing caused by burns? Eur. J. Med. Chem. 2015, 7, 243–250. [Google Scholar] [CrossRef]
- Khorsandi, K.; Hosseinzadeh, R.; Esfahani, H.S.; Keyvani-Ghamsari, S.; Rahman, S.U. Nanomaterials as drug delivery systems with antibacterial properties: Current trends and future priorities. Expert Rev. Anti-Infect. Ther. 2021, 19, 1299–1323. [Google Scholar] [CrossRef] [PubMed]
- Pattabi, R.M.; Pattabi, M. Antibacterial Applications of Silver Nanoparticles synthesized by aqueous extract of Azadirachta indica (neem). J. Biomed. Nanotechnol. 2013, 754, 131–142. [Google Scholar]
- Zimoch-Korzycka, A.; Jarmoluk, A. The use of chitosan, lysozyme, and the nano-silver as antimicrobial ingredients of edible protective hydrosols applied into the surface of meat. J. Food Sci. Tech. 2015, 52, 5996–6002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiu, Z.M.; Zhang, Q.O.; Puppala, H.L.; Colvin, V.L.; Alvarez, P.J.J. Negligible particle-specific antibacterial activity of silver nanoparticles. Nano Lett. 2012, 12, 4271–4275. [Google Scholar] [CrossRef] [PubMed]
- Putri, G.E.; Arief, S.; Jamarun, N.; Gusti, F.; Sary, A.N.R. Characterization of enhanced antibacterial effects of silver loaded cerium oxide catalyst. Orient. J. Chem. 2018, 34, 2895–2901. [Google Scholar] [CrossRef]
- Yang, X.T.; Yu, Q.J.; Gao, W.; Tang, X.N.; Yi, H.H.; Tang, X.L. The mechanism of metal-based antibacterial materials and the progress of food packaging applications: A review. Ceram. Int. 2022, 48, 34148–34168. [Google Scholar] [CrossRef]
- Valsakumari, M.K.; Anushkannan, N.K.; Anusuya, M.; Chinnaiyan, S.K.; Haldar, B.; Jayapriya, M.; Ramachandran, K. Biomolecules and microwaves directed fabrication of Ag/CeO2 nanocomposite: A versatile candidate for the degradation of textile dye mixtures and antibacterial studies. Res. Chem. Intermed. 2022, 48, 5169–5186. [Google Scholar] [CrossRef]
- Trung, D.B.; Nguyen, H.B.; Nguyen, P.A.; Phan, H.P.; Tien-Thanh, N.; Nguyen, T. Green-synthesized silver nanoparticles decorated on ceria nanorods for room-temperature p-nitrophenol hydrogenation. Green Chem. Lett. Rev. 2022, 15, 449–459. [Google Scholar]
- Maleki, P.; Nemati, F.; Gholoobi, A.; Hashemzadeh, A.; Sabouri, Z.; Darroudi, M. Green facile synthesis of silver-doped cerium oxide nanoparticles and investigation of theircytotoxicity and antibacterial activity. Inorg. Chem. Commun. 2021, 131, 108762. [Google Scholar] [CrossRef]
- Jiménez, L.E.P.; Delgado, F.P.; Castellanos, L.N.M.; Blanco, L.R.; Alejandro, E.M.L.; Morales, E.R. Effect of Ag content on the nanostructure and antimicrobial activity of CeO2. Environ. Sci. Pollut. Res. 2023, 30, 57811–57820. [Google Scholar] [CrossRef]
- Ahmad, A.; Javed, M.S.; Khan, S.; Almutairi, T.M.; Mohammed, A.A.A.; Luque, R. Green synthesized Ag decorated CeO2 nanoparticles: Efficient photocatalysts and potential antibacterial agents. Chemosphere 2023, 310, 136841–136851. [Google Scholar] [CrossRef] [PubMed]
- Kayama, T.; Yamazaki, K.; Shinjoh, H. Nanostructured Ceria−Silver Synthesized in a One-Pot Redox Reaction Catalyzes Carbon Oxidation. J. Am. Chem. Soc. 2010, 132, 13154–13155. [Google Scholar] [CrossRef]
- Neal, C.J.; Fox, C.R.; Sakthivel, T.S.; Kumar, U.; Fu, Y.F.; Drake, C.; Parks, G.D.; Seal, S. Metal-Mediated Nanoscale Cerium Oxide Inactivates Human Coronavirus and Rhinovirus by Surface Disruption. ACS Nano 2021, 15, 14544–14556. [Google Scholar] [CrossRef]
- Wang, L.; He, H.; Yu, Y.B.; Sun, L.; Liu, S.J.; Zhang, C.B.; He, L. Morphology-dependent bactericidal activities of Ag/CeO2 catalysts against Escherichia coli. J. Inorg. Biochem. 2014, 135, 45–53. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.H.; Zhou, R.; Li, G.Q.; Li, X.Y. Selective laser welding in liquid: A strategy for preparation of high-antibacterial activity nanozyme against Staphylococcus aureus. J. Adv. Res. 2023, 44, 81–90. [Google Scholar] [CrossRef]
- Muhammad, F.; Huang, F.T.; Cheng, Y.; Chen, X.W.; Wang, Q.; Zhu, C.X.; Zhang, Y.H.; Yang, X.H.; Wang, P.; Wei, H. Nanoceria as an Electron Reservoir: Spontaneous Deposition of Metal Nanoparticles on Oxides and Their Anti-inflammatory Activities. ACS Nano 2022, 16, 20567–20576. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, M.; Khan, R.; Afridi, K.; Nadhman, A.; Ullah, S.; Faisal, S.; Mabood, Z.U.; Hano, C.; Abbasi, B.H. Green synthesis of cerium oxide nanoparticles (CeO2 NPs) and their antimicrobial applications: A review. Int. J. Nanomed. 2020, 15, 5951–5961. [Google Scholar] [CrossRef] [PubMed]
- He, G.W.; Li, F.F.; Wen, J.B. DMAc used as a reducer for preparation of spherical silver nanoparticles with high dispersion. J. Cent. South Univ. 2015, 22, 445–449. [Google Scholar] [CrossRef]
- Wang, C.Y.; Zhang, W.Y.; Qian, Y.T. Preparation of nanocrystalline ceria in CCl4. Mater. Sci. Eng. B-ADV 2002, 94, 170–175. [Google Scholar]
- Pal, S.; Tak, Y.K.; Joardar, J.; Kim, W.; Lee, J.E.; Han, M.S.; Song, J.M. Nanocrystalline silver supported on activated carbon matrix from hydrosol: Antibacterial mechanism under prolonged incubation conditions. J. Nanosci. Nanotechnol. 2009, 9, 2092–2103. [Google Scholar] [CrossRef]
- Pei, S.Z.; You, S.J.; Ma, J.; Chen, X.D.; Ren, N.Q. Electron spin resonance evidence for electro-generated hydroxyl radicals. Environ. Sci. Technol. 2020, 54, 13333–13343. [Google Scholar] [CrossRef]
- van Deelen, T.W.; Mejía, C.H.; de Jong, K.P. Control of metal-support interactions in heterogeneous catalysts to enhance activity and selectivity. Nat. Catal. 2019, 2, 955–970. [Google Scholar] [CrossRef]
- Zhang, M.Z.; Zhang, C.; Zhai, X.Y.; Luo, F.; Du, Y.P.; Yan, C.H. Antibacterial mechanism and activity of cerium oxide nanoparticles. Sci. China-Mater. 2019, 62, 1727–1739. [Google Scholar] [CrossRef] [Green Version]
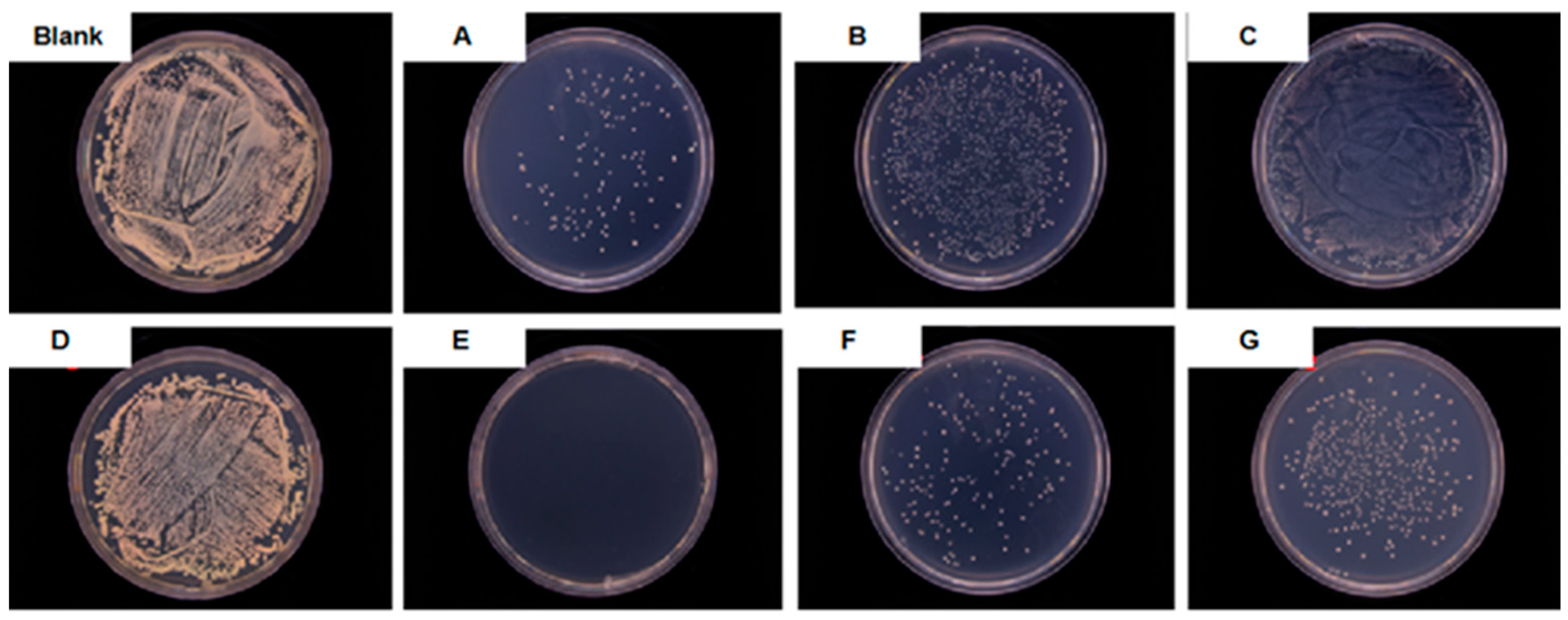
| Samples | Blank | A | B | C | D | E | F | G |
|---|---|---|---|---|---|---|---|---|
| Ag | 0 | 35 | 17.5 | 8.75 | 0 | 35 | 17.5 | 8.75 |
| CeO2 Concentration (µg/mL) | 0 | 0 | 0 | 0 | 2800 | 2800 | 2800 | 2800 |
| Antibacterial Rate (%) | ≈0 | 96 | 70 | ≈0 | ≈0 | ≈100 | 95 | 85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, M.; Liu, M.; Shen, G.; Gong, Y.; Wang, Z.; Ji, D.; Li, J.; Yuan, M.; Wang, Q. Preparation and Characterization of an Antibrowning Nanosized Ag-CeO2 Composite with Synergistic Antibacterial Ability. Materials 2023, 16, 5505. https://doi.org/10.3390/ma16165505
Yang M, Liu M, Shen G, Gong Y, Wang Z, Ji D, Li J, Yuan M, Wang Q. Preparation and Characterization of an Antibrowning Nanosized Ag-CeO2 Composite with Synergistic Antibacterial Ability. Materials. 2023; 16(16):5505. https://doi.org/10.3390/ma16165505
Chicago/Turabian StyleYang, Min, Mi Liu, Genli Shen, Yan Gong, Zhen Wang, Daiyu Ji, Jianqiang Li, Min Yuan, and Qi Wang. 2023. "Preparation and Characterization of an Antibrowning Nanosized Ag-CeO2 Composite with Synergistic Antibacterial Ability" Materials 16, no. 16: 5505. https://doi.org/10.3390/ma16165505








